N-Terminal Fatty Acids of NEFMUT Are Required for the CD8+ T-Cell Immunogenicity of In Vivo Engineered Extracellular Vesicles
Abstract
1. Introduction
2. Materials and Methods
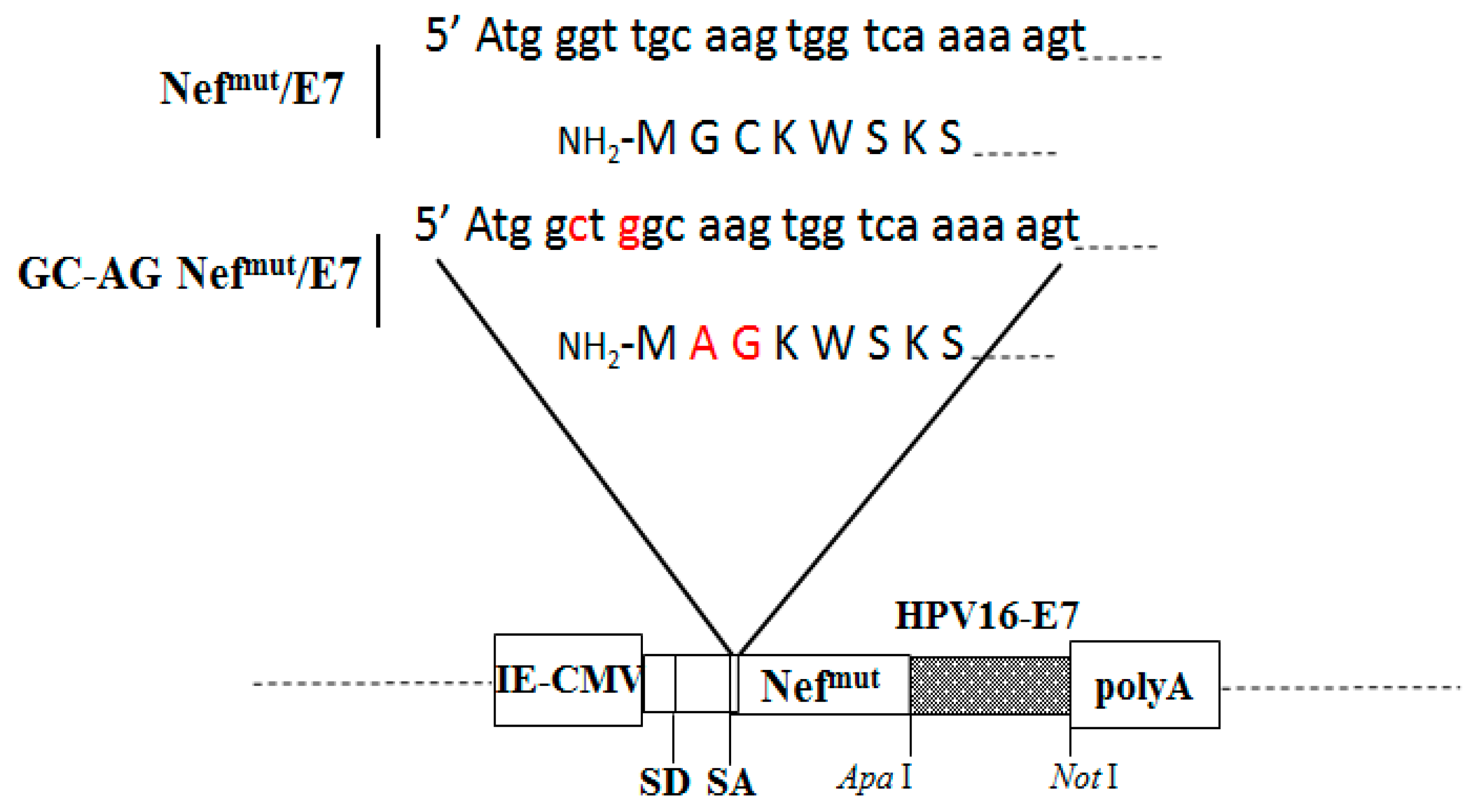
2.1. DNA Constructs
2.2. Cell Cultures and Transfection
2.3. Exosome Purification
2.4. Confocal Microscope Analysis
2.5. Western Blot
2.6. Mice Immunization and IFN-γ ELISpot Assay
2.7. Statistical Analysis
3. Results
3.1. The Lack of Fatty Acids at the Nefmut/E7 N-Terminus Affects the Intracellular Localization but Not the Protein Stability
3.2. N-Terminal Fatty Acids Are Required for Exosome Incorporation of Nefmut/E7
3.3. Nefmut-Mediated EV Engineering Is Necessary for the Induction of CD8+ T-Cell Immunity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pennock, N.D.; Kedl, J.D.; Kedl, R.M. T Cell Vaccinology: Beyond the Reflection of Infectious Responses. Trends Immunol. 2016, 37, 170–180. [Google Scholar] [CrossRef]
- Beura, L.K.; Jameson, S.C.; Masopust, D. Is a Human CD8 T-Cell Vaccine Possible, and If So, What Would It Take? CD8 T-Cell Vaccines: To B or Not to B? Cold Spring Harb. Perspect. Biol. 2018, 10. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Lattanzi, L.; Federico, M. A Strategy of Antigen Incorporation into Exosomes: Comparing Cross-Presentation Levels of Antigens Delivered by Engineered Exosomes and by Lentiviral Virus-like Particles. Vaccine 2012, 30, 7229–7237. [Google Scholar] [CrossRef]
- Anticoli, S.; Manfredi, F.; Chiozzini, C.; Arenaccio, C.; Olivetta, E.; Ferrantelli, F.; Capocefalo, A.; Falcone, E.; Ruggieri, A.; Federico, M. An Exosome-Based Vaccine Platform Imparts Cytotoxic T Lymphocyte Immunity Against Viral Antigens. Biotechnol. J. 2018, 13, e1700443. [Google Scholar] [CrossRef]
- Di Bonito, P.; Chiozzini, C.; Arenaccio, C.; Anticoli, S.; Manfredi, F.; Olivetta, E.; Ferrantelli, F.; Falcone, E.; Ruggieri, A.; Federico, M. Antitumor HPV E7-Specific CTL Activity Elicited by in Vivo Engineered Exosomes Produced through DNA Inoculation. Int. J. Nanomed. 2017, 12, 4579–4591. [Google Scholar] [CrossRef]
- Anticoli, S.; Aricò, E.; Arenaccio, C.; Manfredi, F.; Chiozzini, C.; Olivetta, E.; Ferrantelli, F.; Lattanzi, L.; D’Urso, M.T.; Proietti, E.; et al. Engineered Exosomes Emerging from Muscle Cells Break Immune Tolerance to HER2 in Transgenic Mice and Induce Antigen-Specific CTLs upon Challenge by Human Dendritic Cells. J. Mol. Med. 2018, 96, 211–221. [Google Scholar] [CrossRef]
- Di Bonito, P.; Ridolfi, B.; Columba-Cabezas, S.; Giovannelli, A.; Chiozzini, C.; Manfredi, F.; Anticoli, S.; Arenaccio, C.; Federico, M. HPV-E7 Delivered by Engineered Exosomes Elicits a Protective CD8+ T Cell-Mediated Immune Response. Viruses 2015, 7, 1079–1099. [Google Scholar] [CrossRef]
- Di Bonito, P.; Grasso, F.; Mochi, S.; Petrone, L.; Fanales-Belasio, E.; Mei, A.; Cesolini, A.; Laconi, G.; Conrad, H.; Bernhard, H.; et al. Anti-Tumor CD8+ T Cell Immunity Elicited by HIV-1-Based Virus-like Particles Incorporating HPV-16 E7 Protein. Virology 2009, 395, 45–55. [Google Scholar] [CrossRef]
- Arenaccio, C.; Chiozzini, C.; Columba-Cabezas, S.; Manfredi, F.; Federico, M. Cell Activation and HIV-1 Replication in Unstimulated CD4+ T Lymphocytes Ingesting Exosomes from Cells Expressing Defective HIV-1. Retrovirology 2014, 11, 46. [Google Scholar] [CrossRef]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and Characterization of Exosomes from Cell Culture Supernatants and Biological Fluids. Curr. Protoc. Cell Biol. 2006. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Heeg, K.; Wagner, H.; Lipford, G.B. Identification of H-2Kb Binding and Immunogenic Peptides from Human Papilloma Virus Tumour Antigens E6 and E7. Scand. J. Immunol. 1995, 42, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.; Holst, P.J.; Bukh, J.; Thomsen, A.R.; Christensen, J.P. Enhanced and Sustained CD8+ T Cell Responses with an Adenoviral Vector-Based Hepatitis C Virus Vaccine Encoding NS3 Linked to the MHC Class II Chaperone Protein Invariant Chain. J. Immunol. 2011, 186, 2355–2364. [Google Scholar] [CrossRef] [PubMed]
- Chowers, M.Y.; Spina, C.A.; Kwoh, T.J.; Fitch, N.J.; Richman, D.D.; Guatelli, J.C. Optimal Infectivity in Vitro of Human Immunodeficiency Virus Type 1 Requires an Intact Nef Gene. J. Virol. 1994, 68, 2906–2914. [Google Scholar] [CrossRef] [PubMed]
- Ferrantelli, F.; Arenaccio, C.; Manfredi, F.; Olivetta, E.; Chiozzini, C.; Leone, P.; Percario, Z.; Ascione, A.; Flego, M.; Di Bonito, P.; et al. The Intracellular Delivery of Anti-HPV16 E7 ScFvs through Engineered Extracellular Vesicles Inhibits the Proliferation of HPV-Infected Cells. Int. J. Nanomed. 2019, 14, 8755–8768. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, I.; Nickerson, J.; Penman, S.; Stanley, M. Human Papillomavirus 16 E7 Protein Is Associated with the Nuclear Matrix. Proc. Natl. Acad. Sci. USA. 1991, 88, 11217–11221. [Google Scholar] [CrossRef]
- Fackler, O.T.; d’Aloja, P.; Baur, A.S.; Federico, M.; Peterlin, B.M. Nef from Human Immunodeficiency Virus Type 1(F12) Inhibits Viral Production and Infectivity. J. Virol. 2001, 75, 6601–6608. [Google Scholar] [CrossRef]
- Schalk, J.A.C.; Mooi, F.R.; Berbers, G.A.M.; van Aerts, L.A.G.J.M.; Ovelgönne, H.; Kimman, T.G. Preclinical and Clinical Safety Studies on DNA Vaccines. Hum. Vaccines 2006, 2, 45–53. [Google Scholar] [CrossRef]
- Dupuis, M.; Denis-Mize, K.; Woo, C.; Goldbeck, C.; Selby, M.J.; Chen, M.; Otten, G.R.; Ulmer, J.B.; Donnelly, J.J.; Ott, G.; et al. Distribution of DNA Vaccines Determines Their Immunogenicity after Intramuscular Injection in Mice. J. Immunol. 2000, 165, 2850–2858. [Google Scholar] [CrossRef]
- Hobernik, D.; Bros, M. DNA Vaccines—How Far from Clinical Use? Int. J. Mol. Sci. 2018, 19, 3605. [Google Scholar] [CrossRef]
- Lopes, A.; Vandermeulen, G.; Préat, V. Cancer DNA Vaccines: Current Preclinical and Clinical Developments and Future Perspectives. J. Exp. Clin. Cancer Res. 2019, 38, 146. [Google Scholar] [CrossRef] [PubMed]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiozzini, C.; Manfredi, F.; Arenaccio, C.; Ferrantelli, F.; Leone, P.; Federico, M. N-Terminal Fatty Acids of NEFMUT Are Required for the CD8+ T-Cell Immunogenicity of In Vivo Engineered Extracellular Vesicles. Vaccines 2020, 8, 243. https://doi.org/10.3390/vaccines8020243
Chiozzini C, Manfredi F, Arenaccio C, Ferrantelli F, Leone P, Federico M. N-Terminal Fatty Acids of NEFMUT Are Required for the CD8+ T-Cell Immunogenicity of In Vivo Engineered Extracellular Vesicles. Vaccines. 2020; 8(2):243. https://doi.org/10.3390/vaccines8020243
Chicago/Turabian StyleChiozzini, Chiara, Francesco Manfredi, Claudia Arenaccio, Flavia Ferrantelli, Patrizia Leone, and Maurizio Federico. 2020. "N-Terminal Fatty Acids of NEFMUT Are Required for the CD8+ T-Cell Immunogenicity of In Vivo Engineered Extracellular Vesicles" Vaccines 8, no. 2: 243. https://doi.org/10.3390/vaccines8020243
APA StyleChiozzini, C., Manfredi, F., Arenaccio, C., Ferrantelli, F., Leone, P., & Federico, M. (2020). N-Terminal Fatty Acids of NEFMUT Are Required for the CD8+ T-Cell Immunogenicity of In Vivo Engineered Extracellular Vesicles. Vaccines, 8(2), 243. https://doi.org/10.3390/vaccines8020243






