Vaccination with Aedes aegypti AgBR1 Delays Lethal Mosquito-Borne Zika Virus Infection in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Viruses and Cell Lines
2.3. Mosquitoes and Animals
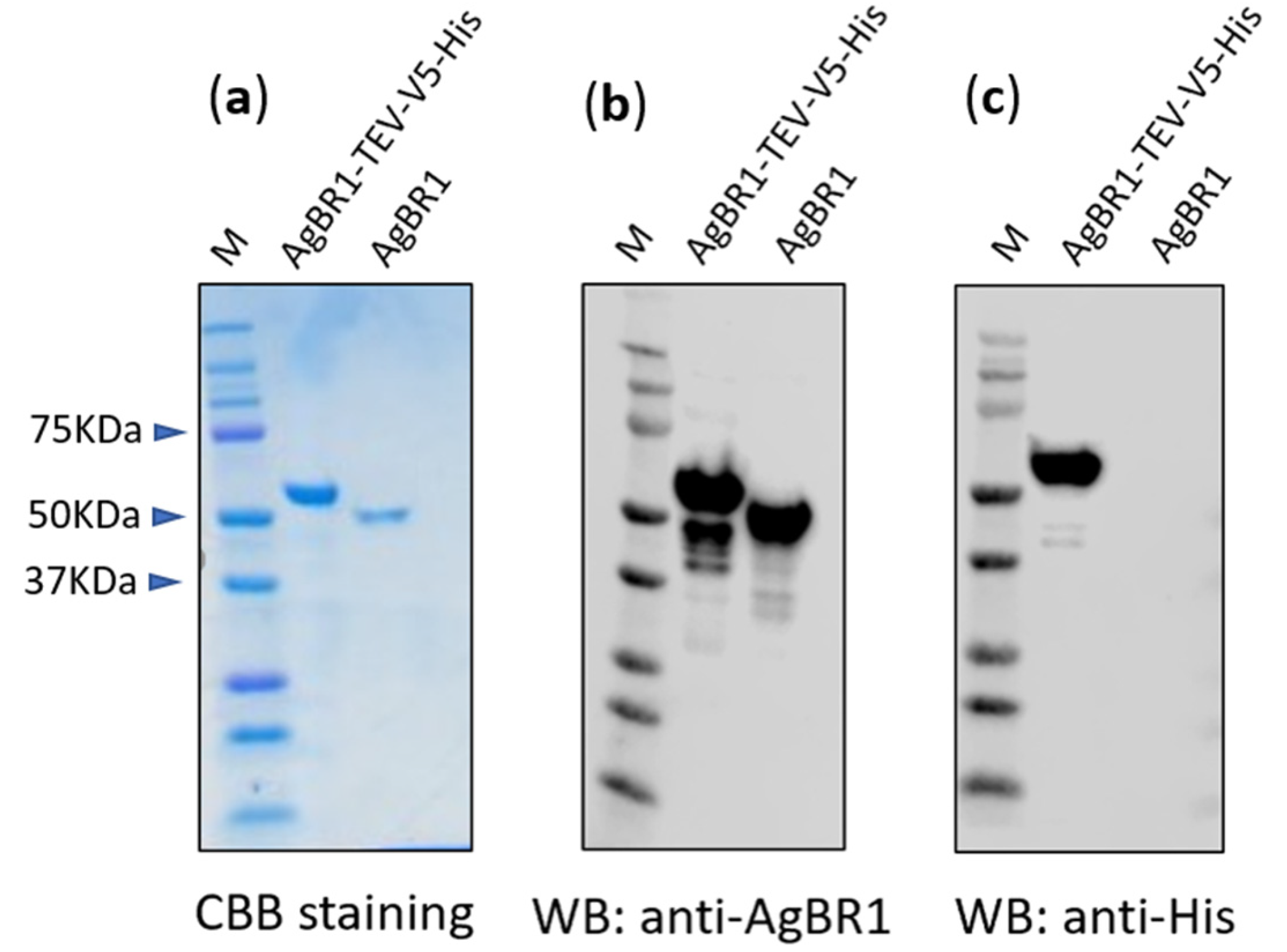
2.4. Plasmids and the Purification of Recombinant Proteins
2.5. Immunoblotting
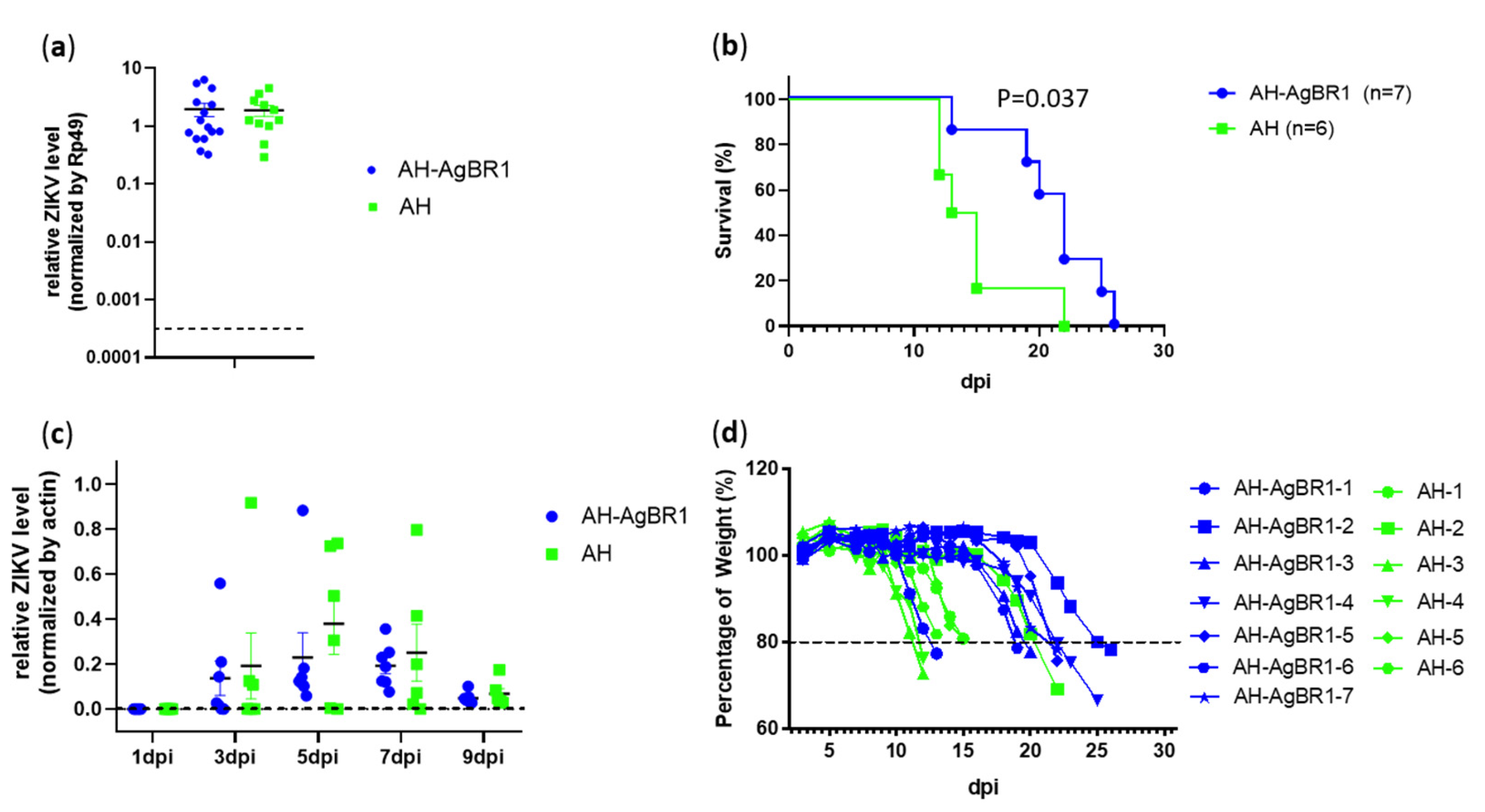
2.6. Active Immunization Studies
2.7. Quantitative Real Time PCR
2.8. ELISA
2.9. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- Huang, Y.J.; Higgs, S.; Horne, K.M.; Vanlandingham, D.L. Flavivirus-mosquito interactions. Viruses 2014, 6, 4703–4730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, S.A.; Jamieson, D.J.; Honein, M.A.; Petersen, L.R. Zika virus and birth defects--reviewing the evidence for causality. N. Engl. J. Med. 2016, 374, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquale, A.; Preiss, S.; Tavares Da Silva, F.; Garcon, N. Vaccine adjuvants: From 1920 to 2015 and beyond. Vaccines (Basel) 2015, 3, 320–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, M.J.; Duehr, J.; Dulin, H.; Broecker, F.; Brown, J.A.; Arumemi, F.O.; Bermudez Gonzalez, M.C.; Leyva-Grado, V.H.; Evans, M.J.; Simon, V.; et al. Human antibodies targeting zika virus ns1 provide protection against disease in a mouse model. Nat. Commun. 2018, 9, 4560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogenesch, H. Mechanism of immunopotentiation and safety of aluminum adjuvants. Front. Immunol. 2012, 3, 406. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.H.; Lee, J.A.; Park, S.Y.; Song, C.S.; Choi, I.S.; Lee, J.B. A review of vaccine development and research for industry animals in korea. Clin. Exp. Vaccine Res. 2012, 1, 18–34. [Google Scholar] [CrossRef] [Green Version]
- Manning, J.E.; Morens, D.M.; Kamhawi, S.; Valenzuela, J.G.; Memoli, M. Mosquito saliva: The hope for a universal arbovirus vaccine? J. Infect. Dis. 2018, 218, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Hastings, A.K.; Uraki, R.; Gaitsch, H.; Dhaliwal, K.; Stanley, S.; Sproch, H.; Williamson, E.; MacNeil, T.; Marin-Lopez, A.; Hwang, J.; et al. Aedes aegypti nest1 protein enhances zika virus pathogenesis by activating neutrophils. J. Virol. 2019, 93, e00395-19. [Google Scholar] [CrossRef] [Green Version]
- Pompon, J.; Manuel, M.; Ng, G.K.; Wong, B.; Shan, C.; Manokaran, G.; Soto-Acosta, R.; Bradrick, S.S.; Ooi, E.E.; Misse, D.; et al. Dengue subgenomic flaviviral rna disrupts immunity in mosquito salivary glands to increase virus transmission. PLoS Pathog. 2017, 13, e1006535. [Google Scholar] [CrossRef]
- Mazeaud, C.; Freppel, W.; Chatel-Chaix, L. The multiples fates of the flavivirus rna genome during pathogenesis. Front. Genet. 2018, 9, 595. [Google Scholar] [CrossRef]
- Jin, L.; Guo, X.; Shen, C.; Hao, X.; Sun, P.; Li, P.; Xu, T.; Hu, C.; Rose, O.; Zhou, H.; et al. Salivary factor ltrin from aedes aegypti facilitates the transmission of zika virus by interfering with the lymphotoxin-beta receptor. Nat. Immunol. 2018, 19, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Colpitts, T.M.; Conway, M.J.; Montgomery, R.R.; Fikrig, E. West nile virus: Biology, transmission, and human infection. Clin. Microbiol. Rev. 2012, 25, 635–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Zhu, Y.; Xiao, X.; Wang, P.; Cheng, G. Progress towards understanding the mosquito-borne virus life cycle. Trends Parasitol. 2019, 35, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Uraki, R.; Hastings, A.K.; Marin-Lopez, A.; Sumida, T.; Takahashi, T.; Grover, J.R.; Iwasaki, A.; Hafler, D.A.; Montgomery, R.R.; Fikrig, E. Aedes aegypti agbr1 antibodies modulate early zika virus infection of mice. Nat. Microbiol. 2019, 4, 948–955. [Google Scholar] [CrossRef]
- Baxter, D. Active and passive immunity, vaccine types, excipients and licensing. Occup. Med. (Lond.) 2007, 57, 552–556. [Google Scholar] [CrossRef] [Green Version]
- Kang, G.; Chen, H.; Ma, F.; Yang, Y.; Wang, Z.; Guo, S.; Song, J. Comparison of the effect of increased hepatitis b vaccine dosage on immunogenicity in healthy children and adults. Hum. Vaccin. Immunother. 2016, 12, 2312–2316. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Ji, Z.; Liang, P.; Wu, Q.; Cui, F.; Wang, F.; Liang, X.; Zhuang, G. The doses of 10 mug should replace the doses of 5 mug in newborn hepatitis b vaccination in china: A cost-effectiveness analysis. Vaccine 2015, 33, 3731–3738. [Google Scholar] [CrossRef]
- Fu, S.; Xu, J.; Li, X.; Xie, Y.; Qiu, Y.; Du, X.; Yu, S.; Bai, Y.; Chen, Y.; Wang, T.; et al. Immunization of mice with recombinant protein cobb or asnc confers protection against brucella abortus infection. PLoS ONE 2012, 7, e29552. [Google Scholar] [CrossRef]
- Coler, R.N.; Skeiky, Y.A.; Bernards, K.; Greeson, K.; Carter, D.; Cornellison, C.D.; Modabber, F.; Campos-Neto, A.; Reed, S.G. Immunization with a polyprotein vaccine consisting of the t-cell antigens thiol-specific antioxidant, leishmania major stress-inducible protein 1, and leishmania elongation initiation factor protects against leishmaniasis. Infect. Immun. 2002, 70, 4215–4225. [Google Scholar] [CrossRef] [Green Version]
- Bs, R.; Hms, K. Optimization of recombinant vaccine antigen dose in mouse model by elisa and immunophenotyping. Glob. Vaccines Immunol. 2017, 2, 1–5. [Google Scholar] [CrossRef]
- Frew, B.C.; Joag, V.R.; Mogridge, J. Proteolytic processing of nlrp1b is required for inflammasome activity. PLoS Pathog. 2012, 8, e1002659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulendran, B.; Artis, D. New paradigms in type 2 immunity. Science 2012, 337, 431–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Bon, A.; Schiavoni, G.; D’Agostino, G.; Gresser, I.; Belardelli, F.; Tough, D.F. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity 2001, 14, 461–470. [Google Scholar] [CrossRef] [Green Version]
- Sumathy, K.; Kulkarni, B.; Gondu, R.K.; Ponnuru, S.K.; Bonguram, N.; Eligeti, R.; Gadiyaram, S.; Praturi, U.; Chougule, B.; Karunakaran, L.; et al. Protective efficacy of zika vaccine in ag129 mouse model. Sci. Rep. 2017, 7, 46375. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.W.; Young, M.P.; Mamidi, A.; Regla-Nava, J.A.; Kim, K.; Shresta, S. A mouse model of zika virus sexual transmission and vaginal viral replication. Cell Rep. 2016, 17, 3091–3098. [Google Scholar] [CrossRef] [Green Version]
- Rossi, S.L.; Tesh, R.B.; Azar, S.R.; Muruato, A.E.; Hanley, K.A.; Auguste, A.J.; Langsjoen, R.M.; Paessler, S.; Vasilakis, N.; Weaver, S.C. Characterization of a novel murine model to study zika virus. Am. J. Trop. Med. Hyg. 2016, 94, 1362–1369. [Google Scholar] [CrossRef] [Green Version]
- Di Guardo, G. Commentary: Zika virus in the americas-yet another arbovirus threat. Front. Microbiol. 2018, 9, 435. [Google Scholar] [CrossRef]
- Plourde, A.R.; Bloch, E.M. A literature review of zika virus. Emerg. Infect. Dis. 2016, 22, 1185–1192. [Google Scholar] [CrossRef] [Green Version]
- Boyer, S.; Calvez, E.; Chouin-Carneiro, T.; Diallo, D.; Failloux, A.B. An overview of mosquito vectors of zika virus. Microbes Infect. 2018, 20, 646–660. [Google Scholar] [CrossRef]

| Mouse Number | AgBR1 Antibody Titiers (Serum Dilution: 1:100, OD450) | Date of Death (Day Post Infection) |
|---|---|---|
| AH | ||
| AH-3 | 0.409 | 12 |
| AH-4 | 0.200 | 12 |
| AH-6 | 0.316 | 13 |
| AH-5 | 0.346 | 15 |
| AH-1 | 0.562 | 15 |
| AH-2 | 0.213 | 22 |
| AH-AgBR1 | ||
| AH-AgBR1-1 | 0.727 | 13 |
| AH-AgBR1-6 | 0.260 | 19 |
| AH-AgBR1-3 | 0.349 | 20 |
| AH-AgBR1-7 | 0.310 | 22 |
| AH-AgBR1-5 | 1.928 | 22 |
| AH-AgBR1-4 | 1.971 | 25 |
| AH-AgBR1-2 | 2.262 | 26 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Marin-Lopez, A.; Jiang, J.; Ledizet, M.; Fikrig, E. Vaccination with Aedes aegypti AgBR1 Delays Lethal Mosquito-Borne Zika Virus Infection in Mice. Vaccines 2020, 8, 145. https://doi.org/10.3390/vaccines8020145
Wang Y, Marin-Lopez A, Jiang J, Ledizet M, Fikrig E. Vaccination with Aedes aegypti AgBR1 Delays Lethal Mosquito-Borne Zika Virus Infection in Mice. Vaccines. 2020; 8(2):145. https://doi.org/10.3390/vaccines8020145
Chicago/Turabian StyleWang, Yuchen, Alejandro Marin-Lopez, Junjun Jiang, Michel Ledizet, and Erol Fikrig. 2020. "Vaccination with Aedes aegypti AgBR1 Delays Lethal Mosquito-Borne Zika Virus Infection in Mice" Vaccines 8, no. 2: 145. https://doi.org/10.3390/vaccines8020145
APA StyleWang, Y., Marin-Lopez, A., Jiang, J., Ledizet, M., & Fikrig, E. (2020). Vaccination with Aedes aegypti AgBR1 Delays Lethal Mosquito-Borne Zika Virus Infection in Mice. Vaccines, 8(2), 145. https://doi.org/10.3390/vaccines8020145




