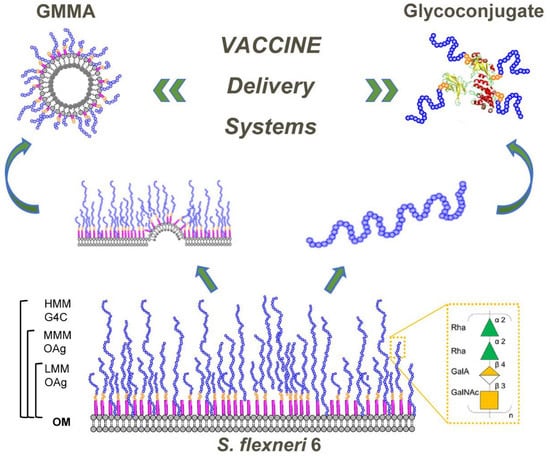
GMMA and Glycoconjugate Approaches Compared in Mice for the Development of a Vaccine against Shigella flexneri Serotype 6
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Mutant Generation and Growth Condition
2.2. OMV/GMMA Production
2.3. GMMA Characterization
2.4. OAg Purification and Characterization
2.5. Glycoconjugates Synthesis and Characterization
2.6. Immunogenicity Studies in Mice
2.7. Statistical Analysis
3. Results
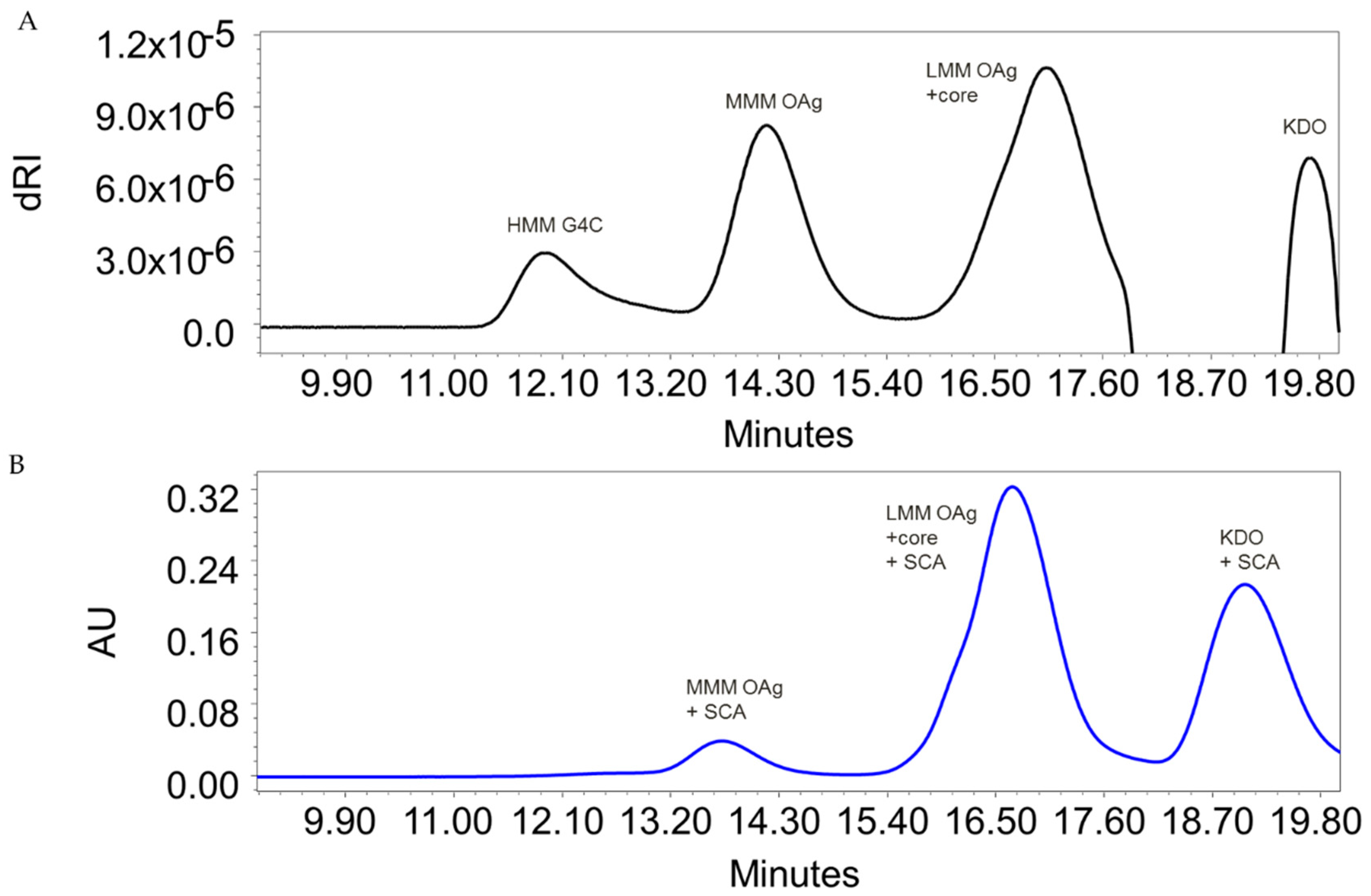
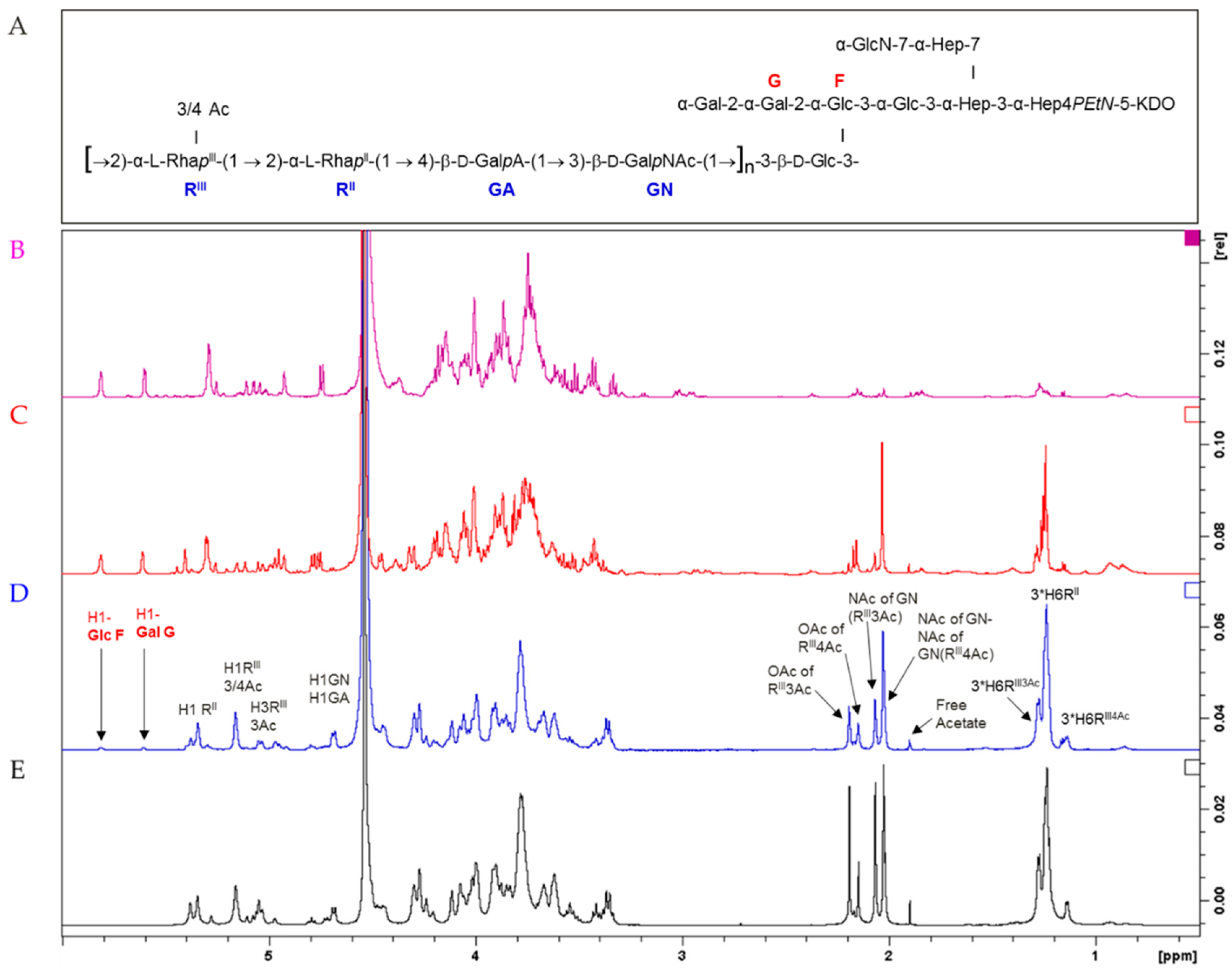
3.1. S. flexneri 6 OAg Characterization
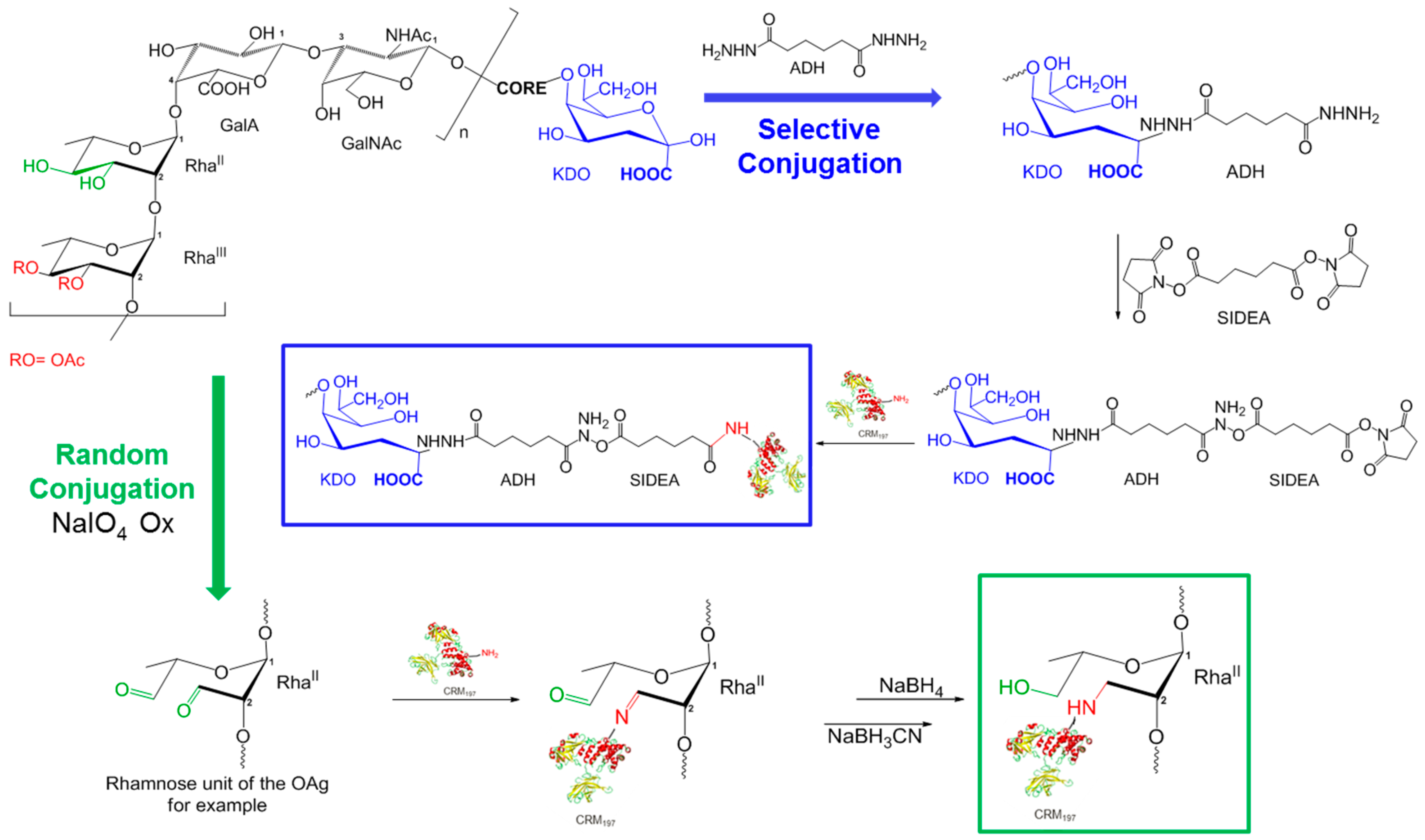
3.2. Synthesis and Characterization of Glycoconjugates Differing in Sugar Length
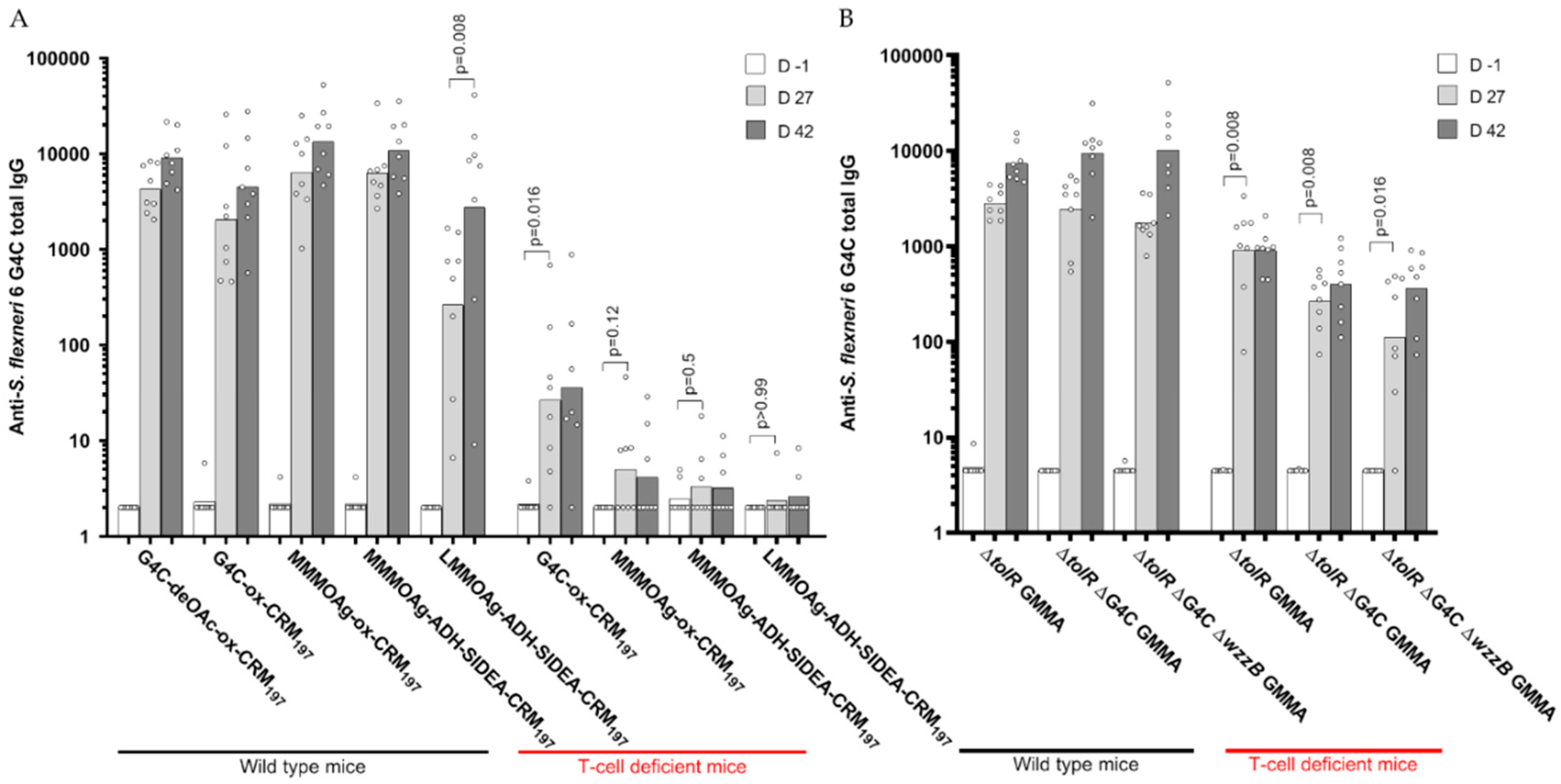
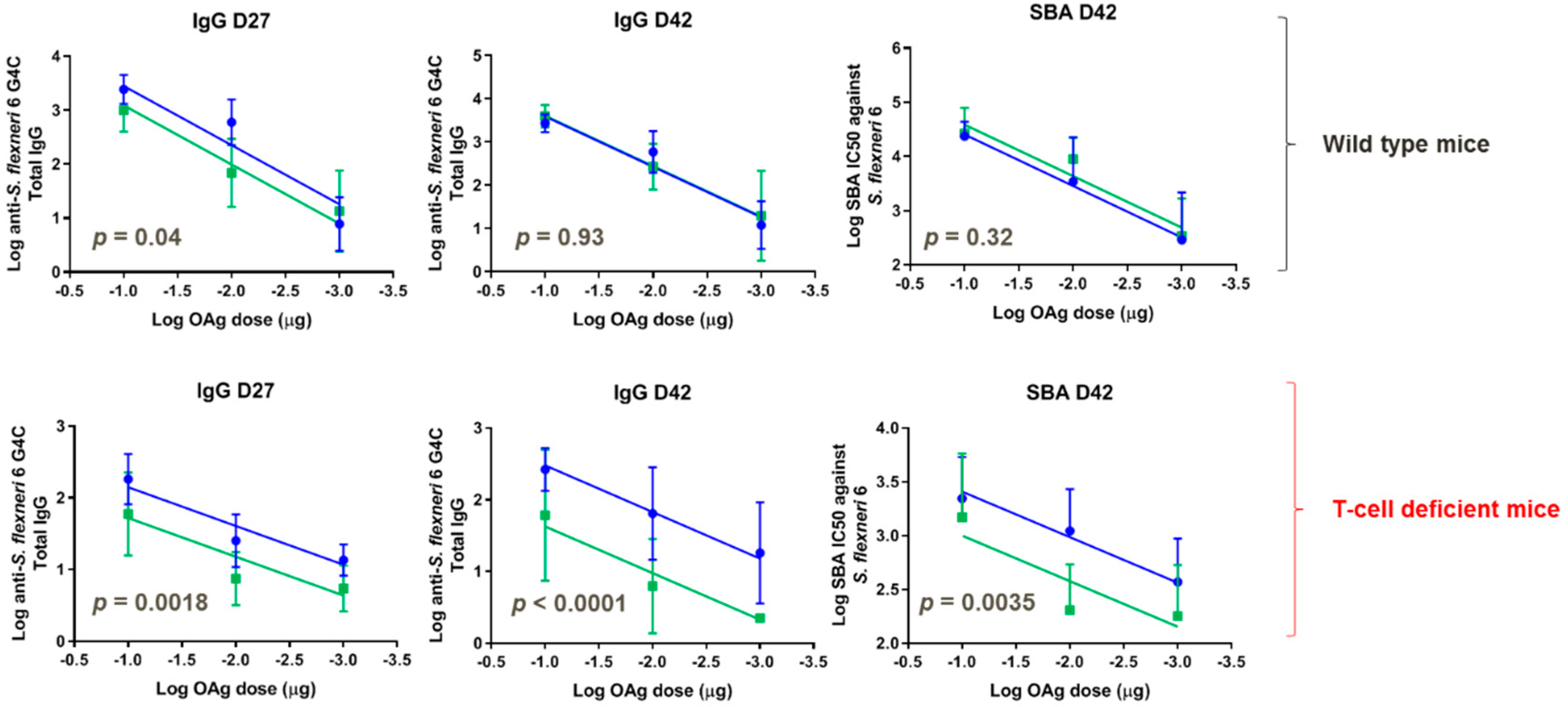
3.3. Immunogenicity of Glycoconjugates in Mice, Investigating Impact of Sugar Length, Conjugation Chemistry, and O-Acetylation on the Immune Response
3.4. Genetic Engineering of Bacteria to Generate GMMA Expressing OAg of Different Lengths
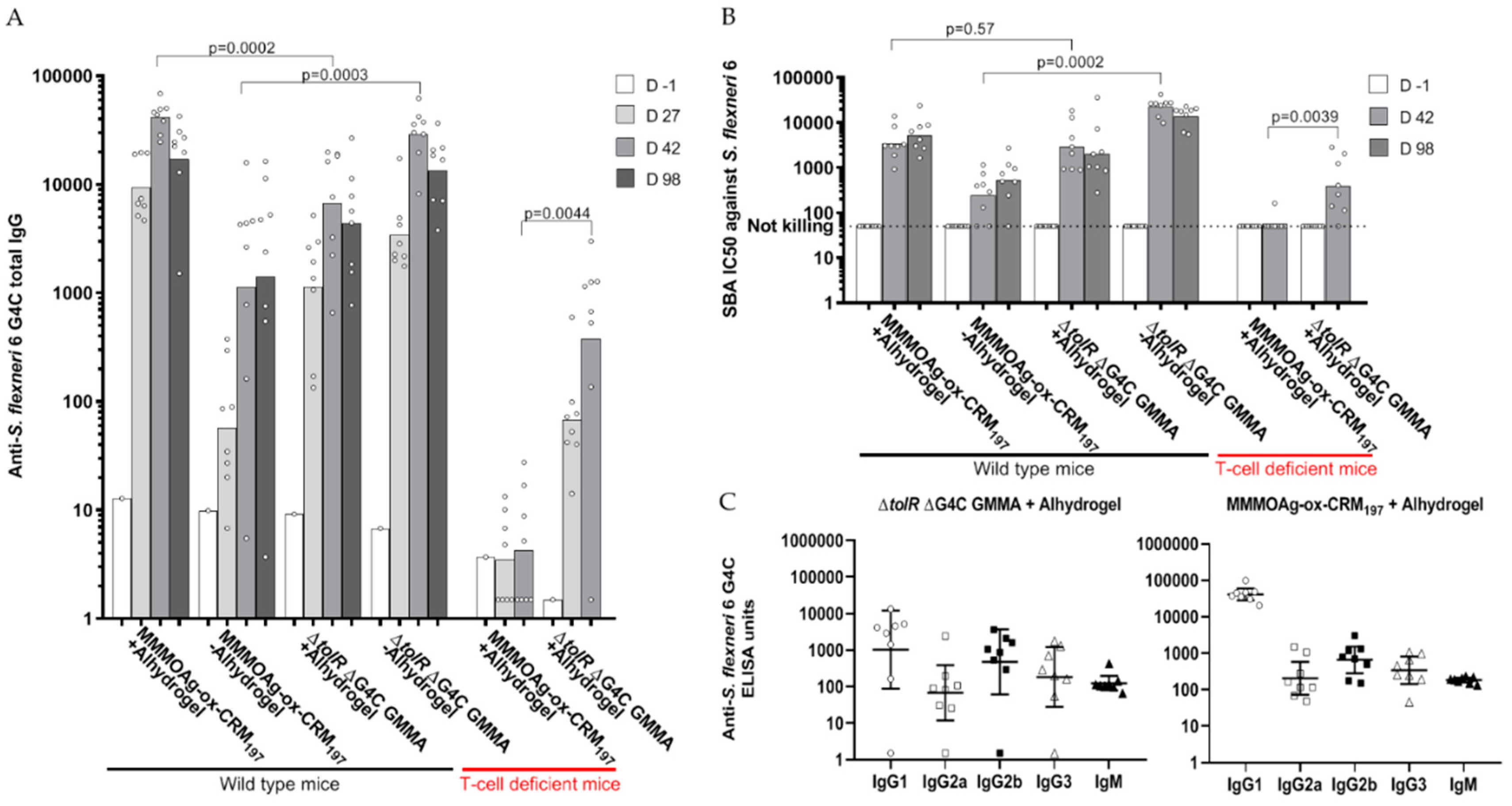
3.5. Immunogenicity of GMMA in Mice, Investigating the Impact of Sugar Length on the Immune Response
3.6. Direct Comparison in Mice of GMMA and Glycoconjugate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schroeder, G.N.; Hilbi, H. Molecular pathogenesis of Shigella spp: Controlling host cell signaling, invasion, and death by type III secretion. Clin. Microbiol. Rev. 2008, 21, 134–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, I.A.; Troeger, C.; Blacker, B.F.; Rao, P.C.; Brown, A.; Atherly, D.E.; Brewer, T.G.; Engmann, C.M.; Houpt, E.R.; Kang, G.; et al. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: The Global Burden of Disease Study 1990–2016. Lancet Infect. Dis. 2018, 18, 1229–1240. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Knirel, Y.A.; Feng, L.; Perepelov, A.V.; Senchenkova, S.Y.N.; Wang, Q.; Reeves, P.R.; Wang, L. Structure and genetics of Shigella O antigens. FEMS Microbiol. Rev. 2008, 32, 627–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Klontz, K.C.; Singh, N. Treatment of drug-resistant Shigella infections. Expert Rev. Anti Infect. 2015, 13, 69–80. [Google Scholar] [CrossRef]
- Puzari, M.; Sharma, M.; Chetia, P. Emergence of antibiotic resistant Shigella species: A matter of concern. J. Infect. Public Health 2018, 11, 451–454. [Google Scholar] [CrossRef]
- CDC. Antibiotic Resistance Threats in the United States, 2019; Department of Health and Human Services: Atlanta, GA, USA, 2019. [Google Scholar]
- World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Mani, S.; Wierzba, T.; Walker, R.I. Status of vaccine research and development for Shigella. Vaccine 2016, 34, 2887–2894. [Google Scholar] [CrossRef] [Green Version]
- Gerke, C.; Colucci, A.M.; Giannelli, C.; Sanzone, S.; Vitali, C.G.; Sollai, L.; Rossi, O.; Martin, L.B.; Auerbach, J.; Di Cioccio, V.; et al. Production of a Shigella sonnei Vaccine Based on Generalized Modules for Membrane Antigens (GMMA), 1790GAHB. PLoS ONE 2015, 10, e0134478. [Google Scholar] [CrossRef]
- Rossi, O.; Caboni, M.; Negrea, A.; Necchi, F.; Alfini, R.; Micoli, F.; Saul, A.; MacLennan, C.A.; Rondini, S.; Gerke, C. Toll-Like Receptor Activation by Generalized Modules for Membrane Antigens from Lipid A Mutants of Salmonella enterica Serovars Typhimurium and Enteritidis. Clin. Vaccine Immunol. 2016, 23, 304–314. [Google Scholar] [CrossRef] [Green Version]
- Rossi, O.; Pesce, I.; Giannelli, C.; Aprea, S.; Caboni, M.; Citiulo, F.; Valentini, S.; Ferlenghi, I.; MacLennan, C.A.; D’Oro, U.; et al. Modulation of endotoxicity of Shigella generalized modules for membrane antigens (GMMA) by genetic lipid A modifications: Relative activation of TLR4 and TLR2 pathways in different mutants. J. Biol. Chem. 2014, 289, 24922–24935. [Google Scholar] [CrossRef] [Green Version]
- Launay, O.; Lewis, D.J.M.; Anemona, A.; Loulergue, P.; Leahy, J.; Scire, A.S.; Maugard, A.; Marchetti, E.; Zancan, S.; Huo, Z.; et al. Safety Profile and Immunologic Responses of a Novel Vaccine Against Shigella sonnei Administered Intramuscularly, Intradermally and Intranasally: Results From Two Parallel Randomized Phase 1 Clinical Studies in Healthy Adult Volunteers in Europe. EBioMedicine 2017, 22, 164–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Launay, O.; Ndiaye, A.G.W.; Conti, V.; Loulergue, P.; Sciré, A.S.; Landre, A.M.; Ferruzzi, P.; Nedjaai, N.; Schütte, L.D.; Auerbach, J.; et al. Booster Vaccination With GVGH Shigella sonnei 1790GAHB GMMA Vaccine Compared to Single Vaccination in Unvaccinated Healthy European Adults: Results From a Phase 1 Clinical Trial. Front. Immunol. 2019, 10, 335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obiero, C.W.; Ndiaye, A.G.W.; Scire, A.S.; Kaunyangi, B.M.; Marchetti, E.; Gone, A.M.; Schutte, L.D.; Riccucci, D.; Auerbach, J.; Saul, A.; et al. A Phase 2a Randomized Study to Evaluate the Safety and Immunogenicity of the 1790GAHB Generalized Modules for Membrane Antigen Vaccine against Shigella sonnei Administered Intramuscularly to Adults from a Shigellosis-Endemic Country. Front. Immunol. 2017, 8, 1884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Micoli, F.; Del Bino, L.; Alfini, R.; Carboni, F.; Romano, M.R.; Adamo, R. Glycoconjugate vaccines: Current approaches towards faster vaccine design. Expert Rev. Vaccines 2019, 18, 881–895. [Google Scholar] [CrossRef]
- Rappuoli, R. Glycoconjugate vaccines: Principles and mechanism. Sci. Transl. Med. 2018, 10, eaat4615. [Google Scholar] [CrossRef]
- Micoli, F.; Adamo, R.; Costantino, P. Protein Carriers for Glycoconjugate Vaccines: History, Selection Criteria, Characterization and New Trends. Molecules 2018, 23, 1451. [Google Scholar] [CrossRef] [Green Version]
- Costantino, P.; Rappuoli, R.; Berti, F. The design of semi-synthetic and synthetic glycoconjugate vaccines. Expert Opin. Drug Discov. 2011, 6, 1045–1066. [Google Scholar] [CrossRef]
- Berti, F.; De Ricco, R.; Rappuoli, R. Role of O-Acetylation in the Immunogenicity of Bacterial Polysaccharide Vaccines. Molecules 2018, 23, 1340. [Google Scholar] [CrossRef] [Green Version]
- Lanzilao, L.; Stefanetti, G.; Saul, A.; MacLennan, C.A.; Micoli, F.; Rondini, S. Strain Selection for Generation of O-Antigen-Based Glycoconjugate Vaccines against Invasive Nontyphoidal Salmonella Disease. PLoS ONE 2015, 10, e0139847. [Google Scholar] [CrossRef] [Green Version]
- Giannini, G.; Rappuoli, R.; Ratti, G. The amino-acid sequence of two non-toxic mutants of diphtheria toxin: CRM45 and CRM197. Nucleic Acids Res. 1984, 12, 4063–4069. [Google Scholar] [CrossRef]
- Perepelov, A.V.; Shekht, M.E.; Liu, B.; Shevelev, S.D.; Ledov, V.A.; Senchenkova, S.N.; L’Vov, V.L.; Shashkov, A.S.; Feng, L.; Aparin, P.G.; et al. Shigella flexneri O-antigens revisited: Final elucidation of the O-acetylation profiles and a survey of the O-antigen structure diversity. FEMS Immunol. Med. Microbiol. 2012, 66, 201–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, S.T.; Lam, J.S. Synthesis of bacterial polysaccharides via the Wzx/Wzy-dependent pathway. Can. J. Microbiol. 2014, 60, 697–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, O.; Baker, K.S.; Phalipon, A.; Weill, F.X.; Citiulo, F.; Sansonetti, P.; Gerke, C.; Thomson, N.R. Draft genomes of Shigella strains used by the STOPENTERICS consortium. Gut Pathog. 2015, 7, 14. [Google Scholar] [CrossRef]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Benedetto, G.; Cescutti, P.; Giannelli, C.; Rizzo, R.; Micoli, F. Multiple Techniques for Size Determination of Generalized Modules for Membrane Antigens from Salmonella typhimurium and Salmonella enteritidis. ACS Omega 2017, 2, 8282–8289. [Google Scholar] [CrossRef] [PubMed]
- De Benedetto, G.; Alfini, R.; Cescutti, P.; Caboni, M.; Lanzilao, L.; Necchi, F.; Saul, A.; MacLennan, C.A.; Rondini, S.; Micoli, F. Characterization of O-antigen delivered by Generalized Modules for Membrane Antigens (GMMA) vaccine candidates against nontyphoidal Salmonella. Vaccine 2017, 35, 419–426. [Google Scholar] [CrossRef]
- Dische, Z.; Shettles, L.B. A specific color reaction of methylpentoses and a spectrophotometric micromethod for their determination. J. Biol. Chem. 1948, 175, 595–603. [Google Scholar]
- Micoli, F.; Ravenscroft, N.; Cescutti, P.; Stefanetti, G.; Londero, S.; Rondini, S.; Maclennan, C.A. Structural analysis of O-polysaccharide chains extracted from different Salmonella Typhimurium strains. Carbohydr. Res. 2014, 385, 1–8. [Google Scholar] [CrossRef]
- Micoli, F.; Rondini, S.; Gavini, M.; Pisoni, I.; Lanzilao, L.; Colucci, A.M.; Giannelli, C.; Pippi, F.; Sollai, L.; Pinto, V.; et al. A scalable method for O-antigen purification applied to various Salmonella serovars. Anal. Biochem. 2013, 434, 136–145. [Google Scholar] [CrossRef] [Green Version]
- Stefanetti, G.; Rondini, S.; Lanzilao, L.; Saul, A.; MacLennan, C.A.; Micoli, F. Impact of conjugation chemistry on the immunogenicity of S. Typhimurium conjugate vaccines. Vaccine 2014, 32, 6122–6129. [Google Scholar] [CrossRef] [Green Version]
- Lei, Q.P.; Shannon, A.G.; Heller, R.K.; Lamb, D.H. Quantification of free polysaccharide in meningococcal polysaccharide-diphtheria toxoid conjugate vaccines. Dev. Biol. 2000, 103, 259–264. [Google Scholar]
- Necchi, F.; Saul, A.; Rondini, S. Setup of luminescence-based serum bactericidal assay against Salmonella Paratyphi A. J. Immunol. Methods 2018, 461, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Kubler-Kielb, J.; Vinogradov, E.; Mocca, C.; Pozsgay, V.; Coxon, B.; Robbins, J.B.; Schneerson, R. Immunochemical studies of Shigella flexneri 2a and 6, and Shigella dysenteriae type 1 O-specific polysaccharide-core fragments and their protein conjugates as vaccine candidates. Carbohydr. Res. 2010, 345, 1600–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Micoli, F.; Rondini, S.; Gavini, M.; Lanzilao, L.; Medaglini, D.; Saul, A.; Martin, L.B. O:2-CRM(197) conjugates against Salmonella Paratyphi A. PLoS ONE 2012, 7, e47039. [Google Scholar] [CrossRef] [Green Version]
- Robbins, J.B.; Kubler-Kielb, J.; Vinogradov, E.; Mocca, C.; Pozsgay, V.; Shiloach, J.; Schneerson, R. Synthesis, characterization, and immunogenicity in mice of Shigella sonnei O-specific oligosaccharide-core-protein conjugates. Proc. Natl. Acad. Sci. USA 2009, 106, 7974–7978. [Google Scholar] [CrossRef] [Green Version]
- Konadu, E.Y.; Lin, F.Y.; Ho, V.A.; Thuy, N.T.; Van Bay, P.; Thanh, T.C.; Khiem, H.B.; Trach, D.D.; Karpas, A.B.; Li, J.; et al. Phase 1 and phase 2 studies of Salmonella enterica serovar paratyphi A O-specific polysaccharide-tetanus toxoid conjugates in adults, teenagers, and 2- to 4-year-old children in Vietnam. Infect. Immun. 2000, 68, 1529–1534. [Google Scholar] [CrossRef] [Green Version]
- Micoli, F.; Rondini, S.; Alfini, R.; Lanzilao, L.; Necchi, F.; Negrea, A.; Rossi, O.; Brandt, C.; Clare, S.; Mastroeni, P.; et al. Comparative immunogenicity and efficacy of equivalent outer membrane vesicle and glycoconjugate vaccines against nontyphoidal Salmonella. Proc. Natl. Acad. Sci. USA 2018, 115, 10428–10433. [Google Scholar] [CrossRef] [Green Version]
- Berlanda Scorza, F.; Colucci, A.M.; Maggiore, L.; Sanzone, S.; Rossi, O.; Ferlenghi, I.; Pesce, I.; Caboni, M.; Norais, N.; Di Cioccio, V.; et al. High yield production process for Shigella outer membrane particles. PLoS ONE 2012, 7, e35616. [Google Scholar] [CrossRef] [Green Version]
- Kis, Z.; Shattock, R.; Shah, N.; Kontoravdi, C. Emerging Technologies for Low-Cost, Rapid Vaccine Manufacture. Biotechnol. J. 2019, 14, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Caboni, M.; Pedron, T.; Rossi, O.; Goulding, D.; Pickard, D.; Citiulo, F.; MacLennan, C.A.; Dougan, G.; Thomson, N.R.; Saul, A.; et al. An O antigen capsule modulates bacterial pathogenesis in Shigella sonnei. PLoS Pathog 2015, 11, e1004749. [Google Scholar] [CrossRef]


| Name in the Study | Phenotype | Country of Infection, Year of Isolation |
|---|---|---|
| Sf6_Sh10.3933 | Wild type | Nigeria, 2010 |
| Sf6_Sh10.6306 | Wild type | India, 2010 |
| Sf6_Sh10.6237 | Wild type | Mexico, 2010 |
| Sf6_Sh10.8537 | Wild type | Egypt, 2010 |
| H130920152 | Wild type | Unknown |
| Sf6_Sh10.8537 ∆tolR | Hyper-blebbing | Produced in this study |
| Sf6_Sh10.8537 ∆tolR ∆ept-etk | Hyper-blebbing, no G4C produced | Produced in this study |
| Sf6_Sh10.8537 ∆tolR ∆ept-etk ∆wzzB | Hyper-blebbing, no G4C produced, short OAg produced | Produced in this study |
| Primer Name | Sequence (5′ → 3′) |
|---|---|
| tolRKO F | ACCGCCAGGCGTTTACCGTTAGCGAGAGCAACAAGGGGTAAGCCATGGCCGTGTAGGCTGGAGCTGCTTC |
| tolRKO R | ACCCGCTCTCTTTCAAGCAAGGGAAACGCAGATGTTTAGATAGGCTGCGTCATATGAATATCCTCCTTAG |
| ept-etkKO F | TTACTCTTTCTCGGAGTAACTATAACCGTAATAGTTATAGCCGTAACTGTGTCTTGAGCGATTGTGTAGG |
| ept-etkKO R | AATATCTATCCCGTCACGCCAGGATTGATTGATCAGTTGCGCGCCAAACCTCCTCCTTAGTTCCTATTCC |
| wzzBKO F | TCCCTTTGTAATAATTCATTATTTTTATCATTTATCCTATAGCATTCATGGTGTAGGCTGGAGCTGCTTC |
| wzzBKO R | CGGGCAAGGTGTCACCACCCTGCCCCTTTTCTTTAAAACCGAAAAGATTACATATGAATATCCTCCTTAG |
| Strains | Average MM (KDa) and relative % * of HMM G4C and MMM OAg | |||
|---|---|---|---|---|
| Bacteria | OMV | |||
| HMM G4C | MMM OAg | HMM G4C | MMM OAg | |
| Sf6_Sh10.3933 | 162.6 (46%) | 17.8 (54%) | 212.7 (52%) | 16.5 (48%) |
| Sf6_Sh10.6306 | 165.0 (41%) | 18.6 (59%) | 197.4 (50%) | 16.5 (50%) |
| Sf6_Sh10.6237 | 171.5 (42%) | 18.0 (58%) | 208.9 (42%) | 16.4 (58%) |
| Sf6_Sh10.8537 | 151.3 (28%) | 16.7 (72%) | 205.2 (43%) | 16.7 (57%) |
| H130920152 | 162.5 (59%) | 17.1 (41%) | 220.4 (64%) | 16.4 (36%) |
| Chemistry | Conjugates | OAg Average Size (KDa) | % OAg O-Acetylation | OAg/Protein w/w Ratio (Molar Ratio) | % Free OAg |
|---|---|---|---|---|---|
| Random | G4C-deOAc-ox-CRM197 | 174 | 0 | 0.29 | 9 |
| G4C-ox-CRM197 | 174 | 63 | 0.38 | 14 | |
| MMMOAg-ox-CRM197 | 22 | 66 | 0.54 | <5 | |
| Selective | MMMOAg-ADH-SIDEA- CRM197 | 22 | 66 | 1.58 (4.2) | <5 |
| LMMOAg-ADH-SIDEA-CRM197 | 1.7 | 52 | 0.085 (4.9) | <10 |
| GMMA | nmol Lipid A/mg GMMA Protein | Total Sugar/Protein w/w Ratio | Z Average Diameter nm (PdI) | 2 x Rw nm |
|---|---|---|---|---|
| ∆tolR | 134.7 | 0.47 | 110.5 (0.1) | 83.2 |
| ∆tolR ∆G4C | 177.4 | 0.53 | 103.2 (0.1) | 82.2 |
| ∆tolR ∆G4C ∆wzzB | 187.6 | 0.11 | 83.0 (0.1) | 72.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raso, M.M.; Gasperini, G.; Alfini, R.; Schiavo, F.; Aruta, M.G.; Carducci, M.; Forgione, M.C.; Martini, S.; Cescutti, P.; Necchi, F.; et al. GMMA and Glycoconjugate Approaches Compared in Mice for the Development of a Vaccine against Shigella flexneri Serotype 6. Vaccines 2020, 8, 160. https://doi.org/10.3390/vaccines8020160
Raso MM, Gasperini G, Alfini R, Schiavo F, Aruta MG, Carducci M, Forgione MC, Martini S, Cescutti P, Necchi F, et al. GMMA and Glycoconjugate Approaches Compared in Mice for the Development of a Vaccine against Shigella flexneri Serotype 6. Vaccines. 2020; 8(2):160. https://doi.org/10.3390/vaccines8020160
Chicago/Turabian StyleRaso, Maria Michelina, Gianmarco Gasperini, Renzo Alfini, Fabiola Schiavo, Maria Grazia Aruta, Martina Carducci, Maria Concetta Forgione, Silvia Martini, Paola Cescutti, Francesca Necchi, and et al. 2020. "GMMA and Glycoconjugate Approaches Compared in Mice for the Development of a Vaccine against Shigella flexneri Serotype 6" Vaccines 8, no. 2: 160. https://doi.org/10.3390/vaccines8020160
APA StyleRaso, M. M., Gasperini, G., Alfini, R., Schiavo, F., Aruta, M. G., Carducci, M., Forgione, M. C., Martini, S., Cescutti, P., Necchi, F., & Micoli, F. (2020). GMMA and Glycoconjugate Approaches Compared in Mice for the Development of a Vaccine against Shigella flexneri Serotype 6. Vaccines, 8(2), 160. https://doi.org/10.3390/vaccines8020160