Technological Approaches for Improving Vaccination Compliance and Coverage
Abstract
1. Introduction
2. Vaccine Formulations for Pulmonary and Nasal Delivery
Dry Powders for Pulmonary Immunisation
3. Oral (Gastrointestinal) Vaccines
4. Cutaneous Immunisation
5. Controlled Antigen Release Delivery Systems for Single-Dose Immunisation
Progress of PLGA Polymer Vaccine Delivery Systems
6. Advanced Vaccine Encapsulation Methods
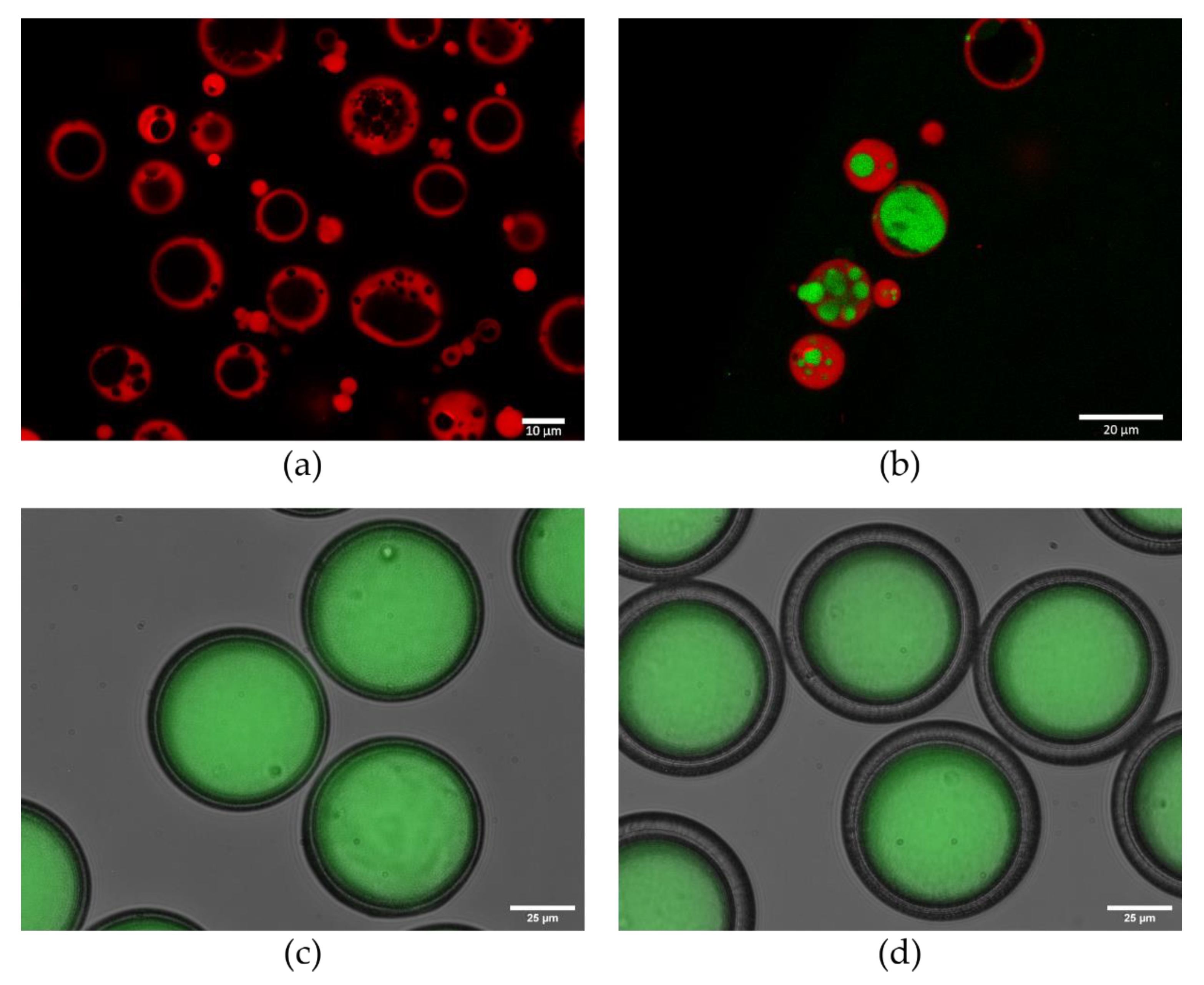
7. Approaches to Encapsulation Using Microfluidics
7.1. Benefits of Using Microfluidics
7.2. Towards Implementing Microfluidics for Vaccine Delivery Systems
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roberts, L. Global polio eradication falters in the final stretch. Science 2020, 367, 14–15. [Google Scholar] [CrossRef]
- Lewnard, J.A.; Lo, N.C.; Arinaminpathy, N.; Frost, I.; Laxminarayan, R. Childhood vaccines and antibiotic use in low- and middle-income countries. Nature 2020, 581, 94–99. [Google Scholar] [CrossRef]
- Vela Ramirez, J.E.; Sharpe, L.A.; Peppas, N.A. Current state and challenges in developing oral vaccines. Adv. Drug. Deliv. Rev. 2017, 114, 116–131. [Google Scholar] [CrossRef]
- Neutra, M.R.; Kozlowski, P.A. Mucosal vaccines: The promise and the challenge. Nat. Rev. Immunol. 2006, 6, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Kiyono, H.; Fukuyama, S. NALT-versus Peyer’s-patch-mediated mucosal immunity. Nat. Rev. Immunol. 2004, 4, 699–710. [Google Scholar] [CrossRef]
- Spit, B.J.; Hendriksen, E.G.; Bruijntjes, J.P.; Kuper, C.F. Nasal lymphoid tissue in the rat. Cell Tissue Res. 1989, 255, 193–198. [Google Scholar] [CrossRef]
- Holmgren, J.; Svennerholm, A.M. Vaccines against mucosal infections. Curr. Opin. Immunol. 2012, 24, 343–353. [Google Scholar] [CrossRef]
- Holmgren, J.; Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med. 2005, 11, S45–S53. [Google Scholar] [CrossRef] [PubMed]
- Ascough, S.; Vlachantoni, I.; Kalyan, M.; Haijema, B.-J.; Wallin-Weber, S.; Dijkstra-Tiekstra, M.; Ahmed, M.S.; van Roosmalen, M.; Grimaldi, R.; Zhang, Q. Local and Systemic Immunity against Respiratory Syncytial Virus Induced by a Novel Intranasal Vaccine. A Randomized, Double-Blind, Placebo-controlled Clinical Trial. Am. J. Respir. Crit. Care Med. 2019, 200, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.A.; Gow, S.P.; Waldner, C.L.; Shields, S.; Wappel, S.; Bowers, A.; Lacoste, S.; Xu, Z.; Ball, E. Comparative efficacy of intranasal and oral vaccines against Bordetella bronchiseptica in dogs. Vet. J. 2016, 212, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Soane, R.J.; Hinchcliffe, M.; Davis, S.S.; Illum, L. Clearance characteristics of chitosan based formulations in the sheep nasal cavity. Int. J. Pharm. 2001, 217, 183–191. [Google Scholar] [CrossRef]
- Ramvikas, M.; Arumugam, M.; Chakrabarti, S.R.; Jaganathan, K.S. Chapter Fifteen-Nasal Vaccine Delivery. In Micro and Nanotechnology in Vaccine Development; Skwarczynski, M., Toth, I., Eds.; William Andrew Publishing: New York, NY, USA, 2017; pp. 279–301. [Google Scholar]
- Mestecky, J.; Russell, M.W.; Elson, C.O. Perspectives on mucosal vaccines: Is mucosal tolerance a barrier? J. Immunol. 2007, 179, 5633–5638. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.A.; O’Hagan, D.T. Designing and building the next generation of improved vaccine adjuvants. J. Control. Release 2014, 190, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Netsomboon, K.; Bernkop-Schnurch, A. Mucoadhesive vs. mucopenetrating particulate drug delivery. Eur. J. Pharm. Biopharm. 2016, 98, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Falkeborn, T.; Hinkula, J.; Olliver, M.; Lindberg, A.; Maltais, A.-K. The intranasal adjuvant Endocine™ enhances both systemic and mucosal immune responses in aged mice immunized with influenza antigen. Virol. J. 2017, 14, 44. [Google Scholar] [CrossRef][Green Version]
- Smith, D.; Streatfield, S.J.; Acosta, H.; Ganesan, S.; Fattom, A. A nanoemulsion-adjuvanted intranasal H5N1 influenza vaccine protects ferrets against homologous and heterologous H5N1 lethal challenge. Vaccine 2019, 37, 6162–6170. [Google Scholar] [CrossRef]
- Nirmal, M.; Khairunnisa, A.G.; Ashwini, K.G.; Michael, R.B.; Michael, F.G.; Mariusz, S.; Istvan, T. Highly Immunogenic Trimethyl Chitosan-based Delivery System for Intranasal Lipopeptide Vaccines against Group A Streptococcus. Curr. Drug Deliv. 2017, 14, 701–708. [Google Scholar] [CrossRef]
- Longet, S.; Lundahl, M.L.E.; Lavelle, E.C. Targeted Strategies for Mucosal Vaccination. Bioconjug. Chem. 2018, 29, 613–623. [Google Scholar] [CrossRef]
- Khan, I.U.; Huang, J.; Li, X.; Xie, J.; Zhu, N. Nasal immunization with RSV F and G protein fragments conjugated to an M cell-targeting ligand induces an enhanced immune response and protection against RSV infection. Antivir. Res. 2018, 159, 95–103. [Google Scholar] [CrossRef]
- Wu, M.; Zhao, H.; Li, M.; Yue, Y.; Xiong, S.; Xu, W. Intranasal Vaccination with Mannosylated Chitosan Formulated DNA Vaccine Enables Robust IgA and Cellular Response Induction in the Lungs of Mice and Improves Protection against Pulmonary Mycobacterial Challenge. Front. Cell. Infect. Microbiol. 2017, 7, 445. [Google Scholar] [CrossRef]
- Willment, J.A.; Marshall, A.S.J.; Reid, D.M.; Williams, D.L.; Wong, S.Y.C.; Gordon, S.; Brown, G.D. The human β-glucan receptor is widely expressed and functionally equivalent to murine Dectin-1 on primary cells. Eur. J. Immunol. 2005, 35, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Soares, E.; Jesus, S.; Borges, O. Chitosan:β-glucan particles as a new adjuvant for the hepatitis B antigen. Eur. J. Pharm. Biopharm. 2018, 131, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Tada, R.; Suzuki, H.; Takahashi, S.; Negishi, Y.; Kiyono, H.; Kunisawa, J.; Aramaki, Y. Nasal vaccination with pneumococcal surface protein A in combination with cationic liposomes consisting of DOTAP and DC-chol confers antigen-mediated protective immunity against Streptococcus pneumoniae infections in mice. Int. Immunopharmacol. 2018, 61, 385–393. [Google Scholar] [CrossRef]
- Mahler, H.C.; Muller, R.; Friess, W.; Delille, A.; Matheus, S. Induction and analysis of aggregates in a liquid IgG1-antibody formulation. Eur. J. Pharm. Biopharm. 2005, 59, 407–417. [Google Scholar] [CrossRef]
- Foged, C. Thermostable Subunit Vaccines for Pulmonary Delivery: How Close Are We? Curr. Pharm. Des. 2016, 22, 2561–2576. [Google Scholar] [CrossRef]
- Nagpal, P.S.; Kesarwani, A.; Sahu, P.; Upadhyay, P. Aerosol immunization by alginate coated mycobacterium (BCG/MIP) particles provide enhanced immune response and protective efficacy than aerosol of plain mycobacterium against M.tb. H37Rv infection in mice. BMC Infect. Dis. 2019, 19, 568. [Google Scholar] [CrossRef]
- Tomar, J.; Patil, H.P.; Bracho, G.; Tonnis, W.F.; Frijlink, H.W.; Petrovsky, N.; Vanbever, R.; Huckriede, A.; Hinrichs, W.L.J. Advax augments B and T cell responses upon influenza vaccination via the respiratory tract and enables complete protection of mice against lethal influenza virus challenge. J. Control. Release 2018, 288, 199–211. [Google Scholar] [CrossRef]
- Thakur, A.; Ingvarsson, P.T.; Schmidt, S.T.; Rose, F.; Andersen, P.; Christensen, D.; Foged, C. Immunological and physical evaluation of the multistage tuberculosis subunit vaccine candidate H56/CAF01 formulated as a spray-dried powder. Vaccine 2018, 36, 3331–3339. [Google Scholar] [CrossRef] [PubMed]
- Kabiri, M.; Sankian, M.; Hosseinpour, M.; Tafaghodi, M. The novel immunogenic chimeric peptide vaccine to elicit potent cellular and mucosal immune responses against HTLV-1. Int. J. Pharm. 2018, 549, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Vemireddy, S.; Pallavi, P.; Sampath Kumar, H.M. Chitosan stabilized nasal emulsion delivery system for effective humoral and cellular response against recombinant tetravalent dengue antigen. Carbohydr. Polym. 2018, 190, 129–138. [Google Scholar] [CrossRef]
- Nevagi, R.J.; Khalil, Z.G.; Hussein, W.M.; Powell, J.; Batzloff, M.R.; Capon, R.J.; Good, M.F.; Skwarczynski, M.; Toth, I. Polyglutamic acid-trimethyl chitosan-based intranasal peptide nano-vaccine induces potent immune responses against group A streptococcus. Acta Biomater. 2018, 80, 278–287. [Google Scholar] [CrossRef]
- Singh, M.; Briones, M.; O’Hagan, D.T. A novel bioadhesive intranasal delivery system for inactivated influenza vaccines. J. Control. Release 2001, 70, 267–276. [Google Scholar] [CrossRef]
- Fukuyama, Y.; Yuki, Y.; Katakai, Y.; Harada, N.; Takahashi, H.; Takeda, S.; Mejima, M.; Joo, S.; Kurokawa, S.; Sawada, S.; et al. Nanogel-based pneumococcal surface protein A nasal vaccine induces microRNA-associated Th17 cell responses with neutralizing antibodies against Streptococcus pneumoniae in macaques. Mucosal. Immunol. 2015, 8, 1144–1153. [Google Scholar] [CrossRef]
- Rose, F.; Wern, J.E.; Gavins, F.; Andersen, P.; Follmann, F.; Foged, C. A strong adjuvant based on glycol-chitosan-coated lipid-polymer hybrid nanoparticles potentiates mucosal immune responses against the recombinant Chlamydia trachomatis fusion antigen CTH522. J. Control. Release 2018, 271, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Yin, Y.; Huang, L.; Yu, Q.; Yang, Q. H9N2 influenza whole inactivated virus combined with polyethyleneimine strongly enhances mucosal and systemic immunity after intranasal immunization in mice. Clin. Vaccine Immunol. 2015, 22, 421–429. [Google Scholar] [CrossRef]
- Jesus, S.; Soares, E.; Costa, J.; Borchard, G.; Borges, O. Immune response elicited by an intranasally delivered HBsAg low-dose adsorbed to poly-epsilon-caprolactone based nanoparticles. Int. J. Pharm. 2016, 504, 59–69. [Google Scholar] [CrossRef]
- Shang, S.; Kats, D.; Cao, L.; Morgun, E.; Velluto, D.; He, Y.; Xu, Q.; Wang, C.R.; Scott, E.A. Induction of Mycobacterium Tuberculosis Lipid-Specific T Cell Responses by Pulmonary Delivery of Mycolic Acid-Loaded Polymeric Micellar Nanocarriers. Front. Immunol. 2018, 9, 2709. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, W.; Luo, Y.; Wang, B.Z. Gold nanoparticles conjugating recombinant influenza hemagglutinin trimers and flagellin enhanced mucosal cellular immunity. Nanomedicine 2018, 14, 1349–1360. [Google Scholar] [CrossRef]
- Thakkar, S.G.; Warnken, Z.N.; Alzhrani, R.F.; Valdes, S.A.; Aldayel, A.M.; Xu, H.; Williams, R.O., 3rd; Cui, Z. Intranasal immunization with aluminum salt-adjuvanted dry powder vaccine. J. Control. Release 2018, 292, 111–118. [Google Scholar] [CrossRef]
- Wang, X.; Yang, D.; Li, S.; Xu, X.; Qin, C.F.; Tang, R. Biomineralized vaccine nanohybrid for needle-free intranasal immunization. Biomaterials 2016, 106, 286–294. [Google Scholar] [CrossRef]
- Zheng, H.; Pan, L.; Lv, J.; Zhang, Z.; Wang, Y.; Hu, W.; Liu, X.; Zhou, P.; Wang, Y.; Zhang, Y. Comparison of immune responses in guinea pigs by intranasal delivery with different nanoparticles-loaded FMDV DNA vaccine. Microb. Pathog. 2020, 142, 104061. [Google Scholar] [CrossRef]
- Hassan, H.A.; Smyth, L.; Wang, J.T.; Costa, P.M.; Ratnasothy, K.; Diebold, S.S.; Lombardi, G.; Al-Jamal, K.T. Dual stimulation of antigen presenting cells using carbon nanotube-based vaccine delivery system for cancer immunotherapy. Biomaterials 2016, 104, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Kuczkowska, K.; Kleiveland, C.R.; Minic, R.; Moen, L.F.; Overland, L.; Tjaland, R.; Carlsen, H.; Lea, T.; Mathiesen, G.; Eijsink, V.G.H. Immunogenic Properties of Lactobacillus plantarum Producing Surface-Displayed Mycobacterium tuberculosis Antigens. Appl. Environ. Microbiol. 2016, 83, e02782-16. [Google Scholar] [CrossRef]
- Kotomina, T.; Korenkov, D.; Matyushenko, V.; Prokopenko, P.; Rudenko, L.; Isakova-Sivak, I. Live attenuated influenza vaccine viral vector induces functional cytotoxic T-cell immune response against foreign CD8+ T-cell epitopes inserted into NA and NS1 genes using the 2A self-cleavage site. Hum. Vaccines Immunother. 2018, 14, 2964–2970. [Google Scholar] [CrossRef]
- Carvalho, A.L.; Miquel-Clopes, A.; Wegmann, U.; Jones, E.; Stentz, R.; Telatin, A.; Walker, N.J.; Butcher, W.A.; Brown, P.J.; Holmes, S.; et al. Use of bioengineered human commensal gut bacteria-derived microvesicles for mucosal plague vaccine delivery and immunization. Clin. Exp. Immunol. 2019, 196, 287–304. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, L.; Sun, H.W.; Guo, H.; Song, Z.; You, Y.; Yang, L.Y.; Tong, Y.N.; Gao, J.N.; Zeng, H.; et al. Epitope-loaded nanoemulsion delivery system with ability of extending antigen release elicits potent Th1 response for intranasal vaccine against Helicobacter pylori. J. Nanobiotechnol. 2019, 17, 1–15. [Google Scholar] [CrossRef]
- Lee, Y.T.; Ko, E.J.; Lee, Y.; Kim, K.H.; Kim, M.C.; Lee, Y.N.; Kang, S.M. Intranasal vaccination with M2e5x virus-like particles induces humoral and cellular immune responses conferring cross-protection against heterosubtypic influenza viruses. PLoS ONE 2018, 13, e0190868. [Google Scholar] [CrossRef] [PubMed]
- Ashhurst, A.S.; McDonald, D.M.; Hanna, C.C.; Stanojevic, V.A.; Britton, W.J.; Payne, R.J. Mucosal Vaccination with a Self-Adjuvanted Lipopeptide Is Immunogenic and Protective against Mycobacterium tuberculosis. J. Med. Chem. 2019, 62, 8080–8089. [Google Scholar] [CrossRef]
- Tomosada, Y.; Chiba, E.; Zelaya, H.; Takahashi, T.; Tsukida, K.; Kitazawa, H.; Alvarez, S.; Villena, J. Nasally administered Lactobacillus rhamnosus strains differentially modulate respiratory antiviral immune responses and induce protection against respiratory syncytial virus infection. BMC Immunol. 2013, 14, 40. [Google Scholar] [CrossRef]
- Hinkula, J.; Nystrom, S.; Devito, C.; Brave, A.; Applequist, S.E. Long-Lasting Mucosal and Systemic Immunity against Influenza A Virus Is Significantly Prolonged and Protective by Nasal Whole Influenza Immunization with Mucosal Adjuvant N3 and DNA-Plasmid Expressing Flagellin in Aging In- and Outbred Mice. Vaccines (Basel) 2019, 7, 64. [Google Scholar] [CrossRef]
- Lin, S.F.; Jiang, P.L.; Tsai, J.S.; Huang, Y.Y.; Lin, S.Y.; Lin, J.H.; Liu, D.Z. Surface assembly of poly(I:C) on polyethyleneimine-modified gelatin nanoparticles as immunostimulatory carriers for mucosal antigen delivery. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Abhyankar, M.M.; Orr, M.T.; Lin, S.; Suraju, M.O.; Simpson, A.; Blust, M.; Pham, T.; Guderian, J.A.; Tomai, M.A.; Elvecrog, J.; et al. Adjuvant composition and delivery route shape immune response quality and protective efficacy of a recombinant vaccine for Entamoeba histolytica. NPJ Vaccines 2018, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, P.; Yu, Y.; Fu, Y.; Jiang, H.; Lu, M.; Sun, Z.; Jiang, S.; Lu, L.; Wu, M.X. Pulmonary surfactant-biomimetic nanoparticles potentiate heterosubtypic influenza immunity. Science 2020, 367, 6480. [Google Scholar] [CrossRef]
- Ye, L.; Ohnemus, A.; Ong, L.C.; Gad, H.H.; Hartmann, R.; Lycke, N.; Staeheli, P. Type I and Type III Interferons Differ in Their Adjuvant Activities for Influenza Vaccines. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Griffin, K.F.; Eyles, J.E.; Spiers, I.D.; Alpar, H.O.; Williamson, E.D. Protection against plague following immunisation with microencapsulated V antigen is reduced by co-encapsulation with IFN-gamma or IL-4, but not IL-6. Vaccine 2002, 20, 3650–3657. [Google Scholar] [CrossRef]
- Matchett, W.E.; Malewana, G.B.R.; Mudrick, H.; Medlyn, M.J.; Barry, M.A. Genetic Adjuvants in Replicating Single-Cycle Adenovirus Vectors Amplify Systemic and Mucosal Immune Responses against HIV-1 Envelope. Vaccines (Basel) 2020, 8, 64. [Google Scholar] [CrossRef]
- Maeto, C.; Rodriguez, A.M.; Holgado, M.P.; Falivene, J.; Gherardi, M.M. Novel mucosal DNA-MVA HIV vaccination in which DNA-IL-12 plus cholera toxin B subunit (CTB) cooperates to enhance cellular systemic and mucosal genital tract immunity. PLoS ONE 2014, 9, e107524. [Google Scholar] [CrossRef]
- Demberg, T.; Boyer, J.D.; Malkevich, N.; Patterson, L.J.; Venzon, D.; Summers, E.L.; Kalisz, I.; Kalyanaraman, V.S.; Lee, E.M.; Weiner, D.B.; et al. Sequential priming with simian immunodeficiency virus (SIV) DNA vaccines, with or without encoded cytokines, and a replicating adenovirus-SIV recombinant followed by protein boosting does not control a pathogenic SIVmac251 mucosal challenge. J. Virol. 2008, 82, 10911–10921. [Google Scholar] [CrossRef] [PubMed]
- Staats, H.F.; Bradney, C.P.; Gwinn, W.M.; Jackson, S.S.; Sempowski, G.D.; Liao, H.X.; Letvin, N.L.; Haynes, B.F. Cytokine requirements for induction of systemic and mucosal CTL after nasal immunization. J.Immunol. 2001, 167, 5386–5394. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Pais, R.; Ohandjo, A.; He, C.; He, Q.; Omosun, Y.; Igietseme, J.U.; Eko, F.O. Comparative evaluation of the protective efficacy of two formulations of a recombinant Chlamydia abortus subunit candidate vaccine in a mouse model. Vaccine 2015, 33, 1865–1872. [Google Scholar] [CrossRef]
- Kang, S.H.; Hong, S.J.; Lee, Y.K.; Cho, S. Oral Vaccine Delivery for Intestinal Immunity-Biological Basis, Barriers, Delivery System, and M Cell Targeting. Polymers (Basel) 2018, 10, 948. [Google Scholar] [CrossRef] [PubMed]
- Kanungo, S.; Chatterjee, P. Oral cholera vaccines: Exploring the farrago of evidence. Lancet Infect. Dis. 2017, 17, 1012–1013. [Google Scholar] [CrossRef][Green Version]
- Marasini, N.; Skwarczynski, M.; Toth, I. Oral delivery of nanoparticle-based vaccines. Expert Rev. Vaccines 2014, 13, 1361–1376. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, T.A.; Teijeiro-Osorio, D.; Rosa, M.; Coulter, I.S.; Alonso, M.J.; Brayden, D.J. Current status of selected oral peptide technologies in advanced preclinical development and in clinical trials. Adv. Drug Deliv. Rev. 2016, 106, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Al-Gousous, J.; Penning, M.; Langguth, P. Molecular insights into shellac film coats from different aqueous shellac salt solutions and effect on disintegration of enteric-coated soft gelatin capsules. Int. J. Pharm. 2015, 484, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.A.; Jepson, M.A.; Simmons, N.L.; Booth, T.A.; Hirst, B.H. Differential expression of lectin-binding sites defines mouse intestinal M-cells. J. Histochem. Cytochem. 1993, 41, 1679–1687. [Google Scholar] [CrossRef]
- Clark, M.A.; Jepson, M.A.; Simmons, N.L.; Hirst, B.H. Selective binding and transcytosis of Ulex europaeus 1 lectin by mouse Peyer’s patch M-cells in vivo. Cell Tissue Res. 1995, 282, 455–461. [Google Scholar] [CrossRef]
- Foster, N.; Clark, M.A.; Jepson, M.A.; Hirst, B.H. Ulex europaeus 1 lectin targets microspheres to mouse Peyer’s patch M-cells in vivo. Vaccine 1998, 16, 536–541. [Google Scholar] [CrossRef]
- Clark, M.A.; Blair, H.; Liang, L.; Brey, R.N.; Brayden, D.; Hirst, B.H. Targeting polymerised liposome vaccine carriers to intestinal M cells. Vaccine 2001, 20, 208–217. [Google Scholar] [CrossRef]
- Hase, K.; Kawano, K.; Nochi, T.; Pontes, G.S.; Fukuda, S.; Ebisawa, M.; Kadokura, K.; Tobe, T.; Fujimura, Y.; Kawano, S.; et al. Uptake through glycoprotein 2 of FimH(+) bacteria by M cells initiates mucosal immune response. Nature 2009, 462, 226–230. [Google Scholar] [CrossRef]
- Shima, H.; Watanabe, T.; Fukuda, S.; Fukuoka, S.; Ohara, O.; Ohno, H. A novel mucosal vaccine targeting Peyer’s patch M cells induces protective antigen-specific IgA responses. Int. Immunol. 2014, 26, 619–625. [Google Scholar] [CrossRef]
- Pappo, J.; Ermak, T.H.; Steger, H.J. Monoclonal antibody-directed targeting of fluorescent polystyrene microspheres to Peyer’s patch M cells. Immunology 1991, 73, 277. [Google Scholar] [PubMed]
- Nochi, T.; Yuki, Y.; Matsumura, A.; Mejima, M.; Terahara, K.; Kim, D.Y.; Fukuyama, S.; Iwatsuki-Horimoto, K.; Kawaoka, Y.; Kohda, T.; et al. A novel M cell-specific carbohydrate-targeted mucosal vaccine effectively induces antigen-specific immune responses. J. Exp. Med. 2007, 204, 2789–2796. [Google Scholar] [CrossRef] [PubMed]
- Garinot, M.; Fievez, V.; Pourcelle, V.; Stoffelbach, F.; des Rieux, A.; Plapied, L.; Theate, I.; Freichels, H.; Jerome, C.; Marchand-Brynaert, J.; et al. PEGylated PLGA-based nanoparticles targeting M cells for oral vaccination. J. Control. Release 2007, 120, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Misumi, S.; Masuyama, M.; Takamune, N.; Nakayama, D.; Mitsumata, R.; Matsumoto, H.; Urata, N.; Takahashi, Y.; Muneoka, A.; Sukamoto, T.; et al. Targeted delivery of immunogen to primate m cells with tetragalloyl lysine dendrimer. J. Immunol. 2009, 182, 6061–6070. [Google Scholar] [CrossRef]
- Davitt, C.J.; Lavelle, E.C. Delivery strategies to enhance oral vaccination against enteric infections. Adv. Drug Deliv. Rev. 2015, 91, 52–69. [Google Scholar] [CrossRef]
- Holmgren, J.; Bourgeois, L.; Carlin, N.; Clements, J.; Gustafsson, B.; Lundgren, A.; Nygren, E.; Tobias, J.; Walker, R.; Svennerholm, A.M. Development and preclinical evaluation of safety and immunogenicity of an oral ETEC vaccine containing inactivated E. coli bacteria overexpressing colonization factors CFA/I, CS3, CS5 and CS6 combined with a hybrid LT/CT B subunit antigen, administered alone and together with dmLT adjuvant. Vaccine 2013, 31, 2457–2464. [Google Scholar] [CrossRef]
- Karlsson, S.L.; Ax, E.; Nygren, E.; Kallgard, S.; Blomquist, M.; Ekman, A.; Benktander, J.; Holmgren, J.; Lebens, M. Development of stable Vibrio cholerae O1 Hikojima type vaccine strains co-expressing the Inaba and Ogawa lipopolysaccharide antigens. PLoS ONE 2014, 9, e108521. [Google Scholar] [CrossRef]
- Leach, S.; Clements, J.D.; Kaim, J.; Lundgren, A. The adjuvant double mutant Escherichia coli heat labile toxin enhances IL-17A production in human T cells specific for bacterial vaccine antigens. PLoS ONE 2012, 7, e51718. [Google Scholar] [CrossRef]
- Lundgren, A.; Bourgeois, L.; Carlin, N.; Clements, J.; Gustafsson, B.; Hartford, M.; Holmgren, J.; Petzold, M.; Walker, R.; Svennerholm, A.M. Safety and immunogenicity of an improved oral inactivated multivalent enterotoxigenic Escherichia coli (ETEC) vaccine administered alone and together with dmLT adjuvant in a double-blind, randomized, placebo-controlled Phase I study. Vaccine 2014, 32, 7077–7084. [Google Scholar] [CrossRef]
- Clements, J.D.; Norton, E.B. The Mucosal Vaccine Adjuvant LT(R192G/L211A) or dmLT. MSphere 2018, 3, e00215-18. [Google Scholar] [CrossRef] [PubMed]
- Agren, L.C.; Ekman, L.; Lowenadler, B.; Lycke, N.Y. Genetically engineered nontoxic vaccine adjuvant that combines B cell targeting with immunomodulation by cholera toxin A1 subunit. J. Immunol. 1997, 158, 3936–3946. [Google Scholar] [PubMed]
- Lycke, N.; Bemark, M. Mucosal adjuvants and long-term memory development with special focus on CTA1-DD and other ADP-ribosylating toxins. Mucosal. Immunol. 2010, 3, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Abautret-Daly, A.E.; Davitt, C.J.; Lavelle, E.C. Harnessing the antibacterial and immunological properties of mucosal-associated invariant T cells in the development of novel oral vaccines against enteric infections. Biochem. Pharmacol. 2014, 92, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Cerundolo, V.; Barral, P.; Batista, F.D. Synthetic iNKT cell-agonists as vaccine adjuvants--finding the balance. Curr. Opin. Immunol. 2010, 22, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Davitt, C.J.; McNeela, E.A.; Longet, S.; Tobias, J.; Aversa, V.; McEntee, C.P.; Rosa, M.; Coulter, I.S.; Holmgren, J.; Lavelle, E.C. A novel adjuvanted capsule based strategy for oral vaccination against infectious diarrhoeal pathogens. J. Control. Release 2016, 233, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Davitt, C.J.H.; Longet, S.; Albutti, A.; Aversa, V.; Nordqvist, S.; Hackett, B.; McEntee, C.P.; Rosa, M.; Coulter, I.S.; Lebens, M.; et al. Alpha-galactosylceramide enhances mucosal immunity to oral whole-cell cholera vaccines. Mucosal. Immunol. 2019, 12, 1055–1064. [Google Scholar] [CrossRef]
- Longet, S.; Abautret-Daly, A.; Davitt, C.J.H.; McEntee, C.P.; Aversa, V.; Rosa, M.; Coulter, I.S.; Holmgren, J.; Raghavan, S.; Lavelle, E.C. An oral alpha-galactosylceramide adjuvanted Helicobacter pylori vaccine induces protective IL-1R- and IL-17R-dependent Th1 responses. NPJ Vaccines 2019, 4, 1–10. [Google Scholar] [CrossRef]
- McDonald, B.F.; Coulter, I.S.; Marison, I.W. Microbeads: A novel multiparticulate drug delivery technology for increasing the solubility and dissolution of celecoxib. Pharm. Dev. Technol. 2015, 20, 211–218. [Google Scholar] [CrossRef]
- Longet, S.; Aversa, V.; O’Donnell, D.; Tobias, J.; Rosa, M.; Holmgren, J.; Coulter, I.S.; Lavelle, E.C. Thermostability of the coating, antigen and immunostimulator in an adjuvanted oral capsule vaccine formulation. Int. J. Pharm. 2017, 534, 60–70. [Google Scholar] [CrossRef]
- Engelke, L.; Winter, G.; Hook, S.; Engert, J. Recent insights into cutaneous immunization: How to vaccinate via the skin. Vaccine 2015, 33, 4663–4674. [Google Scholar] [CrossRef]
- Bragazzi, N.L.; Orsi, A.; Ansaldi, F.; Gasparini, R.; Icardi, G. Fluzone(R) intra-dermal (Intanza(R)/Istivac(R) Intra-dermal): An updated overview. Hum. Vaccin. Immunother. 2016, 12, 2616–2627. [Google Scholar] [CrossRef] [PubMed]
- Caucheteux, S.M.; Piguet, V. New Cutaneous Vaccine Adjuvant that STINGs a Little Less. J. Invest. Dermatol. 2016, 136, 2127–2128. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Rai, B. Electroporation of Skin Stratum Corneum Lipid Bilayer and Molecular Mechanism of Drug Transport: A Molecular Dynamics Study. Langmuir 2018, 34, 5860–5870. [Google Scholar] [CrossRef]
- Diehl, M.C.; Lee, J.C.; Daniels, S.E.; Tebas, P.; Khan, A.S.; Giffear, M.; Sardesai, N.Y.; Bagarazzi, M.L. Tolerability of intramuscular and intradermal delivery by CELLECTRA((R)) adaptive constant current electroporation device in healthy volunteers. Hum. Vaccines Immunother. 2013, 9, 2246–2252. [Google Scholar] [CrossRef] [PubMed]
- Todorova, B.; Adam, L.; Culina, S.; Boisgard, R.; Martinon, F.; Cosma, A.; Ustav, M.; Kortulewski, T.; Le Grand, R.; Chapon, C. Electroporation as a vaccine delivery system and a natural adjuvant to intradermal administration of plasmid DNA in macaques. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Herve, P.L.; Dhelft, V.; Plaquet, C.; Rousseaux, A.; Bouzereau, A.; Gaulme, L.; Tilleul, S.; Ligouis, M.; Donne, N.; Lambert, P.H.; et al. Epidermal micro-perforation potentiates the efficacy of epicutaneous vaccination. J. Control. Release 2019, 298, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Scheiblhofer, S.; Strobl, A.; Hoepflinger, V.; Thalhamer, T.; Steiner, M.; Thalhamer, J.; Weiss, R. Skin vaccination via fractional infrared laser ablation-Optimization of laser-parameters and adjuvantation. Vaccine 2017, 35, 1802–1809. [Google Scholar] [CrossRef]
- Badizadegan, K.; Goodson, J.L.; Rota, P.A.; Thompson, K.M. The potential role of using vaccine patches to induce immunity: Platform and pathways to innovation and commercialization. Expert Rev. Vaccines 2020, 19, 175–194. [Google Scholar] [CrossRef]
- Leone, M.; Monkare, J.; Bouwstra, J.A.; Kersten, G. Dissolving Microneedle Patches for Dermal Vaccination. Pharm. Res. 2017, 34, 2223–2240. [Google Scholar] [CrossRef]
- Hao, Y.; Li, W.; Zhou, X.; Yang, F.; Qian, Z. Microneedles-Based Transdermal Drug Delivery Systems: A Review. J. Biomed. Nanotechnol. 2017, 13, 1581–1597. [Google Scholar] [CrossRef]
- Engert, J.; Anamur, C.; Engelke, L.; Fellner, C.; Lell, P.; Henke, S.; Stadler, J.; Zols, S.; Ritzmann, M.; Winter, G. A pilot study using a novel pyrotechnically driven prototype applicator for epidermal powder immunization in piglets. Int. J. Pharm. 2018, 545, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Etzl, E.E. Collapse Dried Protein Powders for Needle-Free Ballistic Injection. Ph.D. Thesis, Ludwig-Maximilians-Universität, München, Germany, 2016. [Google Scholar]
- Tomar, J.; Born, P.A.; Frijlink, H.W.; Hinrichs, W.L. Dry influenza vaccines: Towards a stable, effective and convenient alternative to conventional parenteral influenza vaccination. Expert Rev. Vaccines 2016, 15, 1431–1447. [Google Scholar] [CrossRef] [PubMed]
- Wallis, J.; Shenton, D.P.; Carlisle, R.C. Novel approaches for the design, delivery and administration of vaccine technologies. Clin. Exp. Immunol. 2019, 196, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Hogan, N.C.; Taberner, A.J.; Jones, L.A.; Hunter, I.W. Needle-free delivery of macromolecules through the skin using controllable jet injectors. Expert. Opin. Drug Deliv. 2015, 12, 1637–1648. [Google Scholar] [CrossRef]
- Mittal, A.; Raber, A.S.; Lehr, C.M.; Hansen, S. Particle based vaccine formulations for transcutaneous immunization. Hum. Vaccines Immunother. 2013, 9, 1950–1955. [Google Scholar] [CrossRef][Green Version]
- Chen, Z.; Lv, Y.; Qi, J.; Zhu, Q.; Lu, Y.; Wu, W. Overcoming or circumventing the stratum corneum barrier for efficient transcutaneous immunization. Drug Discov. Today 2018, 23, 181–186. [Google Scholar] [CrossRef]
- Gamazo, C.; Pastor, Y.; Larraneta, E.; Berzosa, M.; Irache, J.M.; Donnelly, R.F. Understanding the basis of transcutaneous vaccine delivery. Ther. Deliv. 2019, 10, 63–80. [Google Scholar] [CrossRef]
- Pielenhofer, J.; Sohl, J.; Windbergs, M.; Langguth, P.; Radsak, M.P. Current Progress in Particle-Based Systems for Transdermal Vaccine Delivery. Front. Immunol. 2020, 11, 266. [Google Scholar] [CrossRef]
- Hansen, S.; Lehr, C.M. Transfollicular delivery takes root: The future for vaccine design? Expert Rev. Vaccines 2014, 13, 5–7. [Google Scholar] [CrossRef][Green Version]
- O’Hagan, D.T.; Rahman, D.; McGee, J.P.; Jeffery, H.; Davies, M.C.; Williams, P.; Davis, S.S.; Challacombe, S.J. Biodegradable microparticles as controlled release antigen delivery systems. Immunology 1991, 73, 239. [Google Scholar]
- Preis, I.; Langer, R.S. A single-step immunization by sustained antigen release. J. Immunol. Methods 1979, 28, 193–197. [Google Scholar] [CrossRef]
- Park, K. The Controlled Drug Delivery Systems: Past Forward and Future Back. J. Control. Release Off. J. Control. Release Soc. 2014, 190, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J. Single-administration vaccines: Controlled-release technology to mimic repeated immunizations. Trends Biotechnol. 1999, 17, 25–29. [Google Scholar] [CrossRef]
- Leleux, J.; Roy, K. Micro and Nanoparticle-Based Delivery Systems for Vaccine Immunotherapy: An Immunological and Materials Perspective. Adv. Healthc. Mater. 2013, 2, 72–94. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.L.; Soema, P.C.; Slütter, B.; Ossendorp, F.; Jiskoot, W. PLGA particulate delivery systems for subunit vaccines: Linking particle properties to immunogenicity. Hum. Vaccines Immunother. 2016, 12, 1056–1069. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zhang, W.; Liu, Q.; Yang, T.; Wang, L.; Ma, G. Adjuvanticity Regulation by Biodegradable Polymeric Nano/microparticle Size. Mol. Pharm. 2017, 14, 14–22. [Google Scholar] [CrossRef]
- Katare, Y.K.; Muthukumaran, T.; Panda, A.K. Influence of particle size, antigen load, dose and additional adjuvant on the immune response from antigen loaded PLA microparticles. Int. J. Pharm. 2005, 301, 149–160. [Google Scholar] [CrossRef]
- Slütter, B.; Jiskoot, W. Sizing the optimal dimensions of a vaccine delivery system: A particulate matter. Expert Opin. Drug Deliv. 2016, 13, 167–170. [Google Scholar] [CrossRef]
- Oyewumi, M.O.; Kumar, A.; Cui, Z. Nano-microparticles as immune adjuvants: Correlating particle sizes and the resultant immune responses. Expert Rev. Vaccines 2010, 9, 1095–1107. [Google Scholar] [CrossRef]
- Storni, T.; Kundig, T.; Senti, G.; Johansen, P. Immunity in response to particulate antigen-delivery systems. Adv. Drug Deliv. Rev. 2005, 57, 333–355. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, L.; Yang, T.; Liu, Y.; Chen, X.; Liu, Q.; Jia, J.; Ma, G. Immunopotentiator-Loaded Polymeric Microparticles as Robust Adjuvant to Improve Vaccine Efficacy. Pharm. Res. 2015, 32, 2837–2850. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, P.B.; McGinity, J.W. Preparation of microspheres by the solvent evaporation technique. Adv. Drug Deliv. Rev. 1997, 28, 25–42. [Google Scholar] [CrossRef]
- Jiang, W.; Gupta, R.; Deshpande, M.; Schwendeman, S. Biodegradable poly(lactic-co-glycolic acid) microparticles for injectable delivery of vaccine antigens. Adv. Drug Deliv. Rev. 2005, 57, 391–410. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Liu, L.; Ma, P.; Wang, H.; Zhang, C.; Sun, H.; Wang, C.; Song, C.; Leng, X.; Kong, D.; Ma, G. Immune responses to vaccines delivered by encapsulation into and/or adsorption onto cationic lipid-PLGA hybrid nanoparticles. J. Control. Release 2016, 225, 230–239. [Google Scholar] [CrossRef]
- Joshi, V.B.; Geary, S.M.; Salem, A.K. Biodegradable Particles as Vaccine Delivery Systems: Size Matters. AAPS J. 2013, 15, 85–94. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, N.; Amer, S.; Qian, M.; Lv, M.; Zhao, Y.; Su, X.; Cao, J.; He, H.; Zhao, B. Protective efficacy of PLGA microspheres loaded with divalent DNA vaccine encoding the ompA gene of Aeromonas veronii and the hly gene of Aeromonas hydrophila in mice. Vaccine 2013, 31, 5754–5759. [Google Scholar] [CrossRef]
- Nayak, B.; Panda, A.K.; Ray, P.; Ray, A.R. Formulation, characterization and evaluation of rotavirus encapsulated PLA and PLGA particles for oral vaccination. J. Microencapsul. 2009, 26, 154–165. [Google Scholar] [CrossRef]
- Katare, Y.; Panda, A. Immunogenicity and lower dose requirement of polymer entrapped tetanus toxoid co-administered with alum. Vaccine 2006, 24, 3599–3608. [Google Scholar] [CrossRef]
- Yeh, M.K.; Chiang, C.H. Inactive Vibrio cholerae whole-cell vaccine-loaded biodegradable microparticles: In vitro release and oral vaccination. J. Microencapsul. 2004, 21, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Mata, E.; Igartua, M.; Patarroyo, M.E.; Pedraz, J.L.; Hernández, R.M. Enhancing immunogenicity to PLGA microparticulate systems by incorporation of alginate and RGD-modified alginate. Eur. J. Pharm. Sci. 2011, 44, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xiong, F.; He, J.; Dai, X.; Wang, G. Surface-functionalized, pH-responsive poly(lactic-co-glycolic acid)-based microparticles for intranasal vaccine delivery: Effect of surface modification with chitosan and mannan. Eur. J. Pharm. Biopharm. 2016, 109, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Kremer, B.; Roukema, R.; Leede, L. Advances in Single-Shot Vaccine Development. BioPharm Int. 2009, 2009, 28–34. [Google Scholar]
- Kirby, D.J.; Rosenkrands, I.; Agger, E.M.; Andersen, P.; Coombes, A.G.A.; Perrie, Y. PLGA microspheres for the delivery of a novel subunit TB vaccine. J. Drug Target. 2008, 16, 282–293. [Google Scholar] [CrossRef]
- Watkins, H.C.; Pagan, C.L.; Childs, H.R.; Posada, S.; Chau, A.; Rios, J.; Guarino, C.; DeLisa, M.P.; Whittaker, G.R.; Putnam, D. A single dose and long lasting vaccine against pandemic influenza through the controlled release of a heterospecies tandem M2 sequence embedded within detoxified bacterial outer membrane vesicles. Vaccine 2017, 35, 5373–5380. [Google Scholar] [CrossRef]
- Fu, K.; Harrell, R.; Zinski, K.; Um, C.; Jaklenec, A.; Frazier, J.; Lotan, N.; Burke, P.; Klibanov, A.M.; Langer, R. A potential approach for decreasing the burst effect of protein from PLGA microspheres. J. Pharm. Sci. 2003, 92, 1582–1591. [Google Scholar] [CrossRef]
- Han, F.Y.; Thurecht, K.J.; Whittaker, A.K.; Smith, M.T. Bioerodable PLGA-Based Microparticles for Producing Sustained-Release Drug Formulations and Strategies for Improving Drug Loading. Front. Pharmacol. 2016, 7. [Google Scholar] [CrossRef]
- Qi, F.; Wu, J.; Li, H.; Ma, G. Recent research and development of PLGA/PLA microspheres/nanoparticles: A review in scientific and industrial aspects. Front. Chem. Sci. Eng. 2019, 13, 14–27. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, Y.; Li, R.; Zeng, J.; Li, Z.; Tang, Y.; Sun, D. RhBMP-2-loaded Poly(lactic-co-glycolic acid) microspheres fabricated by coaxial electrospraying for protein delivery. J. Biomater. Sci. Polym. Ed. 2017, 28, 2205–2219. [Google Scholar] [CrossRef]
- Tzeng, S.Y.; McHugh, K.J.; Behrens, A.M.; Rose, S.; Sugarman, J.L.; Ferber, S.; Langer, R.; Jaklenec, A. Stabilized single-injection inactivated polio vaccine elicits a strong neutralizing immune response. Proc. Natl. Acad. Sci. USA 2018, 115, E5269–E5278. [Google Scholar] [CrossRef] [PubMed]
- Guarecuco, R.; Lu, J.; McHugh, K.J.; Norman, J.J.; Thapa, L.S.; Lydon, E.; Langer, R.; Jaklenec, A. Immunogenicity of pulsatile-release PLGA microspheres for single-injection vaccination. Vaccine 2018, 36, 3161–3168. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yang, T.; Jia, J.; Lu, T.; Wang, H.; Yan, X.; Wang, L.; Yu, L.; Zhao, Y. Fabrication and characterization of DDAB/PLA-alginate composite microcapsules as single-shot vaccine. RSC Adv. 2018, 8, 13612–13624. [Google Scholar] [CrossRef]
- Jeong, H.H.; Yelleswarapu, V.R.; Yadavali, S.; Issadore, D.; Lee, D. Kilo-scale droplet generation in three-dimensional monolithic elastomer device (3D MED). Lab Chip 2015, 15, 4387–4392. [Google Scholar] [CrossRef] [PubMed]
- Rinker, T.E.; Philbrick, B.D.; Temenoff, J.S. Core-shell microparticles for protein sequestration and controlled release of a protein-laden core. Acta Biomater. 2017, 56, 91–101. [Google Scholar] [CrossRef]
- McHugh, K.J.; Nguyen, T.D.; Linehan, A.R.; Yang, D.; Behrens, A.M.; Rose, S.; Tochka, Z.L.; Tzeng, S.Y.; Norman, J.J.; Anselmo, A.C.; et al. Fabrication of fillable microparticles and other complex 3D microstructures. Science 2017, 357, 1138–1142. [Google Scholar] [CrossRef]
- Lee, T.Y.; Ku, M.; Kim, B.; Lee, S.; Yang, J.; Kim, S.H. Microfluidic Production of Biodegradable Microcapsules for Sustained Release of Hydrophilic Actives. Small 2017, 13, 1700646. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Hribar, K.C.; Brugarolas, T.; Kamat, N.P.; Burdick, J.A.; Lee, D. Harnessing Interfacial Phenomena to Program the Release Properties of Hollow Microcapsules. Adv. Funct. Mater. 2012, 22, 131–138. [Google Scholar] [CrossRef]
- Montazeri, L.; Bonakdar, S.; Taghipour, M.; Renaud, P.; Baharvand, H. Modification of PDMS to fabricate PLGA microparticles by a double emulsion method in a single microfluidic device. Lab Chip 2016, 16, 2596–2600. [Google Scholar] [CrossRef]
- Seo, M.; Paquet, C.; Nie, Z.; Xu, S.; Kumacheva, E. Microfluidic consecutive flow-focusing droplet generators. Soft Matter 2007, 3, 986–992. [Google Scholar] [CrossRef]
- Keohane, K.; Brennan, D.; Galvin, P.; Griffin, B.T. Silicon microfluidic flow focusing devices for the production of size-controlled PLGA based drug loaded microparticles. Int. J. Pharm. 2014, 467, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Hashimoto, M.; Dang, T.T.; Hoare, T.; Kohane, D.S.; Whitesides, G.M.; Langer, R.; Anderson, D.G.; David, H. Preparation of monodisperse biodegradable polymer microparticles using a microfluidic flow-focusing device for controlled drug delivery. Small 2009, 5, 1575–1581. [Google Scholar] [CrossRef]
- Pessi, J.; Santos, H.A.; Miroshnyk, I.; Joukoyliruusi; Weitz, D.A.; Mirza, S. Microfluidics-assisted engineering of polymeric microcapsules with high encapsulation efficiency for protein drug delivery. Int. J. Pharm. 2014, 472, 82–87. [Google Scholar] [CrossRef]
- Loizou, K.; Wong, V.-L.; Hewakandamby, B. Examining the Effect of Flow Rate Ratio on Droplet Generation and Regime Transition in a Microfluidic T-Junction at Constant Capillary Numbers. Inventions 2018, 3, 54. [Google Scholar] [CrossRef]
- Bardin, D.; Kendall, M.R.; Dayton, P.A.; Lee, A.P. Parallel generation of uniform fine droplets at hundreds of kilohertz in a flow-focusing module. Biomicrofluidics 2013, 7, 034112. [Google Scholar] [CrossRef] [PubMed]
- Yadavali, S.; Jeong, H.H.; Lee, D.; Issadore, D. Silicon and glass very large scale microfluidic droplet integration for terascale generation of polymer microparticles. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Munira, S.L.; Hendriks, J.T.; Atmosukarto, I.I.; Friede, M.H.; Carter, L.M.; Butler, J.R.G.; Clements, A.C.A. A cost analysis of producing vaccines in developing countries. Vaccine 2019, 37, 1245–1251. [Google Scholar] [CrossRef] [PubMed]



| Study Title | Pathogen/ Condition | Vaccine | Administration Route | Status | Phase | Clinical Trials Gov Identifier |
|---|---|---|---|---|---|---|
| Intrapulmonary | ||||||
| Phase 1 Clinical Trial of the Safety and Immunogenicity of an Adenovirus-Based TB Vaccine Administered by Aerosol | Tuberculosis | Ad5Ag85A | Pulmonary | Recruiting | I | NCT02337270 |
| Investigating Immune Responses to Aerosol Bacillus Calmette–Guérin (BCG) Challenge in Healthy UK Adults | Tuberculosis | BCG Danish | Pulmonary | Recruiting | I | NCT03912207 |
| ChAdOx1 85A Aerosol Versus Intramuscular Vaccination in Healthy Adults (TB039) (TB039) | Tuberculosis | ChadOx1 85A | Pulmonary | Recruiting | I | NCT04121494 |
| Nasal | ||||||
| Evaluating the Safety and Immune Response to a Single Dose of a respiratory syncytial virus (RSV) Vaccine in Infants and Children | RSV infection | RSV ΔNS2 Δ1313 I1314L | Nasal | Recruiting | I | NCT01893554 |
| Safety and Immunogenicity of a RSV Vaccine in RSV-Seropositive Children and RSV-Seronegative Infants and Children | RSV infection | D46cpΔM2-2 vaccine | Nasal | Active, not recruiting | I | NCT02601612 |
| Evaluating the Infectivity, Safety, and Immunogenicity of the Recombinant Live-Attenuated RSV Vaccines in RSV-Seronegative Infants 6 to 24 Months of Age | RSV infection | RSV ΔNS2/Δ1313/I1314L and RSV 276 | Nasal | Active, not recruiting | I | NCT03227029 |
| Evaluating the Infectivity, Safety, and Immunogenicity of a RSV Vaccine in RSV-Seropositive Children and RSV-Seronegative Infants and Children | RSV infection | RSV 6120/∆NS2/1030s | Nasal | Recruiting | I | NCT03387137 |
| Evaluating the Infectivity, Safety, and Immunogenicity of the Recombinant Live-Attenuated RSV Vaccines in RSV-Seronegative Infants and Children 6 to 24 Months of Age | RSV infection | RSV ΔNS2/Δ1313/I1314L and RSV 276 | Nasal | Active, not recruiting | I | NCT03422237 |
| A Study Assessing Colonisation and Immunogenicity after Nasal Inoculation with N. lactamica and Eradication on Day 4 or 14 | Meningitis | Neisseria lactamica | Nasal | Recruiting | Not applicable | NCT03549325 |
| Evaluating the Infectivity, Safety and Immunogenicity of RSV Vaccines in RSV-Seropositive Children and RSV-Seronegative Infants and Children | RSV infection | RSV 6120/∆NS1 and RSV 6120/F1/G2/∆NS1 | Nasal | Recruiting | I | NCT03596801 |
| Mucosal and Systemic Immunity after Viral Challenge of Healthy Volunteers Vaccinated with Inactivated Influenza Vaccine via the Intranasal Versus Intramuscular Route | Influenza | Flucelvax: Inactivated influenza vaccine | Nasal | Recruiting | II | NCT03845231 |
| Safety and Immunogenicity of a Single Dose of the Recombinant Live-Attenuated RSV Vaccines or placebo, delivered as nose drops to RSV-Seronegative Children 6 to 24 Months of Age | RSV infection | RSV ΔNS2/Δ1313/I1314L, RSV 6120/∆NS2/1030s, and RSV 276 | Nasal | Recruiting | I/II | NCT03916185 |
| Nasal and Systemic Immune Responses to Nasal Influenza Vaccine (Flu-M3) | Influenza | Live attenuated influenza vaccine | Nasal | Active, not recruiting | Not applicable | NCT04110366 |
| A Controlled Study to Assess Safety, Colonisation and Immunogenicity of Reconstituted Lyophilised Neisseria lactamica (Lac5-Nasal) | Meningitis | Lyophilised Neisseria lactamica | Nasal | Recruiting | Not applicable | NCT04135053 |
| A Safety and Immunogenicity of Intranasal Nanoemulsion Adjuvanted Recombinant Anthrax Vaccine in Healthy Adults (IN NE-rPA) | Anthrax | BW-1010: a nanoemulsion adjuvanted recombinant protein | Nasal | Recruiting | I | NCT04148118 |
| Live-Attenuated Influenza Vaccine as a Nasal Model for Influenza Infection | Influenza | Flumist quadrivalent nasal vaccine | Nasal | Not yet recruiting | IV | NCT04164212 |
| Delivery System | Pathogen/Antigen | Administration | Animal Model | Immunity Type Generated | Reference |
|---|---|---|---|---|---|
| Liposomes | Mycobacterium tuberculosis (Mtb) H56 antigen | Pulmonary | Mice | Th1; Th17; IgA; IgG | [29] |
| ISCOMs | Human T cell lymphotropic virus type 1 | Nasal | Mice | Th1; IgA; IgG | [30] |
| Chitosan | Dengue virus | Nasal | Mice | CD8+ T cells; IgA; IgG | [31] |
| γ-polyglutamic acid | Group A Streptococcus | Nasal | Mice | IgA; IgG | [32] |
| Hyaluronic acid | Influenza hemagglutinin | Nasal | Mice, Rabbits, Micro-pigs | IgA; IgG | [33] |
| Pullulan | Streptococcus pneumoniae | Nasal | Macaques | Th2; Th17; IgA; IgG | [34] |
| Synthetic polymer-based particles | |||||
| PLGA | Chlamydia trachomatis | Nasal | Mice | Th1; IgA; IgG | [35] |
| PEI | H9N2 Influenza | Nasal | Mice | Th1; CD8+ T cells; IgA; IgG | [36] |
| PCL | Hepatitis B | Nasal | Mice | IgA; IgG | [37] |
| PPS | Mtb | Nasal | Mice | Th1; Th17 | [38] |
| Inorganic particles | |||||
| Gold particles | H3N2 hemagglutinin | Nasal | Mice | Th1; CD8+ T cells; IgA; IgG | [39] |
| Aluminium particles | Ovalbumin | Nasal | Rats | IgA; IgG | [40] |
| Calcium phosphate particles | Chimeric dengue virus serotype 2 | Nasal | Mice | IgA | [41] |
| Silica-based particles | Foot and mouth disease virus | Nasal | Guinea pigs | IgA; IgG | [42] |
| Carbon nanoparticles | Ovalbumin | Nasal | Mice | Th1; CD8+ T cells | [43] |
| Infectious materials | |||||
| Recombinant bacteria | Lactobacillus plantarum vector for Mtb | Nasal | Mice | Th1; IgA | [44] |
| Recombinant virus | Influenza virus vector for respiratory syncytial virus | Pulmonary; Nasal | Mice | CD8+ T cells | [45] |
| Outer membrane vesicles (OMV) | Bacteroides thetaiotaomicron OMV for Yersinia pestis V and F antigen | Nasal | Mice | IgA; IgG | [46] |
| Emulsions | Helicobacter pylori | Nasal | Mice | Th1; IgA; IgG | [47] |
| VLPs | Influenza VLPs | Nasal | Mice | Th1; IgA; IgG | [48] |
| Immunopotentiator | Pathogen/Antigen | Administration | Animal Model | Immunity Type Generated | Reference |
|---|---|---|---|---|---|
| Bacterial TLR agonists | |||||
| Lipopeptides: TLR-1/2 agonists | Mycobacterium tuberculosis | Nasal | Mice | Th1; Th17 | [49] |
| Lipopolysaccharide: TLR-4 agonist | Human T cell lymphotropic virus type 1 | Nasal | Mice | Th1; IgA; IgG | [30] |
| Peptidoglycan: TLR-2/4 agonists | Respiratory syncytial virus | Nasal | Mice | Th1; Th2 | [50] |
| Flagellin: TLR-5 agonist | Influenza A virus | Nasal | Mice | Th1; CD8+ T cells; IgA; IgG | [51] |
| CpG DNA: TLR-9 agonist | Foot and mouth disease virus | Nasal | Guinea pig | IgA; IgG | [42] |
| Viral TLR agonists | |||||
| Double stranded RNA: TLR 3 agonist | Human parainfluenza virus type 3 virus | Nasal | Mice; Cotton rats; Pigs | Th1; IgA | [52] |
| Guanosine analogues: TLR-7/8 agonists | Entamoeba histolytica | Nasal | Mice | Th1; Th17; IgA; IgG | [53] |
| STING agonist: Cyclic dinucleotide GMP–AMP | H1N1, H3N2, H5N1, H7N9 Influenza | Nasal | Mice; Ferrets | Th1; CD8+ T cells; IgA; IgG | [54] |
| Cytokines | |||||
| Type I Interferons (IFN) | Influenza | Nasal | Mice | IgA; IgG | [55] |
| IFN-γ | Yersinia pestis | Nasal | Mice | IgA; IgG | [56] |
| GM-CSF | HIV-1 | Nasal | Mice | IgA; IgG | [57] |
| IL-12 | HIV | Nasal | Mice | Th1; CD8+ T cells; IgA; IgG | [58] |
| IL-15 | Simian immunodeficiency virus | Pulmonary | Mice | Th1; CD8+ T cells; ADCC | [59] |
| IL-18 | HIV | Nasal | Mice | Th1; CD8+ T cells | [60] |
| FLT-3 ligand | Chlamydia abortus | Nasal | Mice | Th1; IgA; IgG | [61] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemoine, C.; Thakur, A.; Krajišnik, D.; Guyon, R.; Longet, S.; Razim, A.; Górska, S.; Pantelić, I.; Ilić, T.; Nikolić, I.; et al. Technological Approaches for Improving Vaccination Compliance and Coverage. Vaccines 2020, 8, 304. https://doi.org/10.3390/vaccines8020304
Lemoine C, Thakur A, Krajišnik D, Guyon R, Longet S, Razim A, Górska S, Pantelić I, Ilić T, Nikolić I, et al. Technological Approaches for Improving Vaccination Compliance and Coverage. Vaccines. 2020; 8(2):304. https://doi.org/10.3390/vaccines8020304
Chicago/Turabian StyleLemoine, Céline, Aneesh Thakur, Danina Krajišnik, Romain Guyon, Stephanie Longet, Agnieszka Razim, Sabina Górska, Ivana Pantelić, Tanja Ilić, Ines Nikolić, and et al. 2020. "Technological Approaches for Improving Vaccination Compliance and Coverage" Vaccines 8, no. 2: 304. https://doi.org/10.3390/vaccines8020304
APA StyleLemoine, C., Thakur, A., Krajišnik, D., Guyon, R., Longet, S., Razim, A., Górska, S., Pantelić, I., Ilić, T., Nikolić, I., Lavelle, E. C., Gamian, A., Savić, S., & Milicic, A. (2020). Technological Approaches for Improving Vaccination Compliance and Coverage. Vaccines, 8(2), 304. https://doi.org/10.3390/vaccines8020304











