Dynamics of Population Immunity Due to the Herd Effect in the COVID-19 Pandemic
Abstract
1. Background and Basis of Herd Immunity
2. Herd, Population, Collective, or Group Immunity
3. Person-to-Person Transmission Dynamics Inducing Specific Immunity
4. Applied Immunoepidemiology for Epidemic Control Strategy Development
5. Herd Immunity Applications in the Current COVID-19 Pandemic
6. Herd Immunity Limitations in the Current COVID-19 Pandemic
6.1. Laissez-Faire Attitudes or Natural Herd Immunity
6.2. Possible Consequences of Obtaining Herd Immunity by Natural Pathways
6.3. Epidemiological Limitations
6.4. Immunological Limitations
7. Herd Immunity Effectiveness
8. Herd Immunity and Vaccines
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Topley, W.W.C.; Wilson, G.S. The spread of bacterial infection. The problem of herd-immunity. Epidemiol. Infect. 1923, 21, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.E. Herd immunity: History, theory, practice. Epidemiol. Rev. 1993, 15, 265–302. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.; Eames, K.; Heymann, D.L. “Herd immunity”: A rough guide. Clin. Infect. Dis. 2011, 52, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Rashid, H.; Khandaker, G.; Booy, R. Vaccination and herd immunity: What more do we know? Curr. Opin. Infect Dis. 2012, 25, 243–249. [Google Scholar] [CrossRef]
- Smith, D.R. Herd Immunity. Vet. Clin. Pract. 2019, 35, 593–604. [Google Scholar] [CrossRef]
- Goncalves, G. Herd immunity: Recent uses in vaccine assessment. Expert Rev. Vaccines 2008, 7, 1493–1506. [Google Scholar] [CrossRef]
- Korppi, M. Universal pneumococcal vaccination provides marked indirect beneficial effects through herd immunity. Acta Paediatr. 2018, 107, 1488–1489. [Google Scholar] [CrossRef]
- Nymark, L.S.; Sharma, T.; Miller, A.; Enemark, U.; Griffiths, U.K. Inclusion of the value of herd immunity in economic evaluations of vaccines. A systematic review of methods used. Vaccine 2017, 35, 6828–6841. [Google Scholar] [CrossRef]
- Ali, M.; Emch, M.; Von Seidlein, L.; Yunus, M.; Sack, D.A.; Rao, M.; Holmgren, J.; Clemens, J.D. Herd immunity conferred by killed oral cholera vaccines in Bangladesh: A reanalysis. Lancet 2005, 366, 44–49. [Google Scholar] [CrossRef]
- Kinoshita, R.; Nishiura, H. Assessing herd immunity against rubella in Japan: A retrospective seroepidemiological analysis of age-dependent transmission dynamics. BMJ Open 2016, 6. [Google Scholar] [CrossRef]
- Smith, D.; Huynh, C.; Moore, A.J.; Frick, A.; Anderson, C.; Porrachia, M.; Scott, B.; Stous, S.; Schooley, R.; Little, S.; et al. Herd Immunity Likely Protected the Men Who Have Sex With Men in the Recent Hepatitis A Outbreak in San Diego, California. Clin. Infect. Dis. 2019, 68, 1228–1230. [Google Scholar] [CrossRef]
- Maver, P.J.; Poljak, M. Progress in prophylactic human papillomavirus (HPV) vaccination in 2016: A literature review. Vaccine 2018, 36, 5416–5423. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.J.; ElSherif, M.; Ye, L.; MacKinnon-Cameron, D.; Ambrose, A.; Hatchette, T.F.; Lang, A.L.S.; Gillis, H.D.; Martin, I.; Demczuk, W.; et al. Streptococcus pneumoniae serotype 3 is masking PCV13-mediated herd immunity in Canadian adults hospitalized with community acquired pneumonia: A study from the Serious Outcomes Surveillance (SOS) Network of the Canadian immunization research Network (CIRN). Vaccine 2019, 37, 5466–5473. [Google Scholar] [CrossRef] [PubMed]
- Payne, P.; Geyrhofer, L.; Barton, N.H.; Bollback, J.P. CRISPR-based herd immunity can limit phage epidemics in bacterial populations. eLife 2018, 7, e32035. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, I.G.C.D.; Marandino, R.; Mendonça, A.P.; Nogueira, R.M.R.; Vasconcelos, P.F.D.C.; Guerra, L.R.; Brandão, B.C.; Mendonça, A.P.; Aguiar, G.R.; Bacco, P.A. Chikungunya virus infection: Report of the first case diagnosed in Rio de Janeiro, Brazil. Rev. Soc. Bras. Med. Trop. 2012, 45, 128–129. [Google Scholar] [CrossRef] [PubMed]
- Kwok, K.O.; Lai, F.; Wei, W.I.; Wong, S.Y.S.; Tang, J.W. Herd immunity–estimating the level required to halt the COVID-19 epidemics in affected countries. J. Infect. 2020, 80, e32–e33. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.P.; Elveback, L.; Scott, W.; Gatewood, L.; Ackerman, E. Herd immunity: Basic concept and relevance to public health immunization practices. Am. J. Epidemiol. 1971, 94, 179–189. [Google Scholar] [CrossRef]
- Singhal, T. A review of coronavirus disease-2019 (COVID-19). Indian J. Pediatr. 2020, 87. [Google Scholar] [CrossRef]
- Peng, X.; Xu, X.; Li, Y.; Cheng, L.; Zhou, X.; Ren, B. Transmission routes of 2019-nCoV and controls in dental practice. Int. J. Oral Sci. 2020, 12, 9. [Google Scholar] [CrossRef]
- Yeo, C.; Kaushal, S.; Yeo, D. Enteric involvement of coronaviruses: Is faecal–oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol. Hepatol. 2020, 5, 335–337. [Google Scholar] [CrossRef]
- Qiao, J. What are the risks of COVID-19 infection in pregnant women? Lancet 2020, 395, 760–762. [Google Scholar] [CrossRef]
- Zhou, G.; Zhao, Q. Perspectives on therapeutic neutralizing antibodies against the Novel Coronavirus SARS-CoV-2. Int. J. Biol. Sci. 2020, 16, 1718. [Google Scholar] [CrossRef] [PubMed]
- Xun, J.; Lu, L.; Jiang, S.; Lu, H.; Wen, Y.; Huang, J. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered 2 patient cohort and their implications. Medrxiv 2020. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, C.; Tang, L.; Hong, Z.; Zhou, J.; Dong, X.; Yin, H.; Xiao, Q.; Tang, Y.; Qu, X.; et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020, 5, 434–435. [Google Scholar] [CrossRef]
- Wu, J.T.; Leung, K.; Bushman, M.; Kishore, N.; Niehus, R.; de Salazar, P.M.; Cowling, B.J.; Lipsitch, M.; Leung, G.M. Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China. Nat. Med. 2020, 26, 506–510. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Mizumoto, K.; Kagaya, K.; Zarebski, A.; Chowell, G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020, 25, 2000180. [Google Scholar] [CrossRef]
- Benvenuto, D.; Giovanetti, M.; Vassallo, L.; Angeletti, S.; Ciccozzi, M. Application of the ARIMA model on the COVID-2019 epidemic dataset. Data Brief 2020, 29, 105340. [Google Scholar] [CrossRef]
- Read, J.M.; Bridgen, J.R.; Cummings, D.A.; Ho, A.; Jewell, C.P. Novel coronavirus 2019-nCoV: Early estimation of epidemiological parameters and epidemic predictions. Medrxiv 2020. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Dong, Y.; Chang, R.; Xu, C.; Yu, X.; Zhang, S.; Tsamlag, L.; Shang, M.; Huang, J.; et al. Phase-adjusted estimation of the number of Coronavirus Disease 2019 cases in Wuhan, China. Cell Discov. 2020, 6, 10. [Google Scholar] [CrossRef]
- Tang, B.; Bragazzi, N.L.; Li, Q.; Tang, S.; Xiao, Y.; Wu, J. An updated estimation of the risk of transmission of the novel coronavirus (2019-nCov). Infect. Dis. Model. 2020, 5, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.M.; Rui, J.; Wang, Q.P.; Zhao, Z.Y.; Cui, J.A.; Yin, L. A mathematical model for simulating the phase-based transmissibility of a novel coronavirus. Infect. Dis. Poverty 2020, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gayle, A.A.; Wilder-Smith, A.; Rocklöv, J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Travel Med. 2020, 27, taaa021. [Google Scholar] [CrossRef] [PubMed]
- Randolph, H.E.; Barreiro, L.B. Herd Immunity: Understanding COVID-19. Cell Press 2020. [Google Scholar] [CrossRef]
- Shim, E.; Tariq, A.; Choi, W.; Lee, Y.; Chowell, G. Transmission potential and severity of COVID-19 in South Korea. Int. J. Infect. Dis. 2020, 93, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.P.; Meng, S.; Wu, Y.J.; Mao, Y.P.; Ye, R.X.; Wang, Q.Z.; Sun, C.; Sylvia, S.; Rozelle, S.; Raat, H.; et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: A scoping review. Infect. Dis. Poverty 2020, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.; Yang, Y.; Rao, Y.; Rao, X. The outbreak of SARS-CoV-2 pneumonia calls for viral vaccines. npj Vaccines 2020, 5, 18. [Google Scholar] [CrossRef]
- Surveillances, V. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China, 2020. China CDC Wkly 2020, 2, 113–122. [Google Scholar]
- WHO Characterizes COVID-19 as a Pandemic. 2020. Available online: https://www.paho.org/hq/index.php?option=com_content&view=article&id=15756&Itemid=39630&lang=en (accessed on 15 May 2020).
- Foddai, A.; Lindberg, A.; Lubroth, J.; Ellis-Iversen, J. Surveillance to improve evidence for community control decisions during the COVID-19 pandemic–opening the animal epidemic toolbox for public health. One Health 2020, 9, 100130. [Google Scholar] [CrossRef]
- de Lusignan, S.; Bernal, J.L.; Zambon, M.; Akinyemi, O.; Amirthalingam, G.; Andrews, N.; Borrow, R.; Byford, R.; Charlett, A.; Dabrera, G.; et al. Emergence of a novel coronavirus (COVID-19): Protocol for extending surveillance used by the Royal College of general practitioners research and surveillance centre and public health England. JMIR Public Health Surveill. 2020, 6, e18606. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Tufan, Z.K.; Kayaaslan, B. Crushing the curve, the role of national and international institutions and policy makers in COVID-19 pandemic. Turk. J. Med. Sci. 2020, 50, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhang, H.; Xie, J.; Lin, M.; Ying, L.; Pang, P.; Ji, W. Sensitivity of chest CT for COVID-19: Comparison to RT-PCR. Radiology 2020, 200432. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, Y.; Liu, Z.H.; Zhao, Y.J.; Zhang, Q.; Zhang, L.; Cheung, T.; Xiang, Y.-T. Progression of Mental Health Services during the COVID-19 Outbreak in China. Int. J. Biol. Sci. 2020, 16, 1732–1738. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Operational Considerations for COVID-19 Surveillance Using GISRS: Interim Guidance, 26 March 2020 (No. WHO/2019-nCoV/Leveraging_GISRS/2020.1); World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Srivastava, N.; Baxi, P.; Ratho, R.K.; Saxena, S.K. Global Trends in Epidemiology of Coronavirus Disease 2019 (COVID-19). In Coronavirus Disease 2019 (COVID-19); Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Peng, F.; Tu, L.; Yang, Y.; Hu, P.; Wang, R.; Hu, Q.; Cao, F.; Jiang, T.; Sun, J.; Xu, G.; et al. Management and Treatment of COVID-19: The Chinese Experience. Can. J. Cardiol. 2020. [Google Scholar] [CrossRef]
- Li, L.; Qin, L.; Xu, Z.; Yin, Y.; Wang, X.; Kong, B.; Bai, J.; Lu, Y.; Fang, Z.; Song, Q.; et al. Artificial intelligence distinguishes covid-19 from community acquired pneumonia on chest ct. Radiology 2020, 200905. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.H.A.; Paiva, L.T.; De Pinho, M.D.R. A SEIR model for control of infectious diseases with constraints. Math. Biosci. Eng. 2014, 11, 761–784. [Google Scholar] [CrossRef]
- Herrmann, H.A.; Schwartz, J.M. Using network science to propose strategies for effectively dealing with pandemics: The COVID-19 example. medRxiv 2020. [Google Scholar] [CrossRef]
- Fresnadillo-Martínez, M.J.; Garcia-Sanchez, E.; Garcia-Merino, E.; García-Sánchez, J.E. Mathematical modelling of the propagation of infectious diseases: Where we came from, and where we are going. Rev. Esp. Quim. 2013, 26, 81–91. [Google Scholar]
- Sambala, E.Z.; Manderson, L. Policy perspectives on post pandemic influenza vaccination in Ghana and Malawi. BMC Public Health 2017, 17, 227. [Google Scholar] [CrossRef]
- Garnett, G.P. Role of herd immunity in determining the effect of vaccines against sexually transmitted disease. J. Infect. Dis. 2005, 191, S97–S106. [Google Scholar] [CrossRef]
- Zhan, C.; Chi, K.T.; Lai, Z.; Chen, X.; Mo, M. General Model for COVID-19 Spreading with Consideration of Intercity Migration, Insufficient Testing and Active Intervention: Application to Study of Pandemic Progression in Japan and USA. medRxiv 2020. [Google Scholar] [CrossRef]
- Flaxman, S.; Mishra, S.; Gandy, A.; Unwin, H.; Coupland, H.; Mellan, T.; Zhu, H.; Berah, T.; Eaton, J.; Perez Guzman, P.; et al. Report 13: Estimating the Number of Infections and the Impact of Non-Pharmaceutical Interventions on COVID-19 in 11 European Countries; Imperial College London: London, UK, 2020. [Google Scholar]
- Karin, O.; Bar-On, Y.M.; Milo, T.; Katzir, I.; Mayo, A.; Korem, Y.; Dudovich, B.; Yashiv, E.; Zehavi, A.J.; Davidovich, N.; et al. Adaptive cyclic exit strategies from lockdown to suppress COVID-19 and allow economic activity. medRxiv 2020. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L.A. The convalescent sera option for containing COVID-19. J. Clin. Investig. 2020, 130, 1545–1548. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.G.; Whittaker, C.; Watson, O.; Baguelin, M.; Ainslie, K.E.C.; Bhatia, S.; Boonyasiri, A.; Boyd, O.; Cattarino, L. The Global Impact of covid-19 and Strategies for Mitigation and Suppression; Imperial College of London: London, UK, 2020. [Google Scholar]
- The Coalition for Epidemic Preparedness Innovations. CEPI welcomes UK Government’s funding and highlights need for $2 billion to develop a vaccine against COVID-19. 2020. Available online: https://cepi.net/news_cepi/2-billion-required-to-develop-a-vaccine-against-the-covid-19-virus/ (accessed on 16 April 2020).
- James, A.; Hendy, S.C.; Plank, M.J.; Steyn, N. Suppression and Mitigation Strategies for Control of COVID-19 in New Zealand. medRxiv 2020. [Google Scholar] [CrossRef]
- Anderson, R.M.; Heesterbeek, H.; Klinkenberg, D.; Hollingsworth, T.D. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet 2020, 395, 931–934. [Google Scholar] [CrossRef]
- Colson, P.; Rolain, J.M.; Lagier, J.C.; Brouqui, P.; Raoult, D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int. J. Antimicrob. Agents 2020, 55, 105932. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Y.; Zhang, F.; Wang, Q.; Li, T.; Liu, Z.; Wang, J.; Qin, Y.; Zhang, X.; Yan, X.; et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The experience of clinical immunologists from China. Clin. Immunol. 2020, 214, 108393. [Google Scholar] [CrossRef]
- Cortegiani, A.; Ingoglia, G.; Ippolito, M.; Giarratano, A.; Einav, S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care 2020, 57, 279–283. [Google Scholar] [CrossRef]
- COVID-19 Reinfection Becoming an Issue in China, Strategist Says. Available online: https://www.cnbc.com/video/2020/03/16/covid-19-reinfection-becoming-an-issue-in-china-strategist-says.html (accessed on 7 April 2020).
- Sanche, S.; Lin, Y.T.; Xu, C.; Romero-Severson, E.; Hengartner, N.; Ke, R. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Armocida, B.; Formenti, B.; Ussai, S.; Palestra, F.; Missoni, E. The Italian health system and the COVID-19 challenge. Lancet Public Health 2020. [Google Scholar] [CrossRef]
- Worst-Case Estimates for U.S. Coronavirus Deaths. Available online: https://www.nytimes.com/2020/03/13/us/coronavirus-deaths-estimate.html (accessed on 7 April 2020).
- Verity, R.; Okell, L.C.; Dorigatti, I.; Winskill, P.; Whittaker, C.; Imai, N.; Cuomo-Dannenburg, G.; Thompson, H.; Walker, P.G.T.; Fu, H.; et al. Estimates of the severity of coronavirus disease 2019: A model-based analysis. Lancet Infect Dis. 2020. [Google Scholar] [CrossRef]
- Kissler, S.M.; Tedijanto, C.; Goldstein, E.; Grad, Y.H.; Lipsitch, M. Projecting the transmission dynamics of SARS-CoV-2 though the postpandemic period. Science 2020. [Google Scholar] [CrossRef]
- Fang, L.; Karakiulakis, G.; Roth, M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020, 8, e21. [Google Scholar] [CrossRef]
- Rothan, H.A.; Byrareddy, S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020, 109, 102433. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, M.J.; Haddadi, S.; Tahvildari, A.; Farsi, Y.; Arbabi, M.; Hasanzadeh, S.; Jamshidi, P.; Murthi, M.; Mirsaeidi, M. COVID-19 clinical characteristics, and sex-specific risk of mortality: Systematic review and meta-analysis. medRxiv 2020. [Google Scholar] [CrossRef]
- Bao, L.; Deng, W.; Gao, H.; Xiao, C.; Liu, J.; Xue, J.; Lv, Q.; Liu, J.; Yu, P.; Xu, Y.; et al. Reinfection could not occur in SARS-CoV-2 infected rhesus macaques. bioRxiv 2020. [Google Scholar] [CrossRef]
- Andre, F.E.; Booy, R.; Bock, H.L. Bulletin of the World Health Organization Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull. World Health Organ. 2008, 86, 140–146. [Google Scholar] [CrossRef]
- John, T.J.; Samuel, R. Herd immunity and herd effect: New insights and definitions. Eur. J. Epidemiol. 2000, 16, 601–606. [Google Scholar] [CrossRef]
- Anderson, R.M.; May, R.M. Vaccination and herd immunity to infectious diseases. Nature 1985, 318, 323–329. [Google Scholar] [CrossRef]
- Adegbola, R.; Secka, O.; Lahai, G.; Lloyd-Evans, N.; Njie, A.; Usen, S.; Oluwalana, C.; Obaro, S.; Weber, M.; Corrah, T.; et al. Elimination of Haemophilus influenzae type b (Hib) disease from the Gambia after introduction of a Hib conjugate vaccine: A prospective study. Lancet 2005, 366, 144–150. [Google Scholar] [CrossRef]
- Moulton, L.H.; Chung, S.; Croll, J.; Reid, R.; Weatherholtz, R.C.; Santosham, M. Estimation of the indirect effect of Haemophilus influenzae type b conjugate vaccine in an American Indian population. Int. J. Epidemiol. 2000, 29, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Schlenker, T.L.; Bain, C.; Baughman, A.L.; Hadler, S.C. Measles herd immunity: The association of attack rates with immunization rates in preschool children. JAMA 1992, 267, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, M.E. Importance of suppression and mitigation measures in managing COVID-19 outbreaks. medRxiv 2020. [Google Scholar] [CrossRef]
- Gautret, P.; Lagier, J.C.; Parola, P.; Hoang, V.T.; Meddeb, L.; Mailhe, M.; Doudier, B.; Courjon, J.; Giordanengo, V.; Vieira, V.E.; et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 2020, 105949. [Google Scholar] [CrossRef]
- Stebbing, J.; Phelan, A.; Griffin, I.; Tucker, C.; Oechsle, O.; Smith, D.; Richardson, P. COVID-19: Combining antiviral and anti-inflammatory treatments. Lancet Infect. Dis. 2020, 20, 400–402. [Google Scholar] [CrossRef]
- Dong, L.; Hu, S.; Gao, J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov. Ther. 2020, 14, 58–60. [Google Scholar] [CrossRef]
- Katul, G.G.; Mrad, A.; Bonetti, S.; Manoli, G.; Parolari, A.J. Global convergence of COVID-19 basic reproduction number and estimation from early-time SIR dynamics. medRxiv 2020. [Google Scholar] [CrossRef]
- Brodin, P. Why is COVID-19 so mild in children? Acta Paediatr. 2020, 109, 1082–1083. [Google Scholar] [CrossRef]
- Pang, J.; Wang, M.X.; Ang, I.Y.; Tan, S.H.; Lewis, R.F.; Chen, J.I.; Gutierrez, R.A.; Gwee, S.X.; Chua, P.E.; Yang, Q.; et al. Potential rapid diagnostics, vaccine and therapeutics for 2019 novel coronavirus (2019-nCoV): A systematic review. J. Clin. Med. 2020, 9, 623. [Google Scholar] [CrossRef]
- Graham, R.L.; Donaldson, E.F.; Baric, R.S. A decade after SARS: Strategies for controlling emerging coronaviruses. Nat. Rev. Microbiol. 2013, 11, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zeng, H.; Gu, J.; Li, H.; Zheng, L.; Zou, Q. Progress and Prospects on Vaccine Development against SARS-CoV-2. Vaccines 2020, 8, 153. [Google Scholar] [CrossRef] [PubMed]
- Benjamin-Chung, J.; Abedin, J.; Berger, D.; Clark, A.; Jimenez, V.; Konagaya, E.; Tran, D.; Arnold, B.F.; Hubbard, A.E.; Luby, S.P.; et al. Spillover effects on health outcomes in low-and middle-income countries: A systematic review. Int. J. Epidemol. 2017, 46, 1251–1276. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Qadri, F.; Kim, D.R.; Islam, T.; Im, J.; Ahmmed, F.; Chon, Y.; Islam Khan, A.; Zaman, K.; Marks, F.; et al. Unmasking herd protection by an oral cholera vaccine in a cluster-randomized trial. Int. J. Epidemol. 2019, 48, 1252–1261. [Google Scholar] [CrossRef]
- Callaway, E. Should scientists infect healthy people with the coronavirus to test vaccines? Nature 2020, 580, 17. [Google Scholar] [CrossRef]
- Plotkin, S.A.; Plotkin, S.A. Correlates of vaccine-induced immunity. Clin. Infect. Dis. 2008, 47, 401–409. [Google Scholar] [CrossRef]
- Callaway, E. The race for coronavirus vaccines: A graphical guide. Nature 2020, 580, 576. [Google Scholar] [CrossRef]
- Lang, P.O.; Aspinall, R. Immunosenescence and herd immunity: With an ever-increasing aging population do we need to rethink vaccine schedules? Expert Rev. Vaccines 2012, 11, 167–176. [Google Scholar] [CrossRef]
- Nicola, D.; Vito, M.; Linda, J.S.; Canio, B. COVID-19 from veterinary medicine and one health perspectives: What animal coronaviruses have taught us. Res. Vet. Sci. 2020, 131, 21–23. [Google Scholar]
- Del Giudice, G.; Goronzy, J.J.; Grubeck-Loebenstein, B.; Lambert, P.H.; Mrkvan, T.; Stoddard, J.J.; Doherty, T.M. Fighting against a protean enemy: Immunosenescence, vaccines, and healthy aging. NPJ Aging Mech. Dis. 2017, 4, 1. [Google Scholar] [CrossRef]
- Jin, Y.; Yang, H.; Ji, W.; Wu, W.; Chen, S.; Zhang, W.; Duan, G. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses 2020, 12, 372. [Google Scholar] [CrossRef] [PubMed]
- Robson, B. Computers and viral diseases. Preliminary bioinformatics studies on the design of a synthetic vaccine and a preventative peptidomimetic antagonist against the SARS-CoV-2 (2019-nCoV, COVID-19) coronavirus. Comput. Biol. Med. 2020, 26, 103670. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.F.; Quadeer, A.A.; McKay, M.R. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses 2020, 12, 254. [Google Scholar] [CrossRef] [PubMed]
- Colgrove, J. Vaccine refusal revisited the limits of public health persuasion and coercion. N. Eng. J. Med. 2016, 375, 1316–1317. [Google Scholar] [CrossRef] [PubMed]
- Dudley, M.Z.; Halsey, N.A.; Omer, S.B.; Orenstein, W.A.; TO’Leary, S.; Limaye, R.J.; Salmon, D.A. The state of vaccine safety science: Systematic reviews of the evidence. Lancet Infect. Dis. 2020, 20, e80–e89. [Google Scholar] [CrossRef]
- Metcalf, C.J.; Ferrari, M.; Graham, A.L.; Grenfell, B.T. Understanding herd immunity. Trends Immunol. 2015, 36, 753–755. [Google Scholar] [CrossRef]
- Betsch, C.; Böhm, R.; Korn, L.; Holtmann, C. On the benefits of explaining herd immunity in vaccine advocacy. Nature Hum. Behav. 2017, 1, 0056. [Google Scholar] [CrossRef]
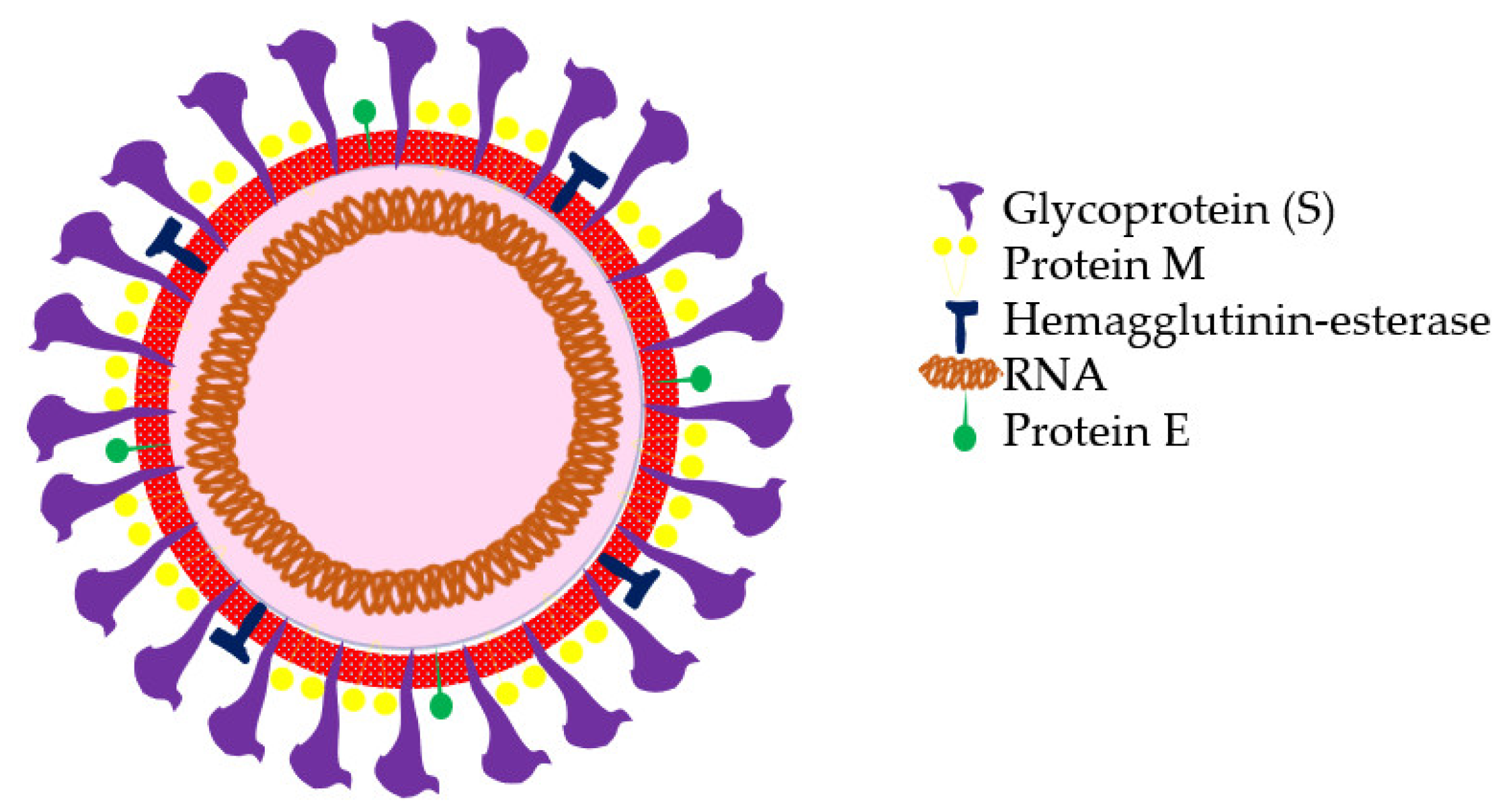
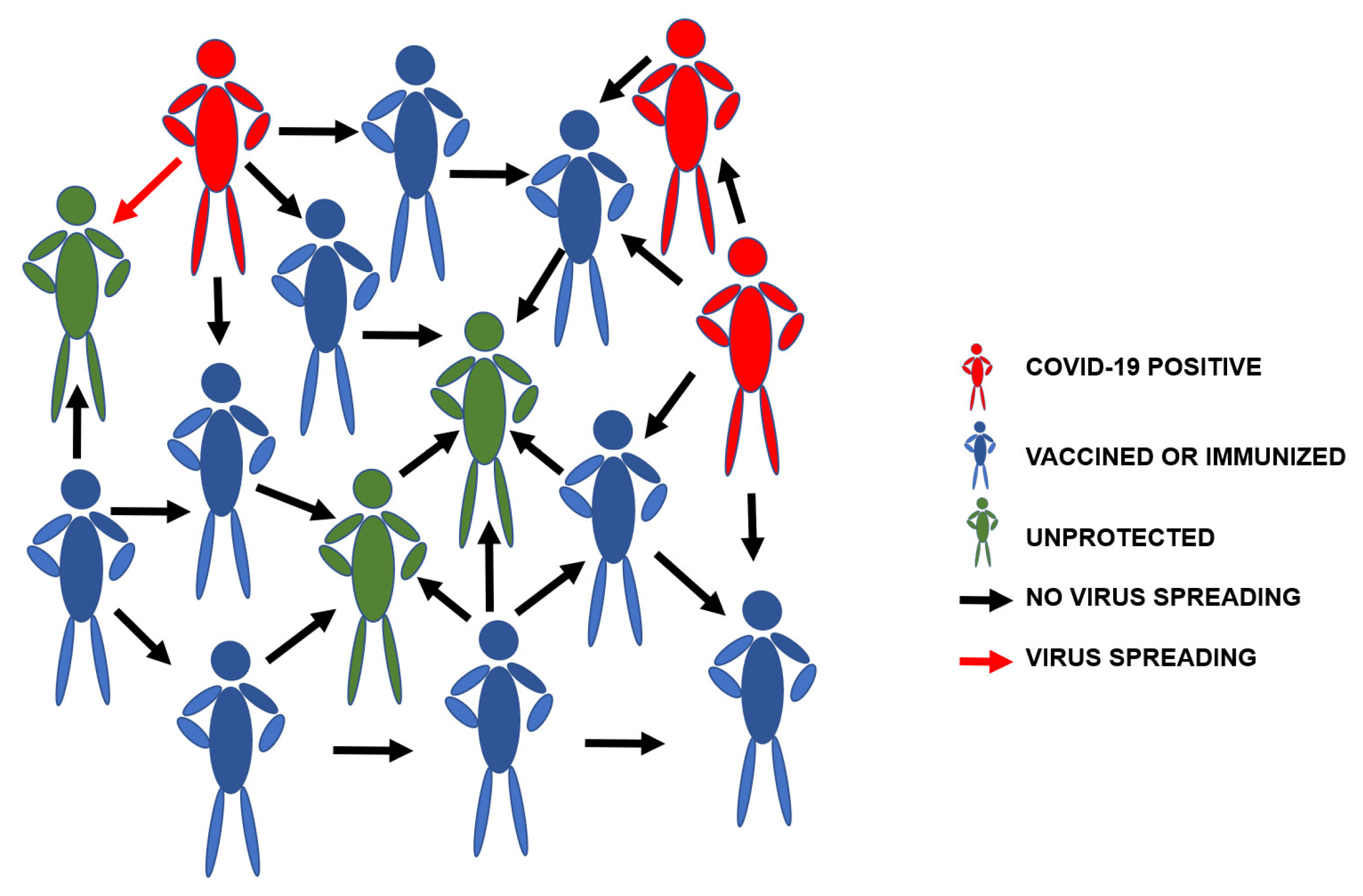
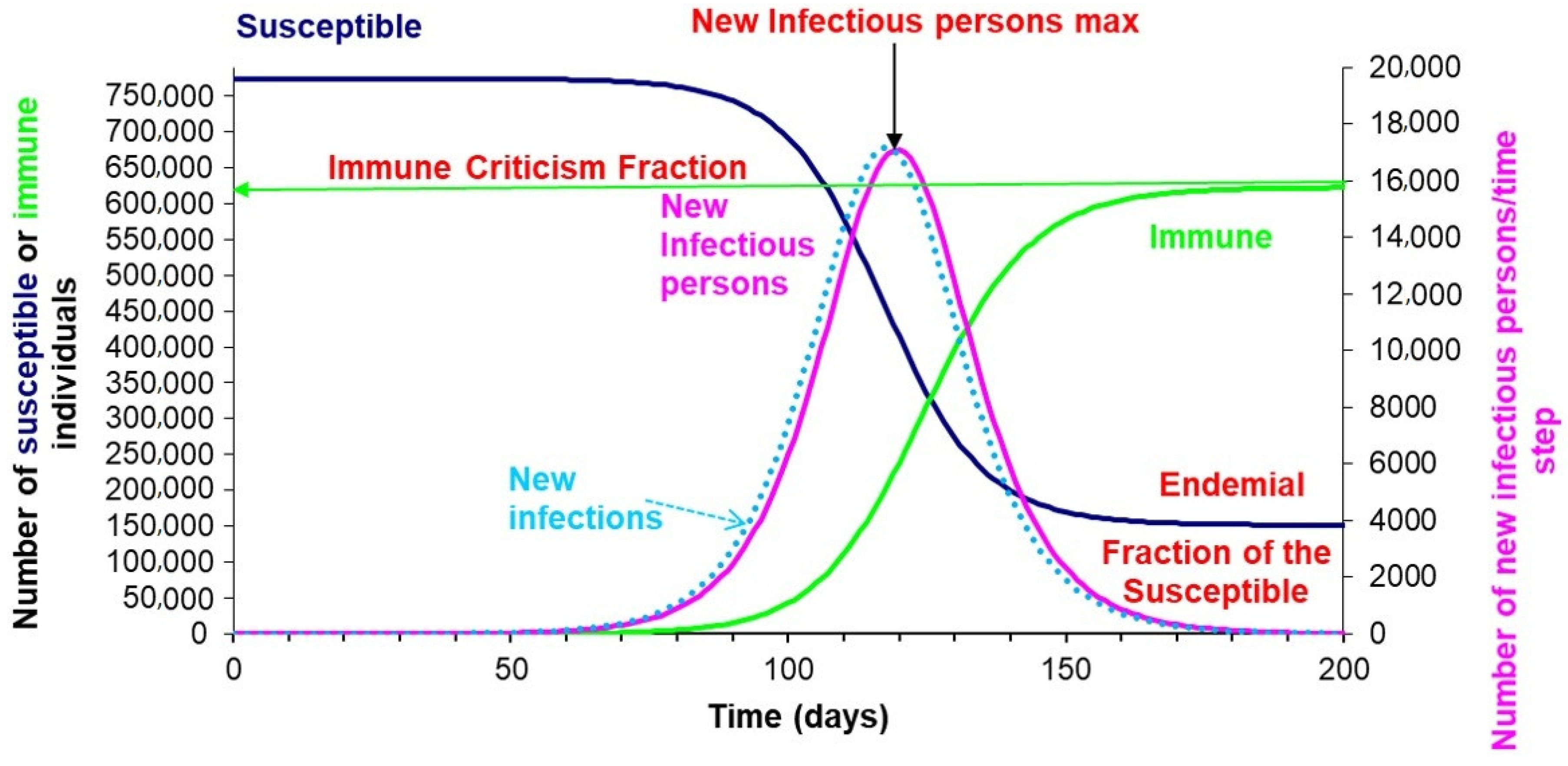

| Country | Rapid Tests Based on Antigens and Antibodies | RT-PCR Screening Tests | Citizen Information Measures | Geo-Position and Tracking Applications | Quarantine Measures | Use of Masks and Other Safety Measures |
|---|---|---|---|---|---|---|
| Canada | Yes. To date (12 April, 2020), Health Canada has approved this measure (shipping only in Canada, not worldwide). Several biomedical private entities received approval for the production of rapid kits. | Government has a guide for public and private institutions. Performed with a rapid test. | Large governmental web page with answer and questions (FAQ). COVID-CANADA App provides a central resource for accessing personalized, trusted, and evidence-based information. | Authorities are still discussing this. No official information available. | 16th of March, borders were quarantined Since the 7th of April, the population has been under lockdown | Suggested mask implementation. Thermal scanning. Plexiglass barriers to ensure social distancing. GPS–Bluetooth technology to determine whether citizens have had contact with a COVID-19 positive subject. |
| China | Yes. Rapid diagnoses and rapid/point-of-care serological tests, especially in Wuhan province, the focus of the onset of COVID-19. | The results of PCR tests are returned the same day in mainland China and performed on even suspected patients. | No information was obtained. | Yes. Using 4g and 5g connections. | Border health quarantine; infectious diseases on 20 January 2020 in Wuhan and other key areas of Hubei. | Strict entry control in buildings (a green health QR code used to grant access) connected to a governmental database that is informed on the health status and movements of the citizens. Mandatory masks. Thermal scanning. Strict disinfection protocols that are shared on social media to engender feelings of security and comfort among the population. |
| Colombia | Yes. With limitations in the ability to obtain rapid detection kits. | Yes. With the participation of University laboratories and state epidemiological surveillance. There are problems with the analysis of samples and the reporting of sample results. | Yes. Limited due to the low diffusion of the government. The use of a WHO WhatsApp bot that delivers real-time information to phones. | No. | Yes. From 24 March till 27 April. | Compulsory use of masks on public transport systems and in public areas, such as stores, outdoor marketplaces, and banks. |
| European Union | Yes. Antigen and antibody-based rapid tests are widely used in the countries of the European Union and the United Kingdom. Their application is recommended to support laboratories that perform RT-PCR tests. The use of commercial tests with a "Conformité Européenne" (CE) seal is recommended. Tests based on direct SARS-CoV-2 antigen detection and indirect antibody detection tests are used. | RT-PCR is the current test methodology applied in EU member states. The ECDC recommends nucleic acid amplification-based tests, such as RT-PCR, as diagnostic tests. | Yes. The use of the WHO WhatsApp bot delivers real-time information to phones. | Europe’s telecom companies are sharing location data with health authorities in Italy, Germany, and Austria to check whether people are remaining at home. The data are aggregated and anonymous, mapping concentrations rather than individuals to respect Europe’s privacy laws. | The authorities of the European Union recommend home isolation for people who have mild symptomatology and quarantine for people who have been potentially exposed. | In the European continent, Bulgaria, Georgia, Luxembourg, Poland, the Czech Republic, and Ukraine have mandatory mask-wearing. Masks are only necessary in enclosed public places, public transport, and shops in Austria, Lithuania, Romania, and Slovenia. From 11 May in France, masks will be mandatory on public transport and recommended for shopping and small areas. In Germany, Spain, and Italy, donning a face mask is also compulsory, including public transport and shops. |
| Russia | No information available. | Yes, since 27 March, AmpliTest SARS-Cov-2 is used to test for the virus in just 2 h and 30 min. | No information available. | Yes. Only patients who have tested positive and have been hospitalized. | 18 March: restrictions on the entry of foreigners. | No information available. |
| Japan | Yes, but rapid tests are applied to a lesser extent than in other countries. Confirmatory tests of PCR RT are preferred. | 86,800 PCR tests have been conducted through to 17 April. Private laboratories can be authorized to perform PCR tests. | Yes. The use of the WHO WhatsApp bot delivers real-time information to phones. | No data available. | Maintained social and economic functions. No cities blockaded as in other countries; only partial quarantine. | Among other countries such as Hong Kong, South Korea, Thailand, and Taiwan with similar cultures, the broad assumption is that anyone could carry the virus; thus, masks are encouraged to be worn at all times and in all places. |
| United Kingdom | Yes. Since 15 March, rapid tests are available for use in community pharmacies or at home. Two million tests have been bought from China as of the 1st April. | National Health Service (NHS) is providing guidance and standard operational procedures for public and private institutions. | Yes. The use of the WHO WhatsApp bot delivers real-time information to phones. | Yes. A governmental App is used to track the progression of medical conditions by citizens self-reporting their symptoms No information on geo-tracking. | Since 23 March. | The use of a mask is not mandatory. Instead, the government urges the public not to wear them in order to ensure sufficient supplies and personal protective equipment (PPE) for healthcare workers. |
| United States of America | Yes. On 2 April, the FDA approved antibody tests. Since then, they have been widely applied among the population. Commercially manufactured serologic tests that check for SARS-CoV-2 antibodies in individuals are becoming increasingly available for use through healthcare providers. | Despite its large population and density, it ranks sixth in performing the most RT-PCR tests, behind South Korea, Canada, Germany, and Italy. | Live information on the government’s web page. The Federal Communications Commission (FCC) supports and encourages citizen information. | $500 million for the Centre for Disease Control (CDC) to launch a new surveillance and data-collection system to monitor the spread of COVID-19. | Depending on the State, the beginning of the quarantine may fluctuate from 19March to 3 April. | Does not specifically advocate the use of surgical masks but does advise the use of “simple cloth face coverings” to slow the spread of the virus and prevent asymptomatic people from transmitting it to others. Mandatory use of masks depends on the state. |
| Venezuela | Yes. With limitations in acquiring rapid detection kits. | Yes; limited due to the excessive centralization of the RT-PCR sample processing in a single government laboratory. There is a mobile laboratory established on the border with Colombia. There is no periodical publication of epidemiological data. | Yes. Highly limited due to low diffusion and information control of government. The use of the WHO WhatsApp bot delivers real-time information to phones. | No | First country in Latin America to establish a lockdown (from 24 March to 27 April). Due to shortages of fuel and food, it was very difficult for the population to respect this order. | Mandatory use of masks. Random temperature check among citizens. China’s measures are being slowly replicated, according to Nicolas Maduro. (e.g., the use of “Carnet Patria”, which is similar to Quick Response (QR) codes but has a digital wallet where the government distributes economic bonds). |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clemente-Suárez, V.J.; Hormeño-Holgado, A.; Jiménez, M.; Benitez-Agudelo, J.C.; Navarro-Jiménez, E.; Perez-Palencia, N.; Maestre-Serrano, R.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. Dynamics of Population Immunity Due to the Herd Effect in the COVID-19 Pandemic. Vaccines 2020, 8, 236. https://doi.org/10.3390/vaccines8020236
Clemente-Suárez VJ, Hormeño-Holgado A, Jiménez M, Benitez-Agudelo JC, Navarro-Jiménez E, Perez-Palencia N, Maestre-Serrano R, Laborde-Cárdenas CC, Tornero-Aguilera JF. Dynamics of Population Immunity Due to the Herd Effect in the COVID-19 Pandemic. Vaccines. 2020; 8(2):236. https://doi.org/10.3390/vaccines8020236
Chicago/Turabian StyleClemente-Suárez, Vicente Javier, Alberto Hormeño-Holgado, Manuel Jiménez, Juan Camilo Benitez-Agudelo, Eduardo Navarro-Jiménez, Natalia Perez-Palencia, Ronald Maestre-Serrano, Carmen Cecilia Laborde-Cárdenas, and Jose Francisco Tornero-Aguilera. 2020. "Dynamics of Population Immunity Due to the Herd Effect in the COVID-19 Pandemic" Vaccines 8, no. 2: 236. https://doi.org/10.3390/vaccines8020236
APA StyleClemente-Suárez, V. J., Hormeño-Holgado, A., Jiménez, M., Benitez-Agudelo, J. C., Navarro-Jiménez, E., Perez-Palencia, N., Maestre-Serrano, R., Laborde-Cárdenas, C. C., & Tornero-Aguilera, J. F. (2020). Dynamics of Population Immunity Due to the Herd Effect in the COVID-19 Pandemic. Vaccines, 8(2), 236. https://doi.org/10.3390/vaccines8020236









