Mycolicibacterium brumae is a Safe and Non-Toxic Immunomodulatory Agent for Cancer Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Cultures
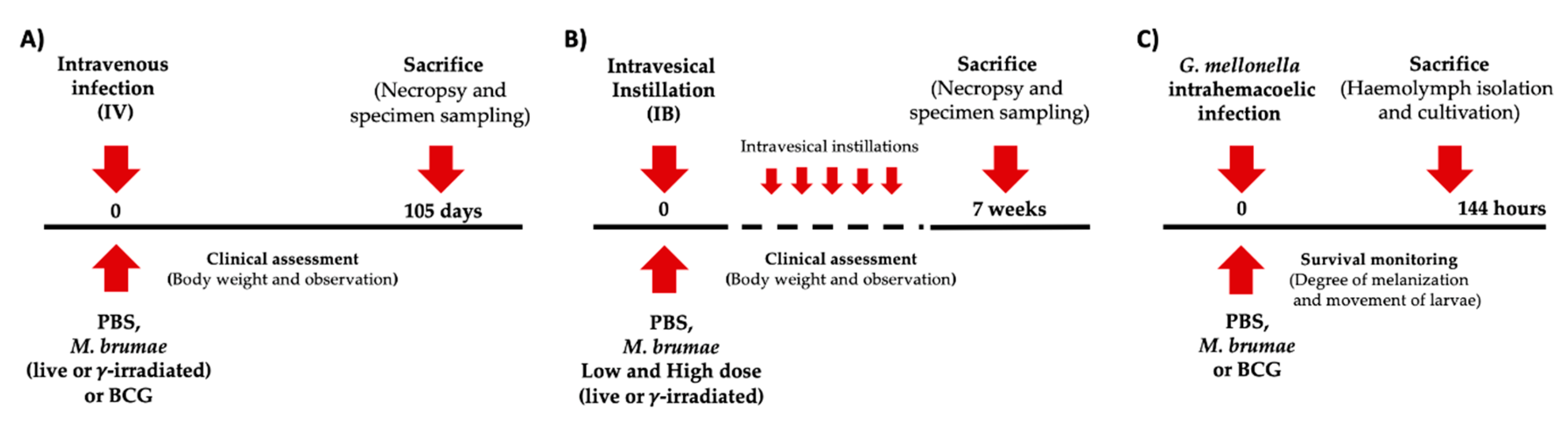
2.2. Intravenous (IV) and Intravesical (IB) Mice Treatments
2.3. G. mellonella Infection Model
2.4. Mycobacteria CFU Counting in Different Organs from Mice and G. mellonella Hemolymph
2.5. Determination of Biochemical and Hematological Parameters
2.6. Histopathological Analysis in Mouse Organs
2.7. Statistical Analysis
3. Results
3.1. M. brumae-Treated Animals Showed 100% Survival Rates after Treatments
3.2. No CFU Were Recovered from M. brumae-Treated Animals at the End of the Experiments
3.3. IV and IB M. brumae Treatments Did not Alter Biochemical and Hematology Parameters Compared to Control Mice
3.4. Histopathology Analysis Reveals the Safety and Lack of Toxicity of M. brumae Treatments in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Gan, C.; Mostafid, H.; Khan, M.; Lewis, D. BCG immunotherapy for bladder cancer—The effects of substrain differences. Nat. Rev. Urol. 2013, 10, 580–588. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [Green Version]
- Sanli, O.; Dobruch, J.; Knowles, M.A.; Burger, M.; Alemozaffar, M.; Nielsen, M.E.; Lotan, Y. Bladder cancer. Nat. Rev. Dis. Prim. 2017, 3, 1–19. [Google Scholar] [CrossRef]
- Woldu, S.L.; Bagrodia, A.; Lotan, Y. Guideline of guidelines: Non-muscle-invasive bladder cancer. BJU Int. 2017, 119, 371–380. [Google Scholar] [CrossRef] [Green Version]
- Noguera-Ortega, E.; Rabanal, R.M.; Secanella-Fandos, S.; Torrents, E.; Luquin, M.; Julián, E. Irradiated Mycobacteria Enhance Survival in Bladder Tumor Bearing Mice Although Less Efficaciously than Live Mycobacteria. J. Urol. 2016, 195, 198–205. [Google Scholar] [CrossRef]
- Sakula, A. BCG: Who were Calmette and Guerin? Thorax 1983, 38, 806–812. [Google Scholar] [CrossRef] [Green Version]
- Boehm, B.E.; Cornell, J.E.; Wang, H.; Mukherjee, N.; Oppenheimer, J.S.; Svatek, R.S. Efficacy of bacillus Calmette-Guérin Strains for Treatment of Nonmuscle Invasive Bladder Cancer: A Systematic Review and Network Meta-Analysis. J. Urol. 2017, 198, 503–510. [Google Scholar] [CrossRef]
- Morales, A.; Eidinger, D.; Bruce, A.W. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J. Urol. 1976, 116, 180–183. [Google Scholar] [CrossRef]
- Herr, H.W.; Laundone, V.P.; Badalament, R.A.; Oettgen, H.F.; Sogani, P.C.; Freedman, B.D.; Melamed, M.R.; Whitmore, W. Bacillus Calmette-Guerin therapy alters the progression of superficial bladder cancer. J. Clin. Oncol. 1988, 6, 1450–1455. [Google Scholar] [CrossRef]
- Herr, H.W.; Morales, A. History of bacillus Calmette-Guerin and bladder cancer: An immunotherapy success story. J. Urol. 2008, 179, 53–56. [Google Scholar] [CrossRef]
- Lamm, D.L.; Blumenstein, B.A.; Crawford, E.D.; Montie, J.E.; Scardino, P.; Grossman, H.B.; Stanistic, T.H.; Smith, J.A.; Sullivan, J.; Sarosdy, M.F.; et al. A randomized trial of intravesical doxorubicin and immunotherapy with bacille Calmette-Guérin for transitional-cell carcinoma of the bladder. N. Engl. J. Med. 1991, 325, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Pettenati, C.; Ingersoll, M.A. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat. Rev. Urol. 2018, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fuge, O.; Vasdev, N.; Allchorne, P.; Green, J.S. Immunotherapy for bladder cancer. Res. Reports Urol. 2015, 7, 65–79. [Google Scholar]
- FitzGerald, J.M. Management of Adverse Reactions to Bacille Calmette-Guérin Vaccine. Clin. Infect. Dis. 2000, 31, S75–S76. [Google Scholar] [CrossRef] [Green Version]
- Green, D.B.; Kawashima, A.; Menias, C.O.; Takashi, T.; Redelman-Sidi, G.; Bhalla, S.; Shah, R.; King, B.F. Complications of Intravesical BCG Immunotherapy for Bladder Cancer. Radio Graph. 2019, 39, 80–94. [Google Scholar] [CrossRef]
- Ehdaie, B.; Sylvester, R.; Herr, H.W. Maintenance bacillus calmette-guérin treatment of non-muscle-invasive bladder cancer: A critical evaluation of the evidence. Eur. Urol. 2013, 64, 579–585. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, J.; Huang, Y.; Ma, L. Clinical Spectrum of Complications Induced by Intravesical Immunotherapy of Bacillus Calmette-Guérin for Bladder Cancer. J. Oncol. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Julián, E.; Noguera-Ortega, E. Microbial Infections and Cancer Therapy, 1st ed.; Chakrabarty, A.M., Fialho, A.M., Eds.; Jenny Stanford Publishing: Boston, MA, USA, 2019; ISBN 9781351041904. [Google Scholar]
- Noguera-Ortega, E.; Julián, E. Mycobacteria-Derived Agents for the Treatment of Urological and Renal Cancers. In Mycobacterium Research and Development; InTechOpen: London, UK, 2018. [Google Scholar]
- Crijnen, J.; De Reijke, T.M. Emerging intravesical drugs for the treatment of non muscle-invasive bladder cancer. Expert Opin. Emerg. Drugs 2018, 23, 135–147. [Google Scholar] [CrossRef]
- Guallar-Garrido, S.; Julián, E. Bacillus Calmette-Guérin (BCG) Therapy for Bladder Cancer: An Update. Immuno Targets Ther. 2020, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kelley, D.R.; Ratliff, T.L.; Catalona, W.J.; Shapiro, A.; Lage, J.M.; Bauer, W.C.; Haaff, E.O.; Dresner, S.M. Intravesical bacillus Calmette-Guerin therapy for superficial bladder cancer: Effect of bacillus Calmette-Guerin viability on treatment results. J. Urol. 1985, 134, 48–53. [Google Scholar] [CrossRef]
- De Boer, E.C.; Rooijakkers, S.J.; Schamhart, D.H.; Kurth, K.-H. Cytokine gene expression in a mouse model: The first instillations with viable bacillus Calmette-Guerin determine the succeeding Th1 response. J. Urol. 2003, 170, 2004–2008. [Google Scholar] [CrossRef] [PubMed]
- Noguera-Ortega, E.; Blanco-Cabra, N.; Rabanal, R.M.; Sánchez-Chardi, A.; Roldán, M.; Guallar-Garrido, S.; Torrents, E.; Luquin, M.; Julián, E. Mycobacteria emulsified in olive oil-in-water trigger a robust immune response in bladder cancer treatment. Sci. Rep. 2016, 6, 27232. [Google Scholar] [CrossRef]
- Noguera-Ortega, E.; Secanella-Fandos, S.; Eraña, H.; Gasión, J.; Rabanal, R.M.; Luquin, M.; Torrents, E.; Julián, E. Nonpathogenic Mycobacterium brumae Inhibits Bladder Cancer Growth In Vitro, Ex Vivo, and In Vivo. Eur. Urol. Focus 2016, 2, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Julian, E.; Roldan, M.; Sanchez-Chardi, A.; Astola, O.; Agusti, G.; Luquin, M. Microscopic Cords, a Virulence-Related Characteristic of Mycobacterium tuberculosis, Are Also Present in Nonpathogenic Mycobacteria. J. Bacteriol. 2010, 162, 1751–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Secanella-Fandos, S.; Luquin, M.; Julián, E. Connaught and russian strains showed the highest direct antitumor effects of different bacillus calmette-guérin substrains. J. Urol. 2013, 189, 711–718. [Google Scholar] [CrossRef]
- Zhang, L.; Ru, H.; Chen, F.; Jin, C.; Sun, R.; Fan, X.; Guo, M.; Mai, J.; Xu, W.; Lin, Q.; et al. Variable Virulence and Efficacy of BCG Vaccine Strains in Mice and Correlation With Genome Polymorphisms. Mol. Ther. 2016, 24, 398–405. [Google Scholar] [CrossRef] [Green Version]
- Velmurugan, K.; Grode, L.; Chang, R.; Fitzpatrick, M.; Laddy, D.; Hokey, D.; Derrick, S.; Morris, S.; McCown, D.; Kidd, R.; et al. Nonclinical Development of BCG Replacement Vaccine Candidates. Vaccines 2013, 1, 120–138. [Google Scholar] [CrossRef]
- Reis, L.O.; Sopena, J.M.G.; Fávaro, W.J.; Martin, M.C.; Simão, A.F.L.; dos Reis, R.B.; de Andrade, M.F.; Domenech, J.D.; Cardo, C.C. Anatomical features of the urethra and urinary bladder catheterization in female mice and rats. An essential translational tool. Acta Cir. Bras. 2011, 26, 106–110. [Google Scholar] [CrossRef]
- Johnson, M.M.; Odell, J.A. Nontuberculous mycobacterial pulmonary infections. J. Thorac. Dis. 2014, 6, 201–220. [Google Scholar]
- Noguera-Ortega, E.; Rabanal, R.M.; Gómez-Mora, E.; Cabrera, C.; Luquin, M.; Julián, E. Intravesical Mycobacterium brumae triggers both local and systemic immunotherapeutic responses against bladder cancer in mice. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Domingos-Pereira, S.; Cesson, V.; Chevalier, M.F.; Derré, L.; Jichlinski, P.; Nardelli-Haefliger, D. Preclinical efficacy and safety of the TY21a vaccine strain for intravesical immunotherapy of non-muscle-invasive bladder cancer. Oncoimmunology 2017, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Felgner, S.; Kocijancic, D.; Frahm, M.; Weiss, S. Bacteria in Cancer Therapy: Renaissance of an Old Concept. Int. J. Microbiol. 2016, 2016, 8451728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, M.R.; Grant, C.; Sanford, T.; Agarwal, P.K. Current clinical trials in non–muscle invasive bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2017, 35, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Lamm, D.L. Efficacy and Safety of Bacille Calmette-Guérin Immunotherapy in Superficial Bladder Cancer. Clin. Infect. Dis. 2000, 31, S86–S90. [Google Scholar] [CrossRef] [PubMed]
- Loxton, A.G.; Knaul, J.K.; Grode, L.; Gutschmidt, A.; Meller, C.; Eisele, B.; Johnstone, H.; van der Spuy, G.; Maertzdorf, J.; Kaufmann, S.H.E.; et al. Safety and Immunogenicity of the Recombinant Mycobacterium bovis BCG Vaccine VPM1002 in HIV-Unexposed Newborn Infants in South Africa. Clin. Vaccine Immunol. 2017, 24, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llorente, C.; Schnabl, B. Fast-Track Clearance of Bacteria from the Liver. Cell Host Microbe 2016, 20, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wei, W.; Shen, Y.X.; Dong, C.; Zhang, L.L.; Wang, N.P.; Yue, L.; Xu, S.Y. Protective effect of melatonin against liver injury in mice induced by Bacillus Calmette-Guerin plus lipopolysaccharide. World J. Gastroenterol. 2004, 10, 2690–2696. [Google Scholar] [CrossRef]
- Egen, J.G.; Rothfuchs, A.G.; Feng, C.G.; Winter, N.; Sher, A.; Germain, R.N. Macrophage and T Cell Dynamics during the Development and Disintegration of Mycobacterial Granulomas. Immunity 2008, 28, 271–284. [Google Scholar] [CrossRef] [Green Version]
- Goldmann, T.; Zissel, G.; Gupta, R.S.; Schlaak, M.; Vollmer, E.; Müller-Quernheim, J. Formation of Granulomas in the Lungs of Severe Combined Immunodeficient Mice after Infection with Bacillus Calmette-Guerin. Am. J. Pathol. 2001, 158, 1890–1891. [Google Scholar] [CrossRef] [Green Version]
- North, R.J.; Izzo, A.A. Granuloma formation in severe combined immunodeficient (SCID) mice in response to progressive BCG infection. Tendency not to form granulomas in the lung is associated with faster bacterial growth in this organ. Am. J. Pathol. 1993, 142, 1959–1966. [Google Scholar]
- Upadhyay, J.; Sudhindra, P.; Abraham, G.; Trivedi, N. Tuberculosis of the Adrenal Gland: A Case Report and Review of the Literature of Infections of the Adrenal Gland. Int. J. Endocrinol. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Lee, J.; Jeon, H.; Kim, D.; Oh, Y.; Kim, Y.; Lim, C. Severe hyperkalemia requiring hospitalization: Predictors of mortality. Crit. Care 2012, 16, R225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherayil, B.J. The role of iron in the immune response to bacterial infection. Immunol. Res. 2011, 50, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva-Gomes, S.; Vale-Costa, S.; Appelberg, R.; Gomes, M.S. Iron in intracellular infection: To provide or to deprive? Front. Cell. Infect. Microbiol. 2013, 3, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eruslanov, E.B.; Lyadova, I.V.; Kondratieva, T.K.; Majorov, K.B.; Scheglov, I.V.; Orlova, M.O.; Apt, A.S. Neutrophil Responses to Mycobacterium tuberculosis infection in genetically suceptible and resistant mice. Infect. Immun. 2005, 73, 1744–1753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leisching, G.R. Susceptibility to Tuberculosis is associated with PI3K-dependent increased mobilization of neutrophils. Front. Immunol. 2018, 9, 1669. [Google Scholar] [CrossRef] [PubMed]
- Bottai, D.; Brosch, R. The BCG strain pool: Diversity matters. Mol. Ther. 2016, 24, 201–203. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, A.M.; Hill-Cawthorne, G.A.; Otto, T.D.; Coll, F.; Guerra-Assunção, J.A.; Gao, G.; Naeem, R.; Ansari, H.; Malas, T.B.; Adroub, S.A.; et al. Genomic expression catalogue of a global collection of BCG vaccine strains show evidence for highly diverged metabolic and cell-wall adaptations. Sci. Rep. 2015, 5, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Spiropoulos, J.; Cooley, W.; Khara, J.S.; Gladstone, C.A.; Asai, M.; Bossé, J.T.; Robertson, B.D.; Newton, S.M.; Langford, P.R. Galleria mellonella-a novel infection model for the Mycobacterium tuberculosis complex. Virulence 2018, 9, 1126–1137. [Google Scholar] [CrossRef] [Green Version]
- Entwistle, F.M.; Coote, P.J. Evaluation of greater wax moth larvae, Galleria mellonella, as a novel in vivo model for non-tuberculosis mycobacteria infections and antibiotic treatments. J. Med. Microbiol. 2018, 67, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Biot, C.; Rentsch, C.A.; Gsponer, J.R.; Birkhauser, F.D.; Jusforgues-Saklani, H.; Lemaitre, F.; Auriau, C.; Bachmann, A.; Bousso, P.; Demangel, C.; et al. Preexisting BCG-Specific T Cells Improve Intravesical Immunotherapy for Bladder Cancer. Sci. Transl. Med. 2012, 4, 137ra72. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Ma, J.; Ni, W.; Wang, F.; Sun, X.; Li, Y.; Li, Q.; Xie, F.; Wang, J.; Zhai, R.; et al. MUC1 and maltose-binding protein recombinant fusion protein combined with Bacillus Calmette-Guerin induces MUC1-specific and nonspecific anti-tumor immunity in mice. Mol. Med. Rep. 2014, 10, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.K.; Kumar, S.; Cannon, A.; Hall, B.; Bhatia, R.; Nasser, M.W.; Mahapatra, S.; Batra, S.K.; Jain, M. MUC4 mucin- a therapeutic target for pancreatic ductal adenocarcinoma. Expert Opin. Ther. Targets 2017, 21, 657–669. [Google Scholar] [CrossRef] [PubMed]




| Treatment | PBS | γ-Irradiated M. brumae | M. brumae | BCG | p Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | |
| ALT (U/L) a | 17.86 ± 5.82 | (11.30–30.80) | 17.89 ± 6.39 1 | (12.20–31.50) | 16.74 ± 3.05 1 | (14.00–22.60) | 61.53 ± 36.81 3 | (27.80–100.80) | < 0.001 |
| AST (U/L) b | 73.50 ± 38.43 | (42.00–163.00) | 51.29 ± 10.81 1 | (42.00–74.00) | 65.86 ± 17.30 1 | (49.00–88.00) | 326.00 ± 74.62 4 | (249.00–428.00) | < 0.0001 |
| Creatinine (mg/dL) | 0.27 ± 0.01 | (0.25–0.29) | 0.27 ± 0.02 | (0.24–0.28) | 0.28 ± 0.01 1 | (0.27–0.30) | 0.23 ± 0.04 5 | (0.17–0.28) | < 0.05, < 0.01 * |
| Iron (µg/dL) | 224.11 ± 40.55 | (158.40–274.10) | 218.26 ± 43.96 | (156.40–276.70) | 203.37 ± 9.89 2 | (188.10–214.50) | 119.84 ± 31.025 | (88.00–157.70) | < 0.01 #, < 0.001 |
| Alkaline Phosphatase (mmol/L) | 72.54 ± 4.71 | (67.20–78.46) | 77.69 ± 11.24 | (68.17–100.46) | 80.10 ± 7.99 1 | (66.90–90.02) | 76.70 ± 11.75 6 | (68.39–85.00) | ns |
| Glucose (mg/mL) | 271.98 ± 26.89 | (232.20–317.40) | 285.51 ± 45.10 | (233.70–352.00) | 269.60 ± 44.31 1 | (213.30–340.10) | 121.37 ± 3.55 3 | (118.20–125.20) | < 0.0001 |
| Potassium (mmol/L) | 4.64 ± 0.74 | (3.61–5.90) | 4.89 ± 0.70 | (3.96–5.75) | 4.27 ± 0.55 | (3.65–5.10) | 5.95 ± 0.66 5 | (5.35–6.84) | < 0.05, < 0.01 &, < 0.0001 ^ |
| Sodium (mmol/L) | 147.00 ± 1.61 | (143.70–148.80) | 146.33 ± 1.32 | (144.50–148.10) | 148.88 ± 1.64 | (146.50–151.10) | 149.14 ± 1.86 5 | (147.00–151.20) | < 0.05 |
| Urea (mg/dL) | 47.01 ± 7.83 | (33.10–53.60) | 44.21 ± 9.36 | (34.20–60.50) | 44.87 ± 8.61 1 | (35.50–60.60) | 68.65 ± 16.27 4 | (55.60–92.20) | < 0.01 |
| Albumin (g/dL) | 2.87 ± 0.12 | (2.72–3.10) | 2.82 ± 0.09 | (2.61–2.89) | 2.82 ± 0.10 1 | (2.71–3.00) | 2.14 ± 0 7 | − | ns |
| Total Protein (g/dL) | 4.88 ± 0.19 | (4.57–5.20) | 4.77 ± 0.18 | (4.38–4.93) | 4.84 ± 0.12 1 | (4.69–5.04) | 4.66 ± 0 7 | − | ns |
| Treatment | PBS | γ-Irradiated M. brumae | M. brumae1 | BCG 1 | p Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | |
| WBCBa (×103 cells/µL) | 0.84 ± 0.23 | (0.46–1.06) | 1.14 ± 0.67 | (0.65–2.55) | 0.71 ± 0.23 | (0.50–1.02) | 11.40 ± 5.13 | (5.00–18.14) | < 0.001 |
| RCBb (×103 cells/µL) | 9.35 ± 0.47 | (8.88–10.23) | 9.84 ± 0.27 | (9.40–10.25) | 9.29 ± 0.29 | (8.97–9.75) | 12.17 ± 0.57 | (11.41–12.80) | < 0.001 |
| Hematocrit (%) | 40.16 ± 1.84 | (37.80–42.20) | 41.67 ± 1.08 | (40.00–43.20) | 40.28 ± 1.06 | (38.9–41.9) | 42.38 ± 2.10 | (40.00–46.30) | ns |
| Platelets (×103 cells/µL) | 1008.14 ± 88.13 | (855.00–1127.00) | 1024.43 ± 110.32 | (861.00–1220.00) | 958.00 ± 211.00 | (588.00–1161.00) | 1502.83 ± 300.46 | (1102.00–1920.00) | < 0.001 |
| Total neutrophils (×103 cells/µL) | 0.48 ± 0.18 | (0.18–0.66) | 0.64 ± 0.39 | (0.21–1.44) | 0.39 ± 0.15 | (0.21–0.58) | 9.99 ± 4.77 | (4.10–16.14) | < 0.0001 |
| Total lymphocytes (×103 cells/µL) | 0.19 ± 0.06 | (0.14–0.31) | 0.22 ± 0.12 | (0.07–0.43) | 0.14 ± 0.05 | (0.07–0.21) | 0.64 ± 0.17 | (0.37–0.84) | < 0.0001 |
| Total basophils (×103 cells/µL) | 0.00 ± 0.00 | (0.00–0.01) | 0.00 ± 0.01 | (0.00–0.01) | 0.00 ± 0.00 | (0.00–0.01) | 0.03 ± 0.02 | (0.01–0.07) | < 0.001 |
| Total eosinophils (×103 cells/µL) | 0.08 ± 0.05 | (0.04–0.17) | 0.14 ± 0.15 | (0.04–0.46) | 0.07 ± 0.03 | (0.03–0.10) | 0.31 ± 0.10 | (0.19–0.46) | < 0.05 *, < 0.01 |
| Total monocytes (×103 cells/µL) | 0.06 ± 0.02 | (0.04–0.09) | 0.08 ± 0.18 | (0.03–0.18) | 0.06 ± 0.03 | (0.02–0.12) | 0.18 ± 0.10 | (0.07–0.31) | < 0.05 #, < 0.01 |
| Treatment | PBS | γ-Irradiated M. brumae (Low Dose) | γ-Irradiated M. brumae (High Dose) | M. brumae (Low Dose) | M. brumae (High Dose) | p Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | |
| ALT (U/L) a | 12.80 ± 3.60 2 | (9.30–19.40) | 16.66 ± 5.10 3 | (11.60–25.10) | 13.08 ± 2.58 2 | (10.04–16.50) | 13.76 ± 6.43 3 | (9.00–23.70) | 14.87 ± 1.71 2 | (12.60–17.70) | ns |
| AST (U/L) b | 59.33 ± 18.33 2 | (38.00–90.00) | 75.60 ± 60.60 3 | (42.00–183.00) | 65.00 ± 16.70 2 | (45.00–94.00) | 67.20 ± 26.00 3 | (51.00–113.00) | 82.71 ± 28.90 1 | (45.00–94.00) | ns |
| Creatinine (mg/dL) | 0.25 ± 0.03 | (0.20–0.29) | 0.28 ± 0.02 2 | (0.26–0.30) | 0.24 ± 0.06 | (0.10–0.30) | 0.27 ± 0.03 1 | (0.25–0.33) | 0.26 ± 0.02 | (0.10–0.30) | ns |
| Iron (µg/dL) | 145.90 ± 50.27 1 | (67.30–207.10) | 160.26 ± 61.10 3 | (113.80–259.30) | 133.06 ± 31.60 1 | (70.90–159.70) | 170.67 ± 21.17 1 | (134.70–219.50) | 165.07 ± 45.90 1 | (70.90–159.70) | ns |
| Alkaline Phosphatase (mmol/L) | 141.87 ± 12.71 1 | (123.73–157.00) | 138.02 ± 14.403 | (112.80–148.65) | 147.95 ± 11.40 2 | (132.30–163.40) | 146.74 ± 19.76 1 | (133.80–174.33) | 149.11 ± 15.95 1 | (132.30–163.40) | ns |
| Glucose (mg/mL) | 247.75 ± 35.92 | (207.70–297.60) | 287.95 ± 60.01 2 | (179.90–351.20) | 256.63 ± 30.90 1 | (224.00–294.20) | 256.63 ± 30.93 1 | (229.30–322.30) | 257.23 ± 49.80 2 | (224.00–294.20) | ns |
| Potassium (mmol/L) | 4.76 ± 0.63 | (3.95–6.01) | 5.04 ± 0.92 2 | (4.07–5.21) | 4.90 ± 0.57 1 | (4.00–5.74) | 4.90 ± 0.57 1 | (3.90–5.65) | 4.69 ± 0.34 2 | (4.00–5.74) | ns |
| Sodium (mmol/L) | 145.58 ± 1.97 | (142.80–149.30) | 144.25 ± 1.58 2 | (142.50–146.80) | 144.66 ± 1.58 1 | (143.50–148.00) | 144.66 ± 1.58 1 | (141.10–153.10) | 146.90 ± 1.59 2 | (143.50–148.00) | ns |
| Urea (mg/dL) | 40.86 ± 5.94 | (32.40–50.60) | 39.63 ± 9.60 2 | (30.10–54.80) | 39.06 ± 7.36 | (29.30–52.10) | 39.06 ± 7.38 1 | (28.20–60.90) | 47.56 ± 9.59 1 | (29.30–52.10) | ns |
| Albumin (g/dL) | 2.59 ± 0.16 1 | (2.30–2.75) | 2.65 ± 0.04 3 | (2.30–2.75) | 2.65 ± 0.04 3 | (2.60–2.71) | 2.65 ± 0.04 1 | (2.33–2.61) | 2.64 ± 0.06 | (2.60–2.71) | ns |
| Total Protein (g/dL) | 4.54 ± 0.24 1 | (4.15–4.89) | 4.74 ± 0.34 3 | (3.99–4.93) | 4.74 ± 0.34 2 | (4.44–5.40) | 4.74 ± 0.34 1 | (4.08–5.02) | 4.60 ± 0.44 | (4.44–5.40) | ns |
| Treatment | PBS | γ-Irradiated M. Brumae (Low Dose) | γ-Irradiated M. Brumae (High Dose) | M. Brumae (Low Dose) 1 | M. Brumae (High Dose) 2 | p Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | |
| WBCBa (×103 cells/µL) | 6.41 ± 1.58 | (4.05–8.42) | 6.59 ± 1.78 | (4.78–9.28) | 5.53 ± 1.35 | (3.57–7.37) | 4.40 ± 1.01 | (2.7–5.28) | 4.79 ± 1.51 | (3.06–6.12) | ns |
| RCBb (×103 cells/µL) | 9.34 ± 0.48 | (8.60–9.87) | 9.13 ± 0.41 | (8.79–9.65) | 9.62 ± 0.43 | (9.02–10.15) | 9.01 ± 0.34 | (8.65–9.43) | 9.12 ± 0.34 | (8.68–9.42) | ns |
| Hematocrit (%) | 41.07 ± 1.60 | (39.40–43.30) | 41.20 ± 2.33 | (38.50–45.40) | 42.3 ± 1.55 | (40.90–44.40) | 40.42 ± 1.82 | (37.9–43) | 40.17 ± 0.67 | (39.30–40.90) | ns |
| Platelets (×103 cells/µL) | 953.00 ± 103.48 | (809.00–1076.00) | 929.00 ± 150.04 | (656.00–1073.00) | 859.33 ± 253.51 | (388.00–1155.00) | 836.00 ± 265.49 | (367.00–996.00) | 854.00 ± 179.37 | (595.00–989.00) | ns |
| Total neutrophils (×103 cells/µL) | 0.94 ± 0.38 | (0.47–1.49) | 0.98 ± 0.25 | (0.70–1.39) | 0.76 ± 0.29 | (0.31–1.10) | 0.53 ± 0.14 | (0.39–0.68) | 0.66 ± 0.26 | (0.39–1.01) | ns |
| Total lymphocytes (×103 cells/µL) | 4.97 ± 1.30 | (3.05–6.27) | 5.21 ± 1.40 | (3.64–7.17) | 4.35 ± 0.98 | (2.80–5.07) | 3.53 ± 0.93 | (2.05–4.59) | 3.56 ± 1.25 | (2.47–4.83) | ns |
| Total basophils (×103 cells/µL) | 0.01 ± 0.01 | (0.00–0.01) | 0.01 ± 0.01 | (0.01–0.02) | 0.01 ± 0.01 | (0.00–0.03) | 0.01 ± 0.01 | (0.00–0.01) | 0.01 ± 0.01 | (0.00 – 0.01) | ns |
| Total eosinophils (×103 cells/µL) | 0.17 ± 0.11 | (0.07–0.37) | 0.14 ± 0.04 | (0.09–0.22) | 0.15 ± 0.02 | (0.12–0.18) | 0.13 ± 0.02 | (0.11–0.15) | 0.33 ± 0.30 | (0.11–0.76) | ns |
| Total monocytes (×103 cells/µL) | 0.13 ± 0.09 | (0.06–0.32) | 0.11 ± 0.03 | (0.06–0.16) | 0.11 ± 0.04 | (0.09–0.20) | 0.05 ± 0.01 | (0.09–0.06) | 0.08 ± 0.04 | (0.06–0.15) | ns |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bach-Griera, M.; Campo-Pérez, V.; Barbosa, S.; Traserra, S.; Guallar-Garrido, S.; Moya-Andérico, L.; Herrero-Abadía, P.; Luquin, M.; Rabanal, R.M.; Torrents, E.; et al. Mycolicibacterium brumae is a Safe and Non-Toxic Immunomodulatory Agent for Cancer Treatment. Vaccines 2020, 8, 198. https://doi.org/10.3390/vaccines8020198
Bach-Griera M, Campo-Pérez V, Barbosa S, Traserra S, Guallar-Garrido S, Moya-Andérico L, Herrero-Abadía P, Luquin M, Rabanal RM, Torrents E, et al. Mycolicibacterium brumae is a Safe and Non-Toxic Immunomodulatory Agent for Cancer Treatment. Vaccines. 2020; 8(2):198. https://doi.org/10.3390/vaccines8020198
Chicago/Turabian StyleBach-Griera, Marc, Víctor Campo-Pérez, Sandra Barbosa, Sara Traserra, Sandra Guallar-Garrido, Laura Moya-Andérico, Paula Herrero-Abadía, Marina Luquin, Rosa Maria Rabanal, Eduard Torrents, and et al. 2020. "Mycolicibacterium brumae is a Safe and Non-Toxic Immunomodulatory Agent for Cancer Treatment" Vaccines 8, no. 2: 198. https://doi.org/10.3390/vaccines8020198






