Influenza Vaccination Experiences of Pregnant Women as a Predictor of the Intention to Become Vaccinated in Future Pregnancies in Spain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Measurements
2.3. Statistical Analysis
2.4. Ethics Approval
3. Results
3.1. Sociodemographic and Obstetric Characteristics of the Interviewees
3.2. Vaccination Self-Declaration and Concordance with the Registry (NVR)
3.3. Beliefs and Opinions about Vaccination in General
3.4. Intention towards Future Vaccination
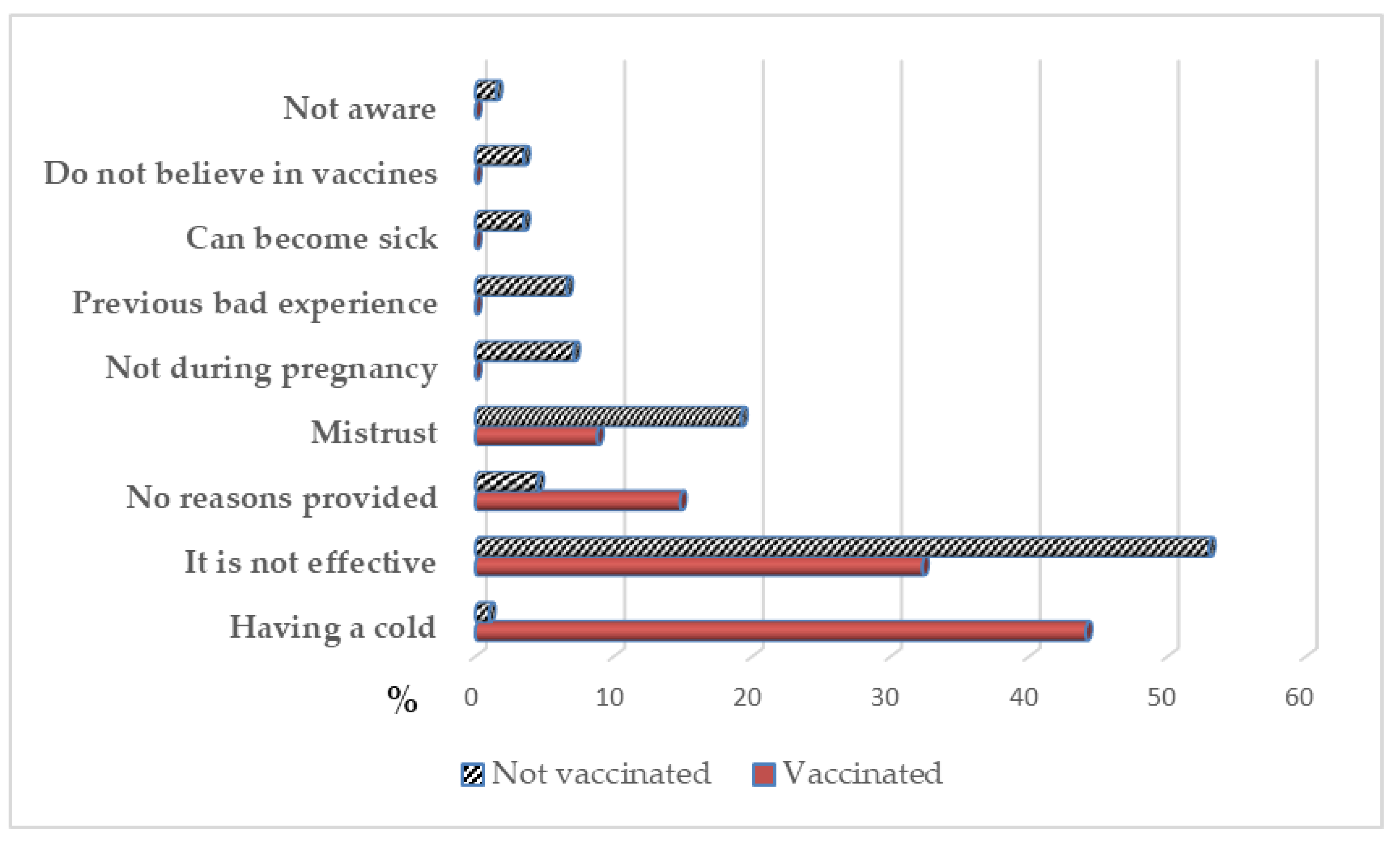
3.5. Secondary Effects after the Vaccination
3.6. Personal Experience Related with the Flu
3.7. Multivariate Analysis with the Future Intent of Non-Vaccination as the Response Variable
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maertens, K.; Orije, M.R.P.; Van Damme, P.; Leuridan, E. Vaccination during pregnancy: Current and possible future recommendations. Eur. J. Pediatr. 2020, 179, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Kachikis, A.; Eckert, L.O.; Englund, J. Who’s the Target: Mother or Baby? Viral Immunol. 2018, 31, 184–194. [Google Scholar] [CrossRef]
- Myers, K.L. Predictors of maternal vaccination in the United States: An integrative review of the literature. Vaccine 2016, 34, 3942–3949. [Google Scholar] [CrossRef] [PubMed]
- Offeddu, V.; Tam, C.C.; Yong, T.T.; Tan, L.K.; Thoon, K.C.; Lee, N.; Tan, T.C.; Yeo, G.; Yung, C.F. Coverage and determinants of influenza vaccine among pregnant women: A cross-sectional study. BMC Public Health. 2019, 19, 890. [Google Scholar] [CrossRef]
- Lassi, Z.S.; Dean, S.V.; Mallick, D.; Bhutta, Z.A. Preconception care: Delivery strategies and packages for care. Reprod. Health. 2014, 11 (Suppl. 3), S7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shorten, A. Midwifery and women’s health. Evid. Based Nurs. 2017, 20, 70–73. [Google Scholar] [CrossRef]
- Gagneur, A.; Lemaître, T.; Gosselin, V.; Farrands, A.; Carrier, N.; Petit, G.; Valiquette, L.; De Wals, P. A postpartum vaccination promotion intervention using motivational interviewing techniques improves short-term vaccine coverage: PromoVac study. BMC Public Health 2018, 18, 811. [Google Scholar] [CrossRef]
- Finale, E.; Leonardi, G.; Auletta, G.; Amadori, R.; Saglietti, C.; Pagani, L.; Guala, A. MMR vaccine in the postpartum does not expose seronegative women to untoward effects. Ann. Ist. Super Sanitain. 2017, 53, 152–156. [Google Scholar] [CrossRef]
- Marchant, A.; Sadarangani, M.; Garand, M.; Dauby, N.; Verhasselt, V.; Pereira, L.; Bjornson, G.; Jones, C.E.; Halperin, S.A.; Edwards, K.M.; et al. Maternal immunisation: Collaborating with mother nature. Lancet Infect. Dis. 2017, 17, e197–e208. [Google Scholar] [CrossRef]
- Chu, H.Y.; Englund, J.A. Maternal immunization. Clin. Infect. Dis. 2014, 59, 560–568. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Blanco, N.; Tuells, J. Knowledge and Attitudes about the Flu Vaccine among Pregnant Women in the Valencian Community (Spain). Medicina (Kaunas) 2019, 55, 467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, L.K.; Donelle, J.; Dodds, L.; Hawken, S.; Wilson, K.; Benchimol, E.I.; Chakraborty, P.; Guttmann, A.; Kwong, J.C.; MacDonald; et al. Health outcomes of young children born to mothers who received 2009 pandemic H1N1 influenza vaccination during pregnancy: Retrospective cohort study. BMJ 2019, 366, l4151. [Google Scholar] [CrossRef] [Green Version]
- WHO. Vaccines against influenza WHO position paper—November 2012. Wkly. Epidemiol. Rec. 2012, 87, 461–476. [Google Scholar]
- Buchy, P.; Badur, S.; Kassianos, G.; Preiss, S.; Tam, J.S. Vaccinating pregnant women against influenza needs to be a priority for all countries: An expert commentary. Int. J. Infect. Dis. 2019, 92, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, H.; McMillan, M.; Andrews, R.M.; Macartney, K.; Edwards, K. Vaccines in pregnancy: The dual benefit for pregnant women and infants. Hum. Vaccin Immunother. 2016, 12, 848–856. [Google Scholar] [CrossRef] [Green Version]
- CDC. Flu Vaccination Coverage, United States, 2018–2019 Influenza Season. 2019. Available online: https://www.cdc.gov/flu/fluvaxview/coverage-1819estimates.htm (accessed on 5 April 2020).
- Ministerio de Sanidad, Consumo y Bienestar Social—Profesionales—Vacunas Coberturas de Vacunación. Available online: https://www.mscbs.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/coberturas.htm (accessed on 5 January 2020).
- Preaud, E.; Durand, L.; Macabeo, B.; Farkas, N.; Sloesen, B.; Palache, A.; Shupo, F.; Samson, S.I.; Vaccines Europe influenza working group. Annual public health and economic benefits of seasonal influenza vaccination: A European estimate. BMC Public Health 2014, 14, 813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Blanco, N.; Tuells, J.; Vila-Candel, R.; Nolasco, A. Adherence and Concordance of Influenza and Pertussis Vaccination Coverage in Pregnant Women in Spain. Int. J. Environ. Res. Public Health 2019, 16, 543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larson, H.J.; Jarrett, C.; Eckersberger, E.; Smith, D.M.D.; Paterson, P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: A systematic review of published literature, 2007–2012. Vaccine 2014, 32, 2150–2159. [Google Scholar] [CrossRef]
- Lutz, C.S.; Carr, W.; Cohn, A.; Rodriguez, L. Understanding barriers and predictors of maternal immunization: Identifying gaps through an exploratory literature review. Vaccine 2018, 36, 7445–7455. [Google Scholar] [CrossRef] [PubMed]
- Danchin, M.H.; Costa-Pinto, J.; Attwell, K.; Willaby, H.; Wiley, K.; Hoq, M.; Leask, J.; Perrett, K.P.; O’Keefe, J.; Giles, M.L.; et al. Vaccine decision-making begins in pregnancy: Correlation between vaccine concerns, intentions and maternal vaccination with subsequent childhood vaccine uptake. Vaccine 2018, 36, 6473–6479. [Google Scholar] [CrossRef]
- Ludolph, R.; Nobile, M.; Hartung, U.; Castaldi, S.; Schulz, P.J. H1N1 Influenza Pandemic in Italy Revisited: Has the Willingness to Get Vaccinated Suffered in the Long Run? J. Public Health Res. 2015, 4, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groom, H.C.; Henninger, M.L.; Smith, N.; Koppolu, P.; Cheetham, T.C.; Glanz, J.M.; Hambidge, S.J.; Jackson, L.A.; Kharbanda, E.O.; Klein, N.P.; et al. Influenza Vaccination During Pregnancy: Influenza Seasons 2002-2012, Vaccine Safety Datalink. Am. J. Prev. Med. 2016, 50, 480–488. [Google Scholar] [CrossRef] [PubMed]
- van Lier, A.; Steens, A.; Ferreira, J.A.; van der Maas, N.A.T.; de Melker, H.E. Acceptance of vaccination during pregnancy: Experience with 2009 influenza A (H1N1) in the Netherlands. Vaccine 2012, 30, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- Regan, A.K.; Blyth, C.C.; Mak, D.B.; Richmond, P.C.; Effler, P.V. Using SMS to monitor adverse events following trivalent influenza vaccination in pregnant women. Aust. N. Z. J. Obstet. Gynaecol. 2014, 54, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Meharry, P.M.; Colson, E.R.; Grizas, A.P.; Stiller, R.; Vázquez, M. Reasons why women accept or reject the trivalent inactivated influenza vaccine (TIV) during pregnancy. Matern. Child. Health J. 2013, 17, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Baum, S.; Hitschold, T.; Becker, A.; Smola, S.; Solomayer, E.; Rody, A.; Rissland, J. Implementation of the Recommendation to Vaccinate Pregnant Women against Seasonal Influenza–Vaccination Rates and Acceptance. Geburtshilfe Frauenheilkd. 2017, 77, 340–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conselleria de Sanitat Universal i Salut Pública. Memòria. 2015. Available online: http://www.san.gva.es/web/comunicacion/memoria-2015 (accessed on 15 January 2020).
- Freund, R.; Le Ray, C.; Charlier, C.; Avenell, C.; Truster, V.; Tréluyer, J.-M.; Skalli, D.; Ville, Y.; Goffinet, F.; Launay, O.; et al. Determinants of non-vaccination against pandemic 2009 H1N1 influenza in pregnant women: A prospective cohort study. PLoS ONE 2011, 6, e20900. [Google Scholar] [CrossRef]
- Hill, L.; Burrell, B.; Walls, T. Factors influencing women’s decisions about having the pertussis-containing vaccine during pregnancy. J. Prim. Health Care 2018, 10, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Böhm, S.; Röbl-Mathieu, M.; Scheele, B.; Wojcinski, M.; Wichmann, O.; Hellenbrand, W. Influenza and pertussis vaccination during pregnancy—Attitudes, practices and barriers in gynaecological practices in Germany. BMC Health Serv. Res. 2019, 19, 616. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, H.; Clarke, M.; Koehler, A.; Watson, M.; Marshall, H. Factors associated with uptake of influenza and pertussis vaccines among pregnant women in South Australia. PLoS ONE 2018, 13, e0197867. [Google Scholar] [CrossRef]
- Lundgren, I.; Berg, M. Central concepts in the midwife-woman relationship. Scand. J. Caring Sci. 2007, 21, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, A.; Nagata, S.; Saitoh, A.; Tsukahara, Y.; Vaida, F.; Sonobe, T.; Kamiya, H.; Naruse, T.; Murashima, S. Perinatal immunization education improves immunization rates and knowledge: A randomized controlled trial. Prev. Med. 2013, 56, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Paterson, P.; Larson, H.J. Strategies to improve maternal vaccination acceptance. BMC Public Health 2019, 19, 342. [Google Scholar] [CrossRef]
- Ishola, D.A.; Permalloo, N.; Cordery, R.J.; Anderson, S.R. Midwives’ influenza vaccine uptake and their views on vaccination of pregnant women. J. Public Health (Oxf) 2013, 35, 570–577. [Google Scholar] [CrossRef] [Green Version]
- Ellingson, M.K.; Dudley, M.Z.; Limaye, R.J.; Salmon, D.A.; O’Leary, S.T.; Omer, S.B. Enhancing uptake of influenza maternal vaccine. Expert Rev. Vaccines 2019, 18, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Elwyn, G.; Charles, C. Shared decision-making: The principles and the competencies. In Evidence-Based Patient Choice: Inevitable or Impossible? Edwards, A.G., Elwyn, G., Eds.; Oxford University Press: Oxford, UK, 2001; pp. 3–16. [Google Scholar]
- Alessandrini, V.; Anselem, O.; Girault, A.; Mandelbrot, L.; Luton, D.; Launay, O.; Goffinet, F. Does the availability of influenza vaccine at prenatal care visits and of immediate vaccination improve vaccination coverage of pregnant women? PLoS ONE 2019, 14, e0220705. [Google Scholar] [CrossRef] [Green Version]
- Leask, J.; Quinn, H.E.; Macartney, K.; Trent, M.; Massey, P.; Carr, C.; Turahui, J. Immunisation attitudes, knowledge and practices of health professionals in regional NSW. Aust. N. Z. J. Public Health 2008, 32, 224–229. [Google Scholar] [CrossRef]
- Yuen, C.Y.S.; Tarrant, M. Determinants of uptake of influenza vaccination among pregnant women—A systematic review. Vaccine 2014, 32, 4602–4613. [Google Scholar] [CrossRef]
- Wu, A.C.; Wisler-Sher, D.J.; Griswold, K.; Colson, E.; Shapiro, E.D.; Holmboe, E.S.; Benin, A.L. Postpartum mothers’ attitudes, knowledge, and trust regarding vaccination. Matern. Child. Health J. 2008, 12, 766–773. [Google Scholar] [CrossRef]
- Cuningham, W.; Geard, N.; Fielding, J.E.; Braat, S.; Madhi, S.A.; Nunes, M.C.; Christian, L.M.; Lin, S.Y.; Lee, C.N.; Yamaguchi, K.; et al. Optimal timing of influenza vaccine during pregnancy: A systematic review and meta-analysis. Influenza Other Respir. Viruses 2019, 13, 438–452. [Google Scholar] [CrossRef] [Green Version]
- Eberhardt, C.S.; Blanchard-Rohner, G.; Lemaître, B.; Boukrid, M.; Combescure, C.; Othenin-Girard, V.; Chilin, A.; Petre, J.; de Tejada, B.M.; Siegrist, C.A. Maternal Immunization Earlier in Pregnancy Maximizes Antibody Transfer and Expected Infant Seropositivity Against Pertussis. Clin. Infect. Dis. 2016, 62, 829–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Influenza | Influenza | Total | p * | |||||
|---|---|---|---|---|---|---|---|---|
| Vaccinated | Unvaccinated | |||||||
| n | % | n | % | n | % | |||
| Department | TV | 189 | 70.8 | 78 | 29.2 | 267 | 39.1 | 0.001 |
| EC | 232 | 55.8 | 184 | 44.2 | 416 | 60.9 | ||
| Country of Origin | Spain | 322 | 60.8 | 208 | 39.2 | 530 | 77.6 | 0.397 |
| Not Spain | 99 | 64.7 | 54 | 35.3 | 153 | 22.4 | ||
| Previous pregnancies | 1 | 11 | 39.3 | 17 | 60.7 | 28 | 40.1 | 0.009 |
| 2 | 406 | 62.9 | 239 | 37.1 | 645 | 94.4 | ||
| ≥3 | 4 | 40.0 | 6 | 60.0 | 10 | 1.5 | ||
| Abortion | No | 415 | 61.9 | 255 | 38.1 | 670 | 98.1 | 0.100 |
| Yes | 6 | 46.2 | 7 | 53.8 | 13 | 1.9 | ||
| Parity | 0 | 14 | 42.4 | 19 | 57.6 | 33 | 4.8 | 0.160 |
| ≥1 | 407 | 62.6 | 243 | 37.4 | 650 | 95.2 | ||
| Trimester of the pregnancy | 1° | 118 | 61.8 | 73 | 38.2 | 191 | 28.0 | 0.220 |
| 2° | 159 | 65.4 | 84 | 34.6 | 243 | 35.6 | ||
| 3° | 144 | 57.8 | 105 | 42.2 | 249 | 36.4 | ||
| Vaccination coverage | 421 | 61.6 | 262 | 38.4 | 683 | 100 | NA | |
| Age | Mean (SD) | 31.1 | (5.6) | 31.8 | (4.8) | |||
| Vaccinated | Unvaccinated | Total | CI 95% | |
|---|---|---|---|---|
| n = 421 (%) | n = 262 (%) | n = 683 (%) | ||
| In general, your opinion about vaccines as a whole is? * | ||||
| Very favorable | 92 (21.9) | 35 (13.4) | 127 (18,6) | [11.8–25.3] |
| Favorable | 301 (71.5) | 173 (66.0) | 474 (69.4) | [65.2–73.5] |
| Indifferent | 21 (5.0) | 36 (13.7) | 57 (8.3) | [1.1–15.4] |
| Unfavorable | 7 (1.7) | 14 (5.3) | 21 (3.1) | [0.0–10.5] |
| Against | 0 (0.0) | 4 (1.5) | 4 (0.6) | [0.0–8.1] |
| Throughout your life, have your received the vaccines scheduled in the vaccination calendar? * | ||||
| Yes | 417 (99.0) | 256 (97.7) | 673 (98.5) | [97.6–99.4] |
| No | 4 (1.0) | 6 (2.3) | 10 (1.5) | [0.0–9.3] |
| Would you get a flu shot if you became pregnant again? * | ||||
| Yes | 387 (91.9) | 64 (24.4) | 451 (66.0) | [61.6–70.3] |
| No | 34 (8.1) | 198 (75.6) | 232 (34.0) | [27.9–40.1] |
| Have you suffered from the flu during pregnancy or while not pregnant? | ||||
| Yes, pregnant | 23 (5.5) | 13 (4.9) | 36 (5.3) | [0.0–12.6] |
| Yes, not pregnant * | 106 (25.2) | 43 (16.4) | 149 (21.8) | [15.2–28.5] |
| Never | 294 (69.8) | 208 (79.3) | 502 (73.5) | [70.0–77.7] |
| Variables | p | OR | CI 95% |
|---|---|---|---|
| Opinion about vaccines Unfavorable Favorable | <0.001 | 4.07 1.00 | (2.01–8.24) |
| Have you suffered from influenza previously? Yes, Pregnant Yes, not Pregnant Never | 0.029 | 3.84 1.03 1.00 | (1.42–10.42) (0.58–1.83) |
| Have you been vaccinated previously? No Yes | <0.001 | 38.4 1.00 | (23.58–62.77) |
| Country of origin Spanish Not Spanish | 0.082 | 1.65 1.00 | (0.94–2.92) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Blanco, N.; Tuells, J.; Nolasco, A. Influenza Vaccination Experiences of Pregnant Women as a Predictor of the Intention to Become Vaccinated in Future Pregnancies in Spain. Vaccines 2020, 8, 291. https://doi.org/10.3390/vaccines8020291
Rodríguez-Blanco N, Tuells J, Nolasco A. Influenza Vaccination Experiences of Pregnant Women as a Predictor of the Intention to Become Vaccinated in Future Pregnancies in Spain. Vaccines. 2020; 8(2):291. https://doi.org/10.3390/vaccines8020291
Chicago/Turabian StyleRodríguez-Blanco, Noelia, José Tuells, and Andreu Nolasco. 2020. "Influenza Vaccination Experiences of Pregnant Women as a Predictor of the Intention to Become Vaccinated in Future Pregnancies in Spain" Vaccines 8, no. 2: 291. https://doi.org/10.3390/vaccines8020291
APA StyleRodríguez-Blanco, N., Tuells, J., & Nolasco, A. (2020). Influenza Vaccination Experiences of Pregnant Women as a Predictor of the Intention to Become Vaccinated in Future Pregnancies in Spain. Vaccines, 8(2), 291. https://doi.org/10.3390/vaccines8020291






