Genetic Adjuvants in Replicating Single-Cycle Adenovirus Vectors Amplify Systemic and Mucosal Immune Responses against HIV-1 Envelope
Abstract
:1. Introduction
2. Materials and Methods
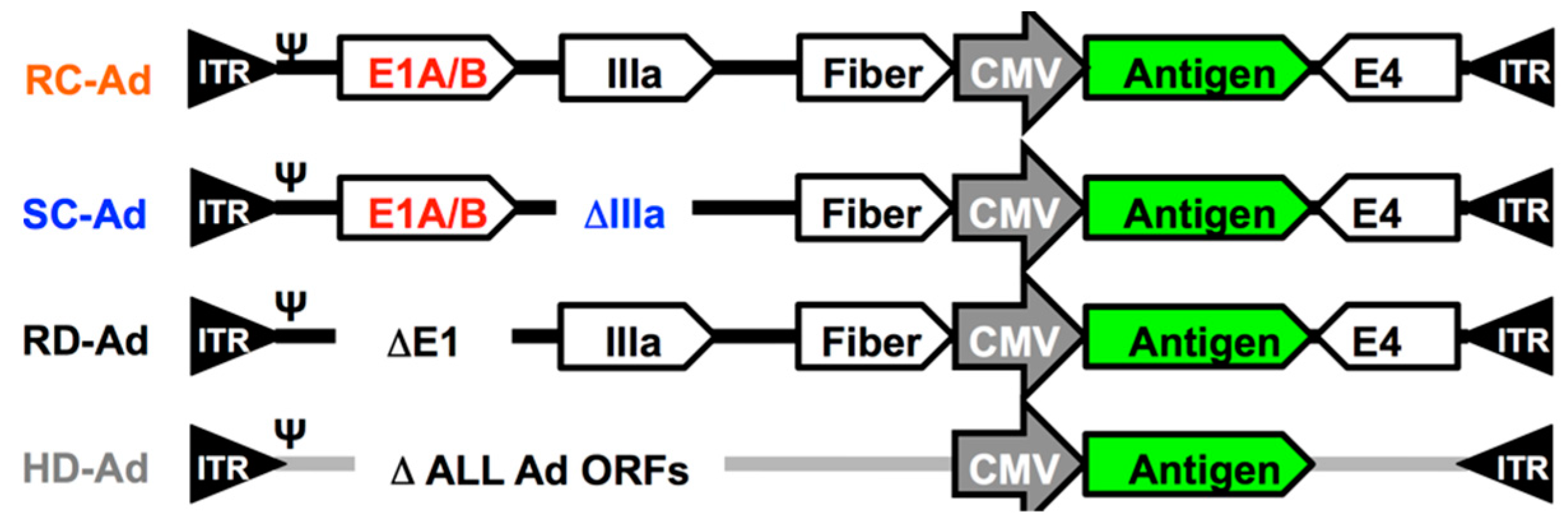
2.1. Single-Cycle Adenovirus Vectors
2.2. SOSIP Protein Vaccine
2.3. Animals
2.4. Immunizations and Sample Collection
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Sub-Isotyping Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Data Analysis
3. Results
3.1. SC-Ad6 Expressing Clade C HIV Envelope and Genetic Adjuvants
3.2. IM or IN Clade C SOSIP Protein Boost of SC-Ad-Env + SC-Ad-Adjuvants
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lehner, T.; Anton, P.A. Mucosal immunity and vaccination against hiv. Aids 2002, 16 (Suppl. S4), S125–S132. [Google Scholar] [CrossRef]
- Haase, A.T. Targeting early infection to prevent hiv-1 mucosal transmission. Nature 2010, 464, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.R.; Fitzgerald, J.C.; Giles-Davis, W.; Gao, G.P.; Wilson, J.M.; Ertl, H.C. Induction of cd8(+) t cells to an hiv-1 antigen through a prime boost regimen with heterologous e1-deleted adenoviral vaccine carriers. J. Immunol. 2003, 171, 6774–6779. [Google Scholar] [CrossRef] [PubMed]
- Barouch, D.H.; Pau, M.G.; Custers, J.H.; Koudstaal, W.; Kostense, S.; Havenga, M.J.; Truitt, D.M.; Sumida, S.M.; Kishko, M.G.; Arthur, J.C.; et al. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-ad5 immunity. J. Immunol. 2004, 172, 6290–6297. [Google Scholar] [CrossRef] [PubMed]
- Lemckert, A.A.; Sumida, S.M.; Holterman, L.; Vogels, R.; Truitt, D.M.; Lynch, D.M.; Nanda, A.; Ewald, B.A.; Gorgone, D.A.; Lifton, M.A.; et al. Immunogenicity of heterologous prime-boost regimens involving recombinant adenovirus serotype 11 (ad11) and ad35 vaccine vectors in the presence of anti-ad5 immunity. J. Virol. 2005, 79, 9694–9701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCoy, K.; Tatsis, N.; Korioth-Schmitz, B.; Lasaro, M.O.; Hensley, S.E.; Lin, S.W.; Li, Y.; Giles-Davis, W.; Cun, A.; Zhou, D.; et al. Effect of preexisting immunity to adenovirus human serotype 5 antigens on the immune responses of nonhuman primates to vaccine regimens based on human- or chimpanzee-derived adenovirus vectors. J. Virol. 2007, 81, 6594–6604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barouch, D.H.; Liu, J.; Li, H.; Maxfield, L.F.; Abbink, P.; Lynch, D.M.; Iampietro, M.J.; SanMiguel, A.; Seaman, M.S.; Ferrari, G.; et al. Vaccine protection against acquisition of neutralization-resistant siv challenges in rhesus monkeys. Nature 2012, 482, 89–93. [Google Scholar] [CrossRef] [Green Version]
- Barouch, D.H.; Alter, G.; Broge, T.; Linde, C.; Ackerman, M.E.; Brown, E.P.; Borducchi, E.N.; Smith, K.M.; Nkolola, J.P.; Liu, J.; et al. Protective efficacy of adenovirus-protein vaccines against siv challenges in rhesus monkeys. Science 2015, 349, 320–324. [Google Scholar] [CrossRef] [Green Version]
- Barouch, D.H.; Tomaka, F.L.; Wegmann, F.; Stieh, D.J.; Alter, G.; Robb, M.L.; Michael, N.L.; Peter, L.; Nkolola, J.P.; Borducchi, E.N.; et al. Evaluation of a mosaic hiv-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (approach) and in rhesus monkeys (nhp 13-19). Lancet 2018, 392, 232–243. [Google Scholar] [CrossRef]
- Mega, E.R. ‘Mosaic’ hiv vaccine to be tested in thousands of people across the world. Nature 2019, 572, 165–166. [Google Scholar] [CrossRef] [Green Version]
- Malkevitch, N.; Patterson, L.J.; Aldrich, K.; Richardson, E.; Alvord, W.G.; Robert-Guroff, M. A replication competent adenovirus 5 host range mutant-simian immunodeficiency virus (siv) recombinant priming/subunit protein boosting vaccine regimen induces broad, persistent siv-specific cellular immunity to dominant and subdominant epitopes in mamu-a*01 rhesus macaques. J. Immunol. 2003, 170, 4281–4289. [Google Scholar] [PubMed]
- Zhao, J.; Lou, Y.; Pinczewski, J.; Malkevitch, N.; Aldrich, K.; Kalyanaraman, V.S.; Venzon, D.; Peng, B.; Patterson, L.J.; Edghill-Smith, Y.; et al. Boosting of siv-specific immune responses in rhesus macaques by repeated administration of ad5hr-sivenv/rev and ad5hr-sivgag recombinants. Vaccine 2003, 21, 4022–4035. [Google Scholar] [CrossRef]
- Patterson, L.J.; Malkevitch, N.; Venzon, D.; Pinczewski, J.; Gomez-Roman, V.R.; Wang, L.; Kalyanaraman, V.S.; Markham, P.D.; Robey, F.A.; Robert-Guroff, M. Protection against mucosal simian immunodeficiency virus siv(mac251) challenge by using replicating adenovirus-siv multigene vaccine priming and subunit boosting. J. Virol. 2004, 78, 2212–2221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, B.; Wang, L.R.; Gomez-Roman, V.R.; Davis-Warren, A.; Montefiori, D.C.; Kalyanaraman, V.S.; Venzon, D.; Zhao, J.; Kan, E.; Rowell, T.J.; et al. Replicating rather than nonreplicating adenovirus-human immunodeficiency virus recombinant vaccines are better at eliciting potent cellular immunity and priming high-titer antibodies. J. Virol. 2005, 79, 10200–10209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinczewski, J.; Zhao, J.; Malkevitch, N.; Patterson, L.J.; Aldrich, K.; Alvord, W.G.; Robert-Guroff, M. Enhanced immunity and protective efficacy against sivmac251 intrarectal challenge following ad-siv priming by multiple mucosal routes and gp120 boosting in mpl-se. Viral Immunol. 2005, 18, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Roman, V.R.; Florese, R.H.; Patterson, L.J.; Peng, B.; Venzon, D.; Aldrich, K.; Robert-Guroff, M. A simplified method for the rapid fluorometric assessment of antibody-dependent cell-mediated cytotoxicity. J. Immunol. Methods 2006, 308, 53–67. [Google Scholar] [CrossRef]
- Gomez-Roman, V.R.; Florese, R.H.; Peng, B.; Montefiori, D.C.; Kalyanaraman, V.S.; Venzon, D.; Srivastava, I.; Barnett, S.W.; Robert-Guroff, M. An adenovirus-based hiv subtype b prime/boost vaccine regimen elicits antibodies mediating broad antibody-dependent cellular cytotoxicity against non-subtype b hiv strains. J. Acquir. Immune Defic. Syndr. 2006, 43, 270–277. [Google Scholar] [CrossRef]
- Peng, B.; Voltan, R.; Cristillo, A.D.; Alvord, W.G.; Davis-Warren, A.; Zhou, Q.; Murthy, K.K.; Robert-Guroff, M. Replicating ad-recombinants encoding non-myristoylated rather than wild-type hiv nef elicit enhanced cellular immunity. Aids 2006, 20, 2149–2157. [Google Scholar] [CrossRef]
- Demberg, T.; Florese, R.H.; Heath, M.J.; Larsen, K.; Kalisz, I.; Kalyanaraman, V.S.; Lee, E.M.; Pal, R.; Venzon, D.; Grant, R.; et al. A replication-competent adenovirus-human immunodeficiency virus (ad-hiv) tat and ad-hiv env priming/tat and envelope protein boosting regimen elicits enhanced protective efficacy against simian/human immunodeficiency virus shiv89.6p challenge in rhesus macaques. J. Virol. 2007, 81, 3414–3427. [Google Scholar]
- Hidajat, R.; Xiao, P.; Zhou, Q.; Venzon, D.; Summers, L.E.; Kalyanaraman, V.S.; Montefiori, D.C.; Robert-Guroff, M. Correlation of vaccine-elicited systemic and mucosal non-neutralizing antibody activities with reduced acute viremia following intrarectal sivmac251 challenge of rhesus macaques. J. Virol. 2009, 83, 791–801. [Google Scholar] [CrossRef] [Green Version]
- Morgan, C.; Marthas, M.; Miller, C.; Duerr, A.; Cheng-Mayer, C.; Desrosiers, R.; Flores, J.; Haigwood, N.; Hu, S.L.; Johnson, R.P.; et al. The use of nonhuman primate models in hiv vaccine development. PLoS medicine 2008, 5, e173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demberg, T.; Robert-Guroff, M. Mucosal immunity and protection against hiv/siv infection: Strategies and challenges for vaccine design. Int. Rev. Immunol. 2009, 28, 20–48. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, H.; Ma, Z.M.; Huang, Y.; Hodge, G.; Thomas, M.A.; DiPasquale, J.; DeSilva, V.; Fritts, L.; Bett, A.J.; Casimiro, D.R.; et al. Low-dose penile sivmac251 exposure of rhesus macaques infected with adenovirus type 5 (ad5) and then immunized with a replication-defective ad5-based siv gag/pol/nef vaccine recapitulates the results of the phase iib step trial of a similar hiv-1 vaccine. J. Virol. 2012, 86, 2239–2250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakhashe, S.K.; Velu, V.; Sciaranghella, G.; Siddappa, N.B.; Dipasquale, J.M.; Hemashettar, G.; Yoon, J.K.; Rasmussen, R.A.; Yang, F.; Lee, S.J.; et al. Prime-boost vaccination with heterologous live vectors encoding siv gag and multimeric hiv-1 gp160 protein: Efficacy against repeated mucosal r5 clade c shiv challenges. Vaccine 2011, 29, 5611–5622. [Google Scholar] [CrossRef] [Green Version]
- Xiao, P.; Patterson, L.J.; Kuate, S.; Brocca-Cofano, E.; Thomas, M.A.; Venzon, D.; Zhao, J.; DiPasquale, J.; Fenizia, C.; Lee, E.M.; et al. Replicating adenovirus-simian immunodeficiency virus (siv) recombinant priming and envelope protein boosting elicits localized, mucosal iga immunity in rhesus macaques correlated with delayed acquisition following a repeated low-dose rectal siv(mac251) challenge. J. Virol. 2012, 86, 4644–4657. [Google Scholar]
- Patterson, L.J.; Kuate, S.; Daltabuit-Test, M.; Li, Q.; Xiao, P.; McKinnon, K.; DiPasquale, J.; Cristillo, A.; Venzon, D.; Haase, A.; et al. Replicating adenovirus-simian immunodeficiency virus (siv) vectors efficiently prime siv-specific systemic and mucosal immune responses by targeting myeloid dendritic cells and persisting in rectal macrophages, regardless of immunization route. Clin. Vaccine Immunol. 2012, 19, 629–637. [Google Scholar] [CrossRef]
- Malkevitch, N.V.; Patterson, L.J.; Aldrich, M.K.; Wu, Y.; Venzon, D.; Florese, R.H.; Kalyanaraman, V.S.; Pal, R.; Lee, E.M.; Zhao, J.; et al. Durable protection of rhesus macaques immunized with a replicating adenovirus-siv multigene prime/protein boost vaccine regimen against a second sivmac251 rectal challenge: Role of siv-specific cd8+ t cell responses. Virology 2006, 353, 83–98. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Roman, V.R.; Robert-Guroff, M. Adenoviruses as vectors for hiv vaccines. AIDS Rev. 2003, 5, 178–185. [Google Scholar]
- Buge, S.L.; Richardson, E.; Alipanah, S.; Markham, P.; Cheng, S.; Kalyan, N.; Miller, C.J.; Lubeck, M.; Udem, S.; Eldridge, J.; et al. An adenovirus-simian immunodeficiency virus env vaccine elicits humoral, cellular, and mucosal immune responses in rhesus macaques and decreases viral burden following vaginal challenge. J. Virol. 1997, 71, 8531–8541. [Google Scholar] [CrossRef] [Green Version]
- Lubeck, M.D.; Natuk, R.; Myagkikh, M.; Kalyan, N.; Aldrich, K.; Sinangil, F.; Alipanah, S.; Murthy, S.C.; Chanda, P.K.; Nigida, S.M., Jr.; et al. Long-term protection of chimpanzees against high-dose hiv-1 challenge induced by immunization. Nat. Med. 1997, 3, 651–658. [Google Scholar] [CrossRef]
- Robert-Guroff, M.; Kaur, H.; Patterson, L.J.; Leno, M.; Conley, A.J.; McKenna, P.M.; Markham, P.D.; Richardson, E.; Aldrich, K.; Arora, K.; et al. Vaccine protection against a heterologous, non-syncytium-inducing, primary human immunodeficiency virus. J. Virol. 1998, 72, 10275–10280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buge, S.L.; Murty, L.; Arora, K.; Kalyanaraman, V.S.; Markham, P.D.; Richardson, E.S.; Aldrich, K.; Patterson, L.J.; Miller, C.J.; Cheng, S.M.; et al. Factors associated with slow disease progression in macaques immunized with an adenovirus-simian immunodeficiency virus (siv) envelope priming-gp120 boosting regimen and challenged vaginally with sivmac251. J. Virol. 1999, 73, 7430–7440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zolla-Pazner, S.; Lubeck, M.; Xu, S.; Burda, S.; Natuk, R.J.; Sinangil, F.; Steimer, K.; Gallo, R.C.; Eichberg, J.W.; Matthews, T.; et al. Induction of neutralizing antibodies to t-cell line-adapted and primary human immunodeficiency virus type 1 isolates with a prime-boost vaccine regimen in chimpanzees. J. Virol. 1998, 72, 1052–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, E.A.; Nehete, P.N.; Buchl, S.S.; Senac, J.S.; Palmer, D.; Ng, P.; Sastry, K.J.; Barry, M.A. Comparison of replication-competent, first generation, and helper-dependent adenoviral vaccines. PLoS ONE 2009, 4, e5059. [Google Scholar] [CrossRef]
- Weaver, E.A.; Barry, M.A. Low seroprevalent species d adenovirus vectors as influenza vaccines. PLoS ONE 2013, 8, e73313. [Google Scholar] [CrossRef]
- Weaver, E.A.; Rubrum, A.M.; Webby, R.J.; Barry, M.A. Protection against divergent influenza h1n1 virus by a centralized influenza hemagglutinin. PLoS ONE 2011, 6, e18314. [Google Scholar] [CrossRef]
- Crosby, C.M.; Barry, M.A. Iiia deleted adenovirus as a single-cycle genome replicating vector. Virology 2014, 462–463, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Crosby, C.M.; Nehete, P.; Sastry, K.J.; Barry, M.A. Amplified and persistent immune responses generated by single-cycle replicating adenovirus vaccines. J. Virol. 2015, 89, 669–675. [Google Scholar] [CrossRef] [Green Version]
- Couch, R.B.; Chanock, R.M.; Cate, T.R.; Lang, D.J.; Knight, V.; Huebner, R.J. Immunization with types 4 and 7 adenovirus by selective infection of the intestinal tract. Am. Rev. Respir. Dis. 1963, 88, 394–403. [Google Scholar]
- Matsuda, K.; Huang, J.; Zhou, T.; Sheng, Z.; Kang, B.H.; Ishida, E.; Griesman, T.; Stuccio, S.; Bolkhovitinov, L.; Wohlbold, T.J.; et al. Prolonged evolution of the memory b cell response induced by a replicating adenovirus-influenza h5 vaccine. Sci. Immunol. 2019, 4. [Google Scholar] [CrossRef]
- Crosby, C.M.; Matchett, W.E.; Anguiano-Zarate, S.S.; Parks, C.A.; Weaver, E.A.; Pease, L.R.; Webby, R.J.; Barry, M.A. Replicating single-cycle adenovirus vectors generate amplified influenza vaccine responses. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crosby, C.M.; Barry, M.A. Transgene expression and host cell responses to replication-defective, single-cycle, and replication-competent adenovirus vectors. Genes 2017, 8, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matchett, W.E.; Anguiano-Zarate, S.S.; Nehete, P.N.; Shelton, K.; Nehete, B.P.; Yang, G.; Dorta-Estremera, S.; Barnette, P.; Xiao, P.; Byrareddy, S.N.; et al. Divergent hiv-1-directed immune responses generated by systemic and mucosal immunization with replicating single-cycle adenoviruses in rhesus macaques. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matchett, W.E.; Anguiano-Zarate, S.S.; Barry, M.A. Comparison of systemic and mucosal immunization with replicating single-cycle adenoviruses. Glob. Vaccines Immunol. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matchett, W.E.; Anguiano-Zarate, S.S.; Barry, M.A. A replicating single cycle adenovirus against clostridium difficile. J. Infectious Dis. under review.
- Barry, M. Single-cycle adenovirus vectors in the current vaccine landscape. Expert Rev. Vaccines 2018, 17, 163–173. [Google Scholar] [CrossRef]
- Anguiano-Zarate, S.S.; Matchett, W.E.; Nehete, P.N.; Sastry, J.K.; Marzi, A.; Barry, M.A. A replicating single-cycle adenovirus vaccine against ebola virus. J. Infect. Dis. 2018, 218, 1883–1889. [Google Scholar] [CrossRef] [Green Version]
- Turner, M.A.; Middha, S.; Hofherr, S.E.; Barry, M.A. Comparison of the life cycles of genetically distant species c and species d human adenoviruses ad6 and ad26 in human cells. J. Virol. 2015, 89, 12401–12417. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Z.; Ertl, H.C. Manipulation of the immune response to a plasmid-encoded viral antigen by coinoculation with plasmids expressing cytokines. Immunity 1995, 2, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Barry, M.A.; Johnston, S.A. Biological features of genetic immunization. Vaccine 1996, 15, 788–791. [Google Scholar] [CrossRef]
- Kuchroo, V.K.; Das, M.P.; Brown, J.A.; Ranger, A.M.; Zamvil, S.S.; Sobel, R.A.; Weiner, H.L.; Nabavi, N.; Glimcher, L.H. B7-1 and b7-2 costimulatory molecules activate differentially the th1/th2 developmental pathways: Application to autoimmune disease therapy. Cell 1995, 80, 707–718. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Boyer, J.D.; Kim, J.; Adagjanyan, M.; Ayyavoo, V.; Ugen, K.E.; Chattergoon, M.; Kudchodkar, S.; Javadian, A.; Frost, P.; et al. DNA Immunization for hiv-1: Enhancement of the Immune Response through Combination Antigen Design and Multiple Plasmid Delivery; Nucleic Acid Vaccines for the Prevention of Infectious Diseases: Bethesda, MD, USA, 1996. [Google Scholar]
- Boyer, J.D.; Ugen, K.E.; Wang, B.; Agadjanyan, M.; Gilbert, L.; Bagarazzi, M.L.; Chattergoon, M.; Frost, P.; Javadian, A.; Williams, W.V.; et al. Protection of chimpanzees from high-dose heterologous hiv-1 challenge by DNA vaccination [see comments]. Nat. Med. 1997, 3, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Barouch, D.H.; Santra, S.; Schmitz, J.E.; Kuroda, M.J.; Fu, T.M.; Wagner, W.; Bilska, M.; Craiu, A.; Zheng, X.X.; Krivulka, G.R.; et al. Control of viremia and prevention of clinical aids in rhesus monkeys by cytokine-augmented DNA vaccination. Science 2000, 290, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Siddappa, N.B.; Song, R.; Kramer, V.G.; Chenine, A.L.; Velu, V.; Ong, H.; Rasmussen, R.A.; Grisson, R.D.; Wood, C.; Zhang, H.; et al. Neutralization-sensitive r5-tropic simian-human immunodeficiency virus shiv-2873nip, which carries env isolated from an infant with a recent hiv clade c infection. J. Virol. 2009, 83, 1422–1432. [Google Scholar] [CrossRef] [Green Version]
- Ringe, R.P.; Yasmeen, A.; Ozorowski, G.; Go, E.P.; Pritchard, L.K.; Guttman, M.; Ketas, T.A.; Cottrell, C.A.; Wilson, I.A.; Sanders, R.W.; et al. Influences on the design and purification of soluble, recombinant native-like hiv-1 envelope glycoprotein trimers. J. Virol. 2015, 89, 12189–12210. [Google Scholar] [CrossRef] [Green Version]
- Alexander, M.R.; Ringe, R.; Sanders, R.W.; Voss, J.E.; Moore, J.P.; Klasse, P.J. What do chaotrope-based avidity assays for antibodies to hiv-1 envelope glycoproteins measure? J. Virol. 2015, 89, 5981–5995. [Google Scholar] [CrossRef] [Green Version]
- Ringe, R.P.; Ozorowski, G.; Yasmeen, A.; Cupo, A.; Cruz Portillo, V.M.; Pugach, P.; Golabek, M.; Rantalainen, K.; Holden, L.G.; Cottrell, C.A.; et al. Improving the expression and purification of soluble, recombinant native-like hiv-1 envelope glycoprotein trimers by targeted sequence changes. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Nehete, P.N.; Yang, G.; He, H.; Nehete, B.; Hanley, P.W.; Barry, M.A.; Sastry, K.J. Enhancement of mucosal immunogenicity of viral vectored vaccines by the nkt cell agonist alpha-galactosylceramide as adjuvant. Vaccines 2014, 2, 686–706. [Google Scholar] [CrossRef]
- Tumpey, T.M.; Renshaw, M.; Clements, J.D.; Katz, J.M. Mucosal delivery of inactivated influenza vaccine induces b-cell-dependent heterosubtypic cross-protection against lethal influenza a h5n1 virus infection. J. Virol. 2001, 75, 5141–5150. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, B.L.; Clements, J.D. Dissociation of escherichia coli heat-labile enterotoxin adjuvanticity from adp-ribosyltransferase activity. Infect. Immun. 1995, 63, 1617–1623. [Google Scholar] [CrossRef] [Green Version]
- Lomada, D.; Gambhira, R.; Nehete, P.N.; Guhad, F.A.; Chopra, A.K.; Peterson, J.W.; Sastry, K.J. A two-codon mutant of cholera toxin lacking adp-ribosylating activity functions as an effective adjuvant for eliciting mucosal and systemic cellular immune responses to peptide antigens. Vaccine 2004, 23, 555–565. [Google Scholar] [CrossRef]
- Pugach, P.; Ozorowski, G.; Cupo, A.; Ringe, R.; Yasmeen, A.; de Val, N.; Derking, R.; Kim, H.J.; Korzun, J.; Golabek, M.; et al. A native-like sosip.664 trimer based on an hiv-1 subtype b env gene. J. Virol. 2015, 89, 3380–3395. [Google Scholar] [CrossRef] [Green Version]
- Cardenas-Freytag, L.; Cheng, E.; Mirza, A. New approaches to mucosal immunization. Adv. Exp. Med. Biol. 1999, 473, 319–337. [Google Scholar]
- Wu, H.Y.; Russell, M.W. Nasal lymphoid tissue, intranasal immunization, and compartmentalization of the common mucosal immune system. Immunol. Res. 1997, 16, 187–201. [Google Scholar] [CrossRef]
- Kim, J.H.; Excler, J.L.; Michael, N.L. Lessons from the rv144 thai phase iii hiv-1 vaccine trial and the search for correlates of protection. Annu. Rev. Med. 2015, 66, 423–437. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Yang, G.; Byrareddy, S.N.; Barry, M.A.; Sastry, K.J. Natural killer t cell and tlr9 agonists as mucosal adjuvants for sublingual vaccination with clade c hiv-1 envelope protein. Vaccine 2014, 32, 6934–6940. [Google Scholar] [CrossRef] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matchett, W.E.; Malewana, G.B.R.; Mudrick, H.; Medlyn, M.J.; Barry, M.A. Genetic Adjuvants in Replicating Single-Cycle Adenovirus Vectors Amplify Systemic and Mucosal Immune Responses against HIV-1 Envelope. Vaccines 2020, 8, 64. https://doi.org/10.3390/vaccines8010064
Matchett WE, Malewana GBR, Mudrick H, Medlyn MJ, Barry MA. Genetic Adjuvants in Replicating Single-Cycle Adenovirus Vectors Amplify Systemic and Mucosal Immune Responses against HIV-1 Envelope. Vaccines. 2020; 8(1):64. https://doi.org/10.3390/vaccines8010064
Chicago/Turabian StyleMatchett, William E., Goda Baddage Rakitha Malewana, Haley Mudrick, Michael J. Medlyn, and Michael A. Barry. 2020. "Genetic Adjuvants in Replicating Single-Cycle Adenovirus Vectors Amplify Systemic and Mucosal Immune Responses against HIV-1 Envelope" Vaccines 8, no. 1: 64. https://doi.org/10.3390/vaccines8010064
APA StyleMatchett, W. E., Malewana, G. B. R., Mudrick, H., Medlyn, M. J., & Barry, M. A. (2020). Genetic Adjuvants in Replicating Single-Cycle Adenovirus Vectors Amplify Systemic and Mucosal Immune Responses against HIV-1 Envelope. Vaccines, 8(1), 64. https://doi.org/10.3390/vaccines8010064




