Is an Increased Risk of Developing Guillain–Barré Syndrome Associated with Seasonal Influenza Vaccination? A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
3. Results
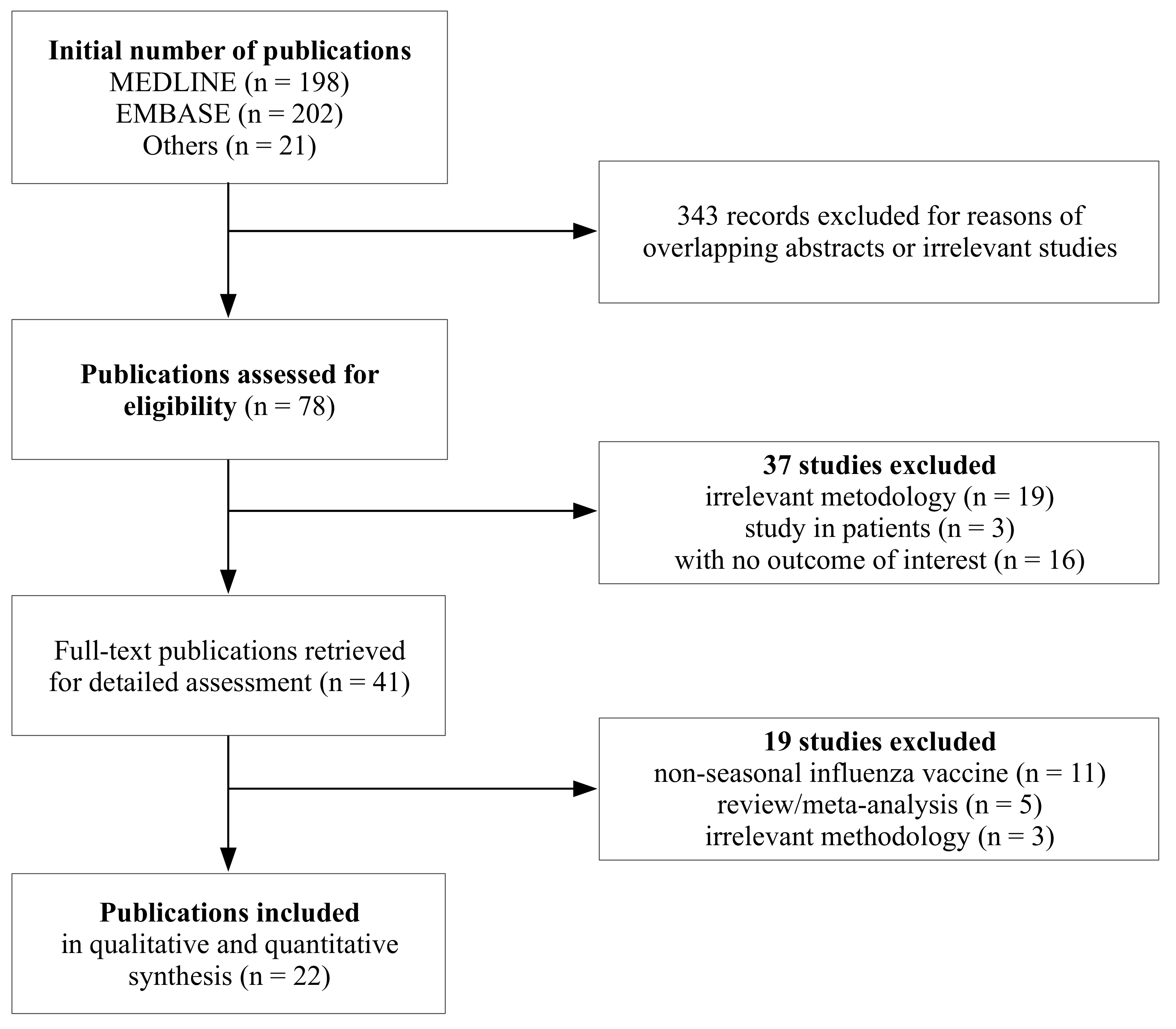
3.1. Selection and Characteristics of Studies
3.2. iTIV Vaccination and GBS Risk
3.3. Estimate of GBS Risk in Specific Subgroups
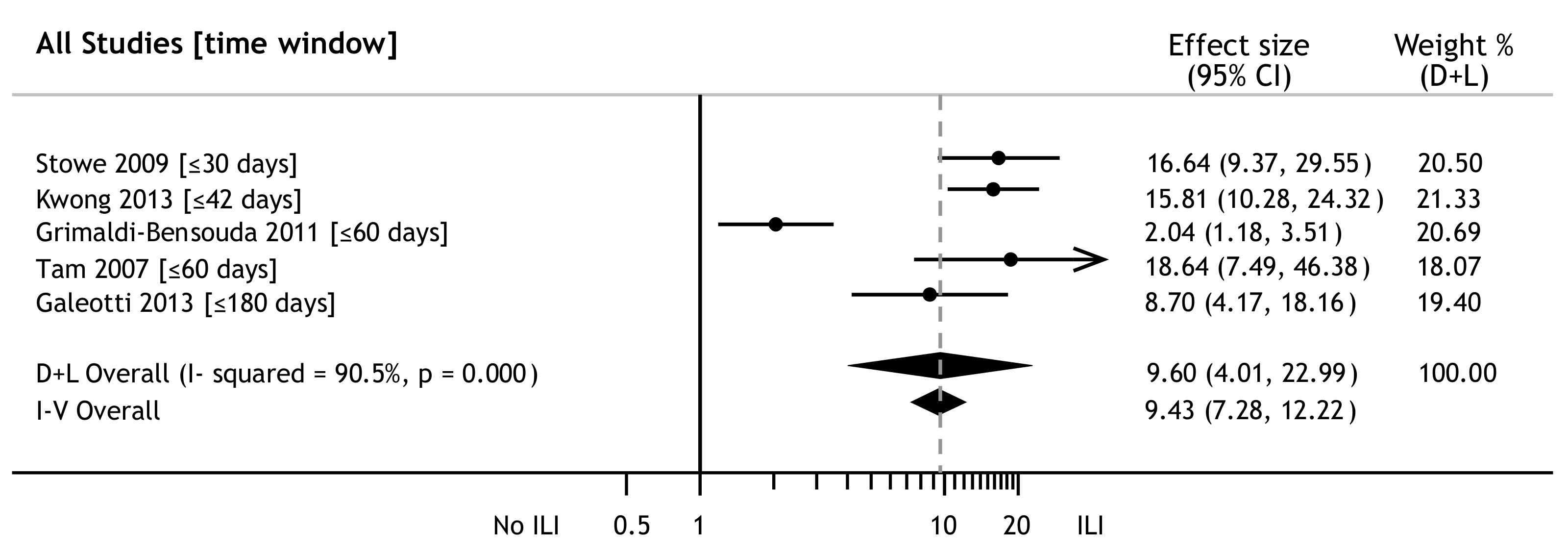
3.4. Influenza and GBS Risk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sejvar, J.J.; Baughman, A.L.; Wise, M.; Morgan, O.W. Population incidence of Guillain-Barre syndrome: A systematic review and meta-analysis. Neuroepidemiology 2011, 36, 123–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shui, I.M.; Rett, M.; Weintraub, E.; Marcy, M.; Amato, A.A.; Sheikh, S.I. Vaccine safety datalink research team. Guillain-Barré syndrome incidence in a large United States cohort (2000–2009). Neuroepidemiology 2012, 39, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, H.C.; Hartung, H.-P.; Kieseier, B.C.; Hughes, R.A.C. Guillain-Barre syndrome after exposure to influenza virus. Lancet Infect. Dis. 2010, 10, 643–651. [Google Scholar] [CrossRef]
- Tam, C.C.; O’Brien, S.J.; Petersen, I.; Islam, A.; Hayward, A.; Rodrigues, L.C. Guillain-Barre syndrome and preceding infection with Campylobacter, influenza and Epstein-Barr virus in the General Practice Research Database. PLoS ONE 2007, 2, e344. [Google Scholar] [CrossRef] [Green Version]
- Tam, C.C.; O’Brien, S.J.; Rodrigues, L.C. Influenza, Campylobacter and Mycoplasma infections, and hospital admissions for Guillain-Barre syndrome. England Emerg. Infect. Dis. 2006, 12, 1880–1887. [Google Scholar] [CrossRef] [PubMed]
- Hardy, T.A.; Blum, S.; McCombe, P.A.; Reddel, S.W. Guillain-Barr syndrome: Modern theories of etiology. Curr. Allergy Asthma Rep. 2011, 11, 197–204. [Google Scholar] [CrossRef]
- Yuki, N.; Hartung, H.P. Guillain-Barre syndrome. N. Engl. J. Med. 2012, 366, 2294–2304. [Google Scholar] [CrossRef]
- Schonberger, L.B.; Bregman, D.J.; Sullivan-Bolyai, J.Z.; Keenlyside, R.A.; Ziegler, D.W.; Retailliau, H.F.; Eddins, D.L.; Bryan, J.A. Guillain-Barré syndrome following vaccination n the National Influenza Immunization Program, United States, 1976–1977. Am. J. Epidemiol. 1979, 110, 105–123. [Google Scholar] [CrossRef]
- Langmuir, A.D.; Bregman, D.J.; Kurland, L.T.; Nathanson, N.; Victor, M. An epidemiologic and clinical evaluation of Guillain-Barré syndrome reported in association with the administration of swine influenza vaccine. Am. J. Epidemiol. 1984, 119, 841–879. [Google Scholar] [CrossRef]
- Safranek, T.J.; Lawrence, D.N.; Kurland, L.T.; Culver, D.H.; Wiederholt, W.C.; Hayner, N.S.; Osterholm, M.T.; O’Brien, P.; Hughes, J.M. Reassessment of the association between Guillain- Barré Syndrome and receipt of swine influenza vaccine in 1976–1977: Results of a two-state study. Expert Neurology Group. Am. J. Epidemiol. 1991, 133, 940–951. [Google Scholar] [CrossRef]
- Hurwitz, E.; Schonberger, L.; Nelson, D.; Holman, R. Guillain-Barré syndrome and the 1978–1979 influenza vaccine. N. Engl. J. Med. 1981, 304, 1557–1561. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.E.; Katona, P.; Hurwitz, E.S.; Schonberger, L.B. Guillain-Barré syndrome in the United States, 1979–1980 and 1980–1981. Lack of an association with influenza vaccination. JAMA 1982, 248, 698–700. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.A.; Charlton, J.; Latinovic, R.; Gulliford, M.C. No association between immunization and Guillain-Barré syndrome in the United Kingdom, 1992 to 2000. Arch. Intern. Med. 2006, 166, 1301–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stowe, J.; Andrews, N.; Wise, L.; Miller, E. Investigation of the temporal association of Guillain-Barré syndrome with influenza vaccine and influenza like illness using the United Kingdom General Practice Research Database. Am. J. Epidemiol. 2009, 169, 382–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burwen, D.R.; Ball, R.; Bryan, W.W.; Izurieta, H.S.; La Voie, L.; Gibbs, N.A.; Kliman, R.; Braun, M.M. Evaluation of Guillain-Barré Syndrome among recipients of influenza vaccine in 2000 and 2001. Am. J. Prev. Med. 2010, 39, 296–304. [Google Scholar] [CrossRef]
- Grimaldi-Bensouda, L.; Alpérovitch, A.; Besson, G.; Vial, C.; Cuisset, J.M.; Papeix, C.; Lyon-Caen, O.; Benichou, J.; Rossignol, M.; Lucien Abenhaim for the GBS-PGRx Study Group. Guillain-Barre syndrome, influenzalike illnesses, and influenza vaccination during seasons with and without circulating A/H1N1 viruses. Am. J. Epidemiol. 2011, 174, 326–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wise, M.E.; Viray, M.; Sejvar, J.J.; Lewis, P.; Baughman, A.L.; Connor, W.; Danila, R.; Giambrone, G.P.; Hale, C.; Hogan, B.C.; et al. Guillain-Barre syndrome during the 2009–2010 H1N1 influenza vaccination campaign: Population-based surveillance among 45 million Americans. Am. J. Epidemiol. 2012, 175, 1110–1119. [Google Scholar] [CrossRef] [Green Version]
- Greene, S.K.; Rett, M.; Weintraub, E.S.; Li, L.; Yin, R.; Amato, A.A.; Ho, D.T.; Sheikh, S.I.; Fireman, B.H.; Daley, M.F.; et al. Risk of confirmed Guillain-Barré syndrome following receipt of monovalent inactivated influenza A (H1N1) and seasonal influenza vaccines in the Vaccine Safety Datalink Project, 2009–2010. Am. J. Epidemiol. 2012, 175, 1100–1109. [Google Scholar] [CrossRef] [Green Version]
- Crawford, N.W.; Cheng, A.; Andrews, N.; Charles, P.G.; Clothier, H.J.; Day, B.; Day, T.; Gates, P.; Macdonell, R.; Roberts, L.; et al. Guillain-Barré syndrome following pandemic (H1N1) 2009 influenza A immunisation in Victoria: A self-controlled case series. Med. J. Aust. 2012, 197, 574–578. [Google Scholar] [CrossRef]
- Baxter, R.; Bakshi, N.; Fireman, B.; Lewis, E.; Ray, P.; Vellozzi, C.; Klein, N.P. Lack of association of Guillain-Barré syndrome with vaccinations. Clin. Infect. Dis. 2013, 57, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Kawai, A.T.; Li, L.; Kulldorff, M.; Vellozzi, C.; Weintraub, E.; Baxter, R.; Belongia, E.A.; Daley, M.F.; Jacobsen, S.J.; Naleway, A.; et al. Absence of associations between influenza vaccines and increased risks of seizures, Guillain-Barré syndrome, encephalitis, or anaphylaxis in the 2012–2013 season. Pharmacoepidemiol. Drug. Saf. 2014, 23, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Lyu, R.K.; Lin, W.T.; Huang, Y.T.; Lin, H.S.; Chang, S.H. Gulllain-Barre Syndrome After Trivalent Influenza Vaccination in Adults. Front. Neurol. 2019, 10, 768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmon, D.A.; Proschan, M.; Forshee, R.; Gargiullo, P.; Bleser, W.; Burwen, D.R.; Cunningham, F.; Garman, P.; Greene, S.K.; Lee, G.M.; et al. Association between Guillain-Barré syndrome and influenza A (H1N1) 2009 monovalent inactivated vaccines in the USA: A meta-analysis. Lancet 2013, 381, 1458–1461. [Google Scholar] [CrossRef]
- Dodd, C.N.; Romio, S.A.; Black, S.; Vellozzi, C.; Andrews, N.; Sturkenboom, M.; Zuber, P.; Hua, W.; Bonhoeffer, J.; Buttery, J.; et al. International collaboration to assess the risk of Guillain Barré Syndrome following Influenza A (H1N1) 2009 monovalent vaccines. Vaccine 2013, 31, 4448–4458. [Google Scholar] [CrossRef] [Green Version]
- Martín Arias, L.H.; Sanz, R.; Sáinz, M.; Treceño, C.; Carvajal, A. Guillain-Barré syndrome and influenza vaccines: A meta-analysis. Vaccine 2015, 33, 3773–3778. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 11 December 2019).
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.; Altman, D.G. Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons: Chichester, UK, 2009; pp. 243–296. [Google Scholar]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Chaimani, A.; Mavridis, D.; Salanti, G. A hands-on practical tutorial on performing meta-analysis with Stata. Evid. Based Ment. Health. 2014, 17, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Lasky, T.; Terracciano, G.J.; Magder, L.; Koski, C.L.; Ballesteros, M.; Nash, D.; Clark, S.; Haber, P.; Stolley, P.D.; Schonberger, L.B.; et al. The Guillain-Barré syndrome and the 1992–1993 and 1993–1994 influenza vaccines. N. Engl. J. Med. 1998, 339, 1797–1802. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.F.; Wu, Z.L.; Wu, H.S.; Wang, Q.Y.; Zhao-Ri, G.T.; Wang, C.Y.; Liang, Z.X.; Cui, S.L.; Zheng, J.D. A case-control study on children with Guillain-Barre syndrome in North China. Biomed. Environ. Sci. 2003, 16, 105–111. [Google Scholar] [PubMed]
- Juurlink, D.N.; Stukel, T.A.; Kwong, J.; Kopp, A.; McGeer, A.; Upshur, R.E.; Manuel, D.G.; Moineddin, R.; Wilson, K. Guillain-Barré syndrome after influenza vaccination in adults: A population-based study. Arch. Intern. Med. 2006, 166, 2217–2221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, T.Y.; Huang, K.Y.; Huang, T.T.; Huang, Y.S.; Ho, H.C.; Chou, P.; Lin, C.H.; Wei, C.K.; Lian, W.C.; Chen, T.C.; et al. The impact of influenza vaccinations on the adverse effects and hospitalization rate in the elderly: A national based study in an Asian country. PLoS ONE 2012, 7, e50337. [Google Scholar] [CrossRef] [PubMed]
- Tokars, J.I.; Lewis, P.; DeStefano, F.; Wise, M.; Viray, M.; Morgan, O.; Gargiullo, P.; Vellozzi, C. The risk of Guillain-Barré syndrome associated with influenza A (H1N1) 2009 monovalent vaccine and 2009–2010 seasonal influenza vaccines: results from self-controlled analyses. Pharmacoepidemiol. Drug. Saf. 2012, 21, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, F.; Massari, M.; D’Alessandro, R.; Beghi, E.; Chiò, A.; Logroscino, G.; Filippini, G.; Benedetti, M.D.; Pugliatti, M.; Santuccio, C.; et al. Risk of Guillain-Barré syndrome after 2010–2011 influenza vaccination. Eur. J. Epidemiol. 2013, 28, 433–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwong, J.C.; Vasa, P.P.; Campitelli, M.A.; Hawken, S.; Wilson, K.; Rosella, L.C.; Stukel, T.A.; Crowcroft, N.S.; McGeer, A.J.; Zinman, L.; et al. Risk of Guillain-Barré syndrome after seasonal influenza vaccination and influenza health-care encounters: A self-controlled study. Lancet Infect. Dis. 2013, 3, 769–776. [Google Scholar] [CrossRef]
- McCarthy, N.L.; Gee, J.; Lin, N.D.; Thyagarajan, V.; Pan, Y.; Su, S.; Turnbull, B.; Chan, K.A.; Weintraub, E. Evaluating the safety of influenza vaccine using a claims-based health system. Vaccine 2013, 31, 5975–5982. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Chu, X.; Xu, Y.; Ma, F. Vaccines and the risk of Guillain-Barré syndrome. Eur. J. Epidemiol. 2019. [Google Scholar] [CrossRef]

| Study, Year [Ref.]; Country; Study Period | Study Design | Participants | iTIV1 (%) | GBS2 Assessment | Time Window (Days) | Effect Size (95% CI3) | Quality |
|---|---|---|---|---|---|---|---|
| Hurwitz 1981 [11]; USA; 1978–1979 | C4 | F9: 44%; Age: 0–95 y10; 544 pts11 | 100 | Committee12 | ≤56 | cRR13:1.4 (0.7–2.7) | N14 |
| Kaplan 1982 [12]; USA; 1979–1981 | C | F: 43%; Age: 15–74 y; 778 pts | 100 | Neurologists | ≤56 | cRR:0.6 (0.29–1.31) S1979–198021cRR:1.4 (0.8–2.5) S1980–1981 | N |
| Lasky 1998 [32]; USA; 1992–1994 | C | F: 38%; Age: ≥18 y; 273 pts | 100 | ICD-9: 357.015 | ≤42 | aRR13:2.0 (<1.0–4.3) S1992–1993aRR:1.5 (0.8–2.9) S1993–1994 | Y14 |
| Liu 2003 [33]; China; ? | C-C5 | F: 49%; Age: 1–14 y; 51 pts and 51 controls | 10016 | Selection criteria17 | Any time | OR18:3.13 (0.27–82.33) | N |
| Hughes 2006 [13]; UK; 1992–2000 | SCCS6 | F: 47%; Age: 0+; 228 pts | 100 | ICD-9: 357.0 | ≤42 | aRR:0.99 (0.32–3.12) | Y |
| Juurlink 2006 [34]; Canada; 1992–2004 | SCCS | F: ?19; Age: ≥18 y; 685 pts | 100 | ICD-9: 357.0 | ≤49 | aRR:1.45 (1.05–1.99) | Y |
| Tam 2007 [4]; UK; 1990–2001 | C-C | F: ?; Age: 0+ y; 553 pts and 5445 controls | 100 | ICD-9: 357.0 | ≤60 | aOR18:0.16 (0.02–1.25) | Y |
| Stowe 2009 [14]; UK; 1990–2005 | SCCS | F: 43%; Age: 0+ y; 690 pts | 100 | ICD-9: 357.0 | ≤30≤180 | aRR20:0.58 (0.18–1.86)aRR:0.80 (0.51–1.27) | Y |
| Burwen 2010 [15]; USA; 2000–2002 | SCRI7 | F: 60%; Age: 0+ y; 164 pts | 100 | BCC: 1,222 | ≤42 | IRR20:0.86 (0.52–1.41) S2000–2001IRR:1.21 (0.79–1.86) S2001–2002 | Y |
| Grimaldi-Bensouda 2011 [16] France; 2007–2010 | C-C | F: 39%; Age: 3–80 y; 145 pts and 1080 controls | 100 | BCC: 1,2,3 | ≤42≤180 | aOR:1.3 (0.41–4.12)cOR4:0.74 (0.46–1.19)23 | Y |
| Ho 2012 [35]; Taiwan; 2008–2009 | C | F: 52%; Age: ≥65 y; 41,986 vaccinated and 51,063 unvaccinated | 100 | ICD-9: 357.0 | ≤365 | aOR:1.64 (0.77–3.49) | Y |
| Tokars 2012 [36]; USA; 2009-2010 | SCRI | F: 58%; Age: 2–88 y; 78 pts | >51,3 | BCC: 1,2,3 | ≤42 | RR:1.5 (0.8–3.0)24 | Y |
| Wise 2012 [17]; USA; 2009–10 | C | F: 48%; Age: 0+ y; 411 pts | >53 | BCC: 1,2,3 | ≤42 | aRR:1.43 (0.94–1.89) | Y |
| Greene 2012 [18]; USA; 2009–2010 | SCRI | F: 63%; Age: 2–83 y; 14 pts | 100 | BCC: 1,2,3 | ≤42 | RR:1.0 (0.3–3.5) | Y |
| Crawford 2012 [19]; Australia; 2010–2011 | SCCS | F: 48%; Age: 7–95 y; 54 pts | 100 | BCC: 1,2,3,4 | ≤42 | IRR:0.69 (0.08–5.64) | Y |
| Baxter 2013 [20]; USA; 1995–2006 | Cc8 | F: 41%; Age: 5–87 y; 451 pts | 100 | BCC: 1,2,3 | ≤42 | aOR:1.11 (0.39–3.08) | Y |
| ≤70 | aOR:0.99 (0.33–2.70) | ||||||
| Galeotti 2013 [37]; Italy; 2010–2011 | C-C | F: 42%; Age: ≥18 y; 140 pts and 308 controls | 100 | BCC: 1,2,3 | ≤42 | aOR:3.8 (1.3–10.5) | Y |
| ≤365 | aOR:1.6 (0.9–2.7) | ||||||
| Kwong 2013 [38]; Canada; 1993–2011 | SCCS | F: 46%; Age: 0+ y; 330 pts | 100 | ICD-9: 357.0 | ≤42 | IRR:1.52 (1.17–1.99) | Y |
| McCarthy 2013 [39]; USA; 2009–2011 | SCRI | F: ?; Age: 0–80 y; 1021 pts | ? | ICD-9: 357.0 | ≤42 | aRR:1.57 (0.61–4.05) S2009–2010aRR:1.00 (0.45–2.23) S2010-2011 | Y |
| Kawai 2014 [21]; USA; 2012–2013 | SCRI | F: 44%; Age: 0+ y; 116 pts | 100 | ICD-9: 357.0 | ≤42 | aRR:0.5 (0.3–0.9) | Y |
| Chang 2019 [22]; Taiwan; 2007–2015 | C-C | F: 38%; Age: ≥50 y; 182 pts and 910 controls | 100 | ICD-9: 357.0 | ≤42≤90 | OR:1.46 (0.56–3.78)OR:1.26 (0.67–2.38) | Y |
| Chen 2019 [40]; China; 2011–2015 | C-C | F: 38%; Age: 0+ y; 1056 pts and 4312 controls | 10016 | BCC: 1,2,3 | ≤42 | aOR:1.03 (0.73–1.45) | Y |
| Critical Variable | High-Quality Studies | All Studies | |||
|---|---|---|---|---|---|
| Study Records | ES1 (95% CI2) | Study Records | ES (95% CI) | ||
| Time window | shorter3 | 17 | 1.19 (0.99–1.44) | 18 | 1.20 (1.00–1.44) |
| longer4 | 7 | 1.08 (0.77–1.52) | 12 | 1.12 (0.89–1.42) | |
| Age (years) | ≥65 | 5 | 1.11 (0.88–1.39) | 5 | 1.11 (0.88–1.39) |
| Study (type) | C-C | 4 | 0.97 (0.62–1.53) | 6 | 1.05 (0.74–1.48) |
| C | 4 | 1.56 (1.16–2.09) | 7 | 1.39 (1.10–1.75) | |
| SCCS | 5 | 1.25 (0.94–1.65) | 5 | 1.25 (0.94–1.65) | |
| SCRI | 7 | 0.98 (0.72–1.34) | 7 | 0.98 (0.72–1.34) | |
| Geographic region | North America | 13 | 1.24 (1.02–1.50) | 16 | 1.22 (1.02–1.45) |
| Europe | 5 | 0.90 (0.58–1.40) | 5 | 0.90 (0.58–1.40) | |
| Influenza season | 2009–2010 | 4 | 1.43 (1.03–1.97) | 4 | 1.43 (1.03–1.97) |
| 2010–2011 | 3 | 1.35 (0.88–2.07) | 3 | 1.35 (0.88–2.07) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petráš, M.; Králová Lesná, I.; Dáňová, J.; Čelko, A.M. Is an Increased Risk of Developing Guillain–Barré Syndrome Associated with Seasonal Influenza Vaccination? A Systematic Review and Meta-Analysis. Vaccines 2020, 8, 150. https://doi.org/10.3390/vaccines8020150
Petráš M, Králová Lesná I, Dáňová J, Čelko AM. Is an Increased Risk of Developing Guillain–Barré Syndrome Associated with Seasonal Influenza Vaccination? A Systematic Review and Meta-Analysis. Vaccines. 2020; 8(2):150. https://doi.org/10.3390/vaccines8020150
Chicago/Turabian StylePetráš, Marek, Ivana Králová Lesná, Jana Dáňová, and Alexander M. Čelko. 2020. "Is an Increased Risk of Developing Guillain–Barré Syndrome Associated with Seasonal Influenza Vaccination? A Systematic Review and Meta-Analysis" Vaccines 8, no. 2: 150. https://doi.org/10.3390/vaccines8020150
APA StylePetráš, M., Králová Lesná, I., Dáňová, J., & Čelko, A. M. (2020). Is an Increased Risk of Developing Guillain–Barré Syndrome Associated with Seasonal Influenza Vaccination? A Systematic Review and Meta-Analysis. Vaccines, 8(2), 150. https://doi.org/10.3390/vaccines8020150






