Mucosal Vaccination with UV-Inactivated Chlamydia suis in Pre-Exposed Outbred Pigs Decreases Pathogen Load and Induces CD4 T-Cell Maturation into IFN-?+ Effector Memory Cells
Abstract
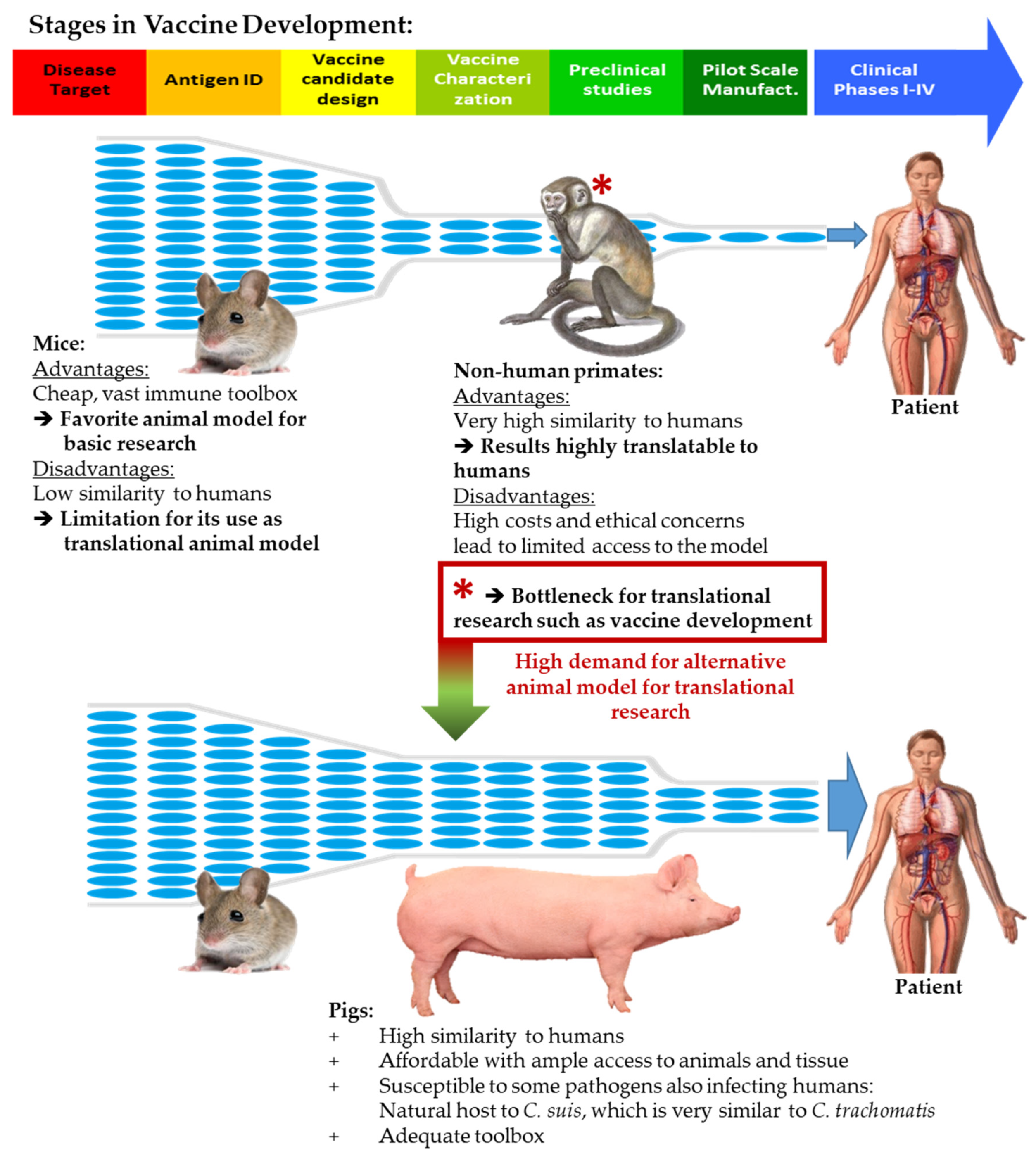
:1. Introduction
2. Materials and Methods
2.1. Chlamydia Suis
2.2. Vaccine and Adjuvant Preparation
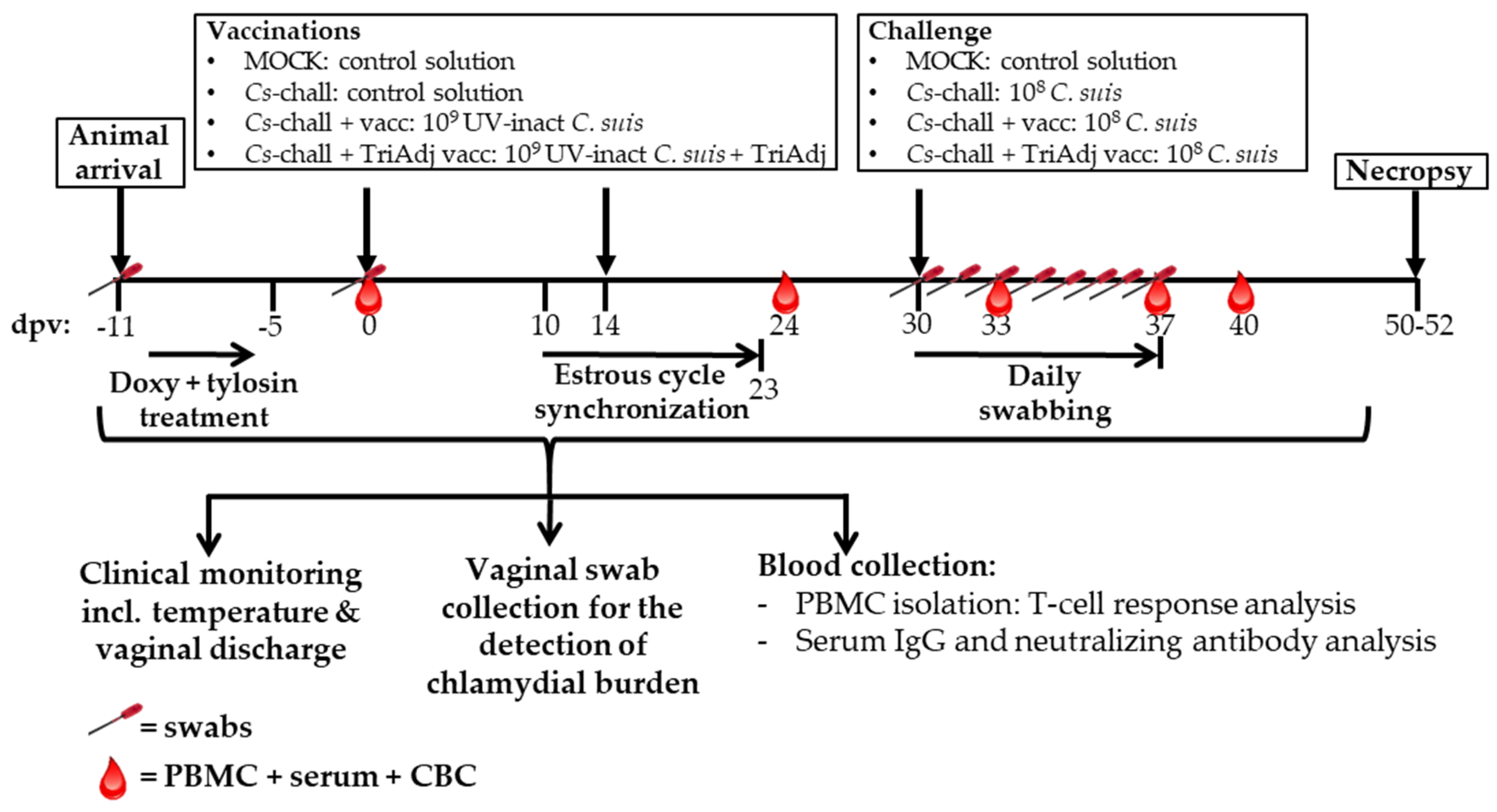
2.3. Pigs and Experimental Design
2.4. Sampling
2.5. Detection of Chlamydia via qPCR
2.6. Serum Anti-Chlamydia Suis Immunoglobulin G Detection
2.7. Neutralizing Antibody Detection
2.8. Chlamydia suis-Specific CD4 T-Cell Proliferation
2.9. Chlamydia suis-Specific IFN-Γ Production by CD4 T Cells
2.10. Blood CD4 T-Cell mRNA Data Acquisition, Processing, and Analysis
2.11. Statistical Analysis
3. Results
3.1. Chlamydia Load in Vaginal Swabs
3.1.1. Pre-exposure and the efficacy of the antibiotic treatment
3.1.2. Effect of Vaccination on the Genital Cs Load
3.2. The Humoral Immune Response to Chlamydia suis Vaccination and Challenge
3.3. The CD4 T-Cell Response to Chlamydia suis Vaccination and Challenge
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garg, R.; Babiuk, L.; Gerdts, V. A novel combination adjuvant platform for human and animal vaccines. Vaccine 2017, 35, 4486–4489. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.; Rowley, J.; Vander Hoorn, S.; Wijesooriya, N.S.; Unemo, M.; Low, N.; Stevens, G.; Gottlieb, S.; Kiarie, J.; Temmerman, M. Global Estimates of the Prevalence and Incidence of Four Curable Sexually Transmitted Infections in 2012 Based on Systematic Review and Global Reporting. PLoS ONE 2015, 10, e0143304. [Google Scholar] [CrossRef] [Green Version]
- Brunham, R.C.; Rey-Ladino, J. Immunology of Chlamydia infection: Implications for a Chlamydia trachomatis vaccine. Nat. Rev. Immunol. 2005, 5, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Käser, T.; Renois, F.; Wilson, H.L.; Cnudde, T.; Gerdts, V.; Dillon, J.R.; Jungersen, G.; Agerholm, J.S.; Meurens, F. Contribution of the swine model in the study of human sexually transmitted infections. Infect. Genet. Evol. 2017, 66, 346–360. [Google Scholar] [CrossRef] [Green Version]
- Dawson, H.D.; Loveland, J.E.; Pascal, G.; Gilbert, J.G.R.; Uenishi, H.; Mann, K.M.; Sang, Y.; Zhang, J.; Carvalho-Silva, D.; Hunt, T.; et al. Structural and functional annotation of the porcine immunome. BMC Genom. 2013, 14, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Roshick, C.; Wood, H.; Caldwell, H.D.; McClarty, G. Comparison of gamma interferon-mediated antichlamydial defense mechanisms in human and mouse cells. Infect. Immun. 2006, 74, 225–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stary, G.; Olive, A.; Radovic-Moreno, A.F.; Gondek, D.; Alvarez, D.; Basto, P.A.; Perro, M.; Vrbanac, V.D.; Tager, A.M.; Shi, J.; et al. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science 2015, 348, 1–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lizarraga, D.; Carver, S.; Timms, P. Navigating to the most promising directions amid complex fields of vaccine development: A chlamydial case study. Expert Rev. Vaccines 2019, 18, 1323–1337. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E.; Kalmar, I.; Vanrompay, D. Animal models for studying female genital tract infection with Chlamydia trachomatis. Infect. Immun. 2013, 81, 3060–3067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boje, S.; Olsen, A.W.; Erneholm, K.; Agerholm, J.S.; Jungersen, G.; Andersen, P.; Follmann, F. A multi-subunit Chlamydia vaccine inducing neutralizing antibodies and strong IFN-gamma(+) CMI responses protects against a genital infection in minipigs. Immunol. Cell Biol. 2016, 94, 185–195. [Google Scholar] [CrossRef] [Green Version]
- Käser, T.; Pasternak, J.A.; Delgado-Ortega, M.; Hamonic, G.; Lai, K.; Erickson, J.; Walker, S.; Dillon, J.R.; Gerdts, V.; Meurens, F. Chlamydia suis and Chlamydia trachomatis induce multifunctional CD4 T cells in pigs. Vaccine 2017, 35, 91–100. [Google Scholar] [CrossRef]
- Schautteet, K.; De Clercq, E.; Jonsson, Y.; Lagae, S.; Chiers, K.; Cox, E.; Vanrompay, D. Protection of pigs against genital Chlamydia trachomatis challenge by parenteral or mucosal DNA immunization. Vaccine 2012, 30, 2869–2881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schautteet, K.; Stuyven, E.; Beeckman, D.S.; Van Acker, S.; Carlon, M.; Chiers, K.; Cox, E.; Vanrompay, D. Protection of pigs against Chlamydia trachomatis challenge by administration of a MOMP-based DNA vaccine in the vaginal mucosa. Vaccine 2011, 29, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Schautteet, K.; Stuyven, E.; Cox, E.; Vanrompay, D. Validation of the Chlamydia trachomatis genital challenge pig model for testing recombinant protein vaccines. J. Med. Microbiol. 2011, 60, 117–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanrompay, D.; Hoang, T.Q.; De Vos, L.; Verminnen, K.; Harkinezhad, T.; Chiers, K.; Morre, S.A.; Cox, E. Specific-pathogen-free pigs as an animal model for studying Chlamydia trachomatis genital infection. Infect. Immun. 2005, 73, 8317–8321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzen, E.; Follmann, F.; Jungersen, G.; Agerholm, J.S. A review of the human vs. porcine female genital tract and associated immune system in the perspective of using minipigs as a model of human genital Chlamydia infection. Vet. Res. 2015, 46, 116. [Google Scholar] [CrossRef]
- Li, L.X.; McSorley, S.J. B cells enhance antigen-specific CD4 T cell priming and prevent bacteria dissemination following Chlamydia muridarum genital tract infection. PLoS Pathog. 2013, 9, e1003707. [Google Scholar] [CrossRef] [Green Version]
- De Puysseleyr, K.; De Puysseleyr, L.; Dhondt, H.; Geens, T.; Braeckman, L.; Morré, S.A.; Cox, E.; Vanrompay, D. Evaluation of the presence and zoonotic transmission of Chlamydia suis in a pig slaughterhouse. BMC Infect. Dis. 2014, 14, 560. [Google Scholar] [CrossRef] [Green Version]
- Longbottom, D.; Coulter, L.J. Animal Chlamydioses and Zoonotic Implications. J. Comp. Pathol. 2003, 128, 217–244. [Google Scholar] [CrossRef]
- Erneholm, K.; Lorenzen, E.; Boje, S.; Olsen, A.W.; Jungersen, G.; Jensen, H.E.; Cassidy, J.P.; Andersen, P.; Agerholm, J.S.; Follmann, F. Genital Infiltrations of CD4(+) and CD8(+) T Lymphocytes, IgA(+) and IgG(+) Plasma Cells and Intra-Mucosal Lymphoid Follicles Associate with Protection Against Genital Chlamydia trachomatis Infection in Minipigs Intramuscularly Immunized with UV-Inactivated Bacteria Adjuvanted With CAF01. Front. Microbiol. 2019, 10, 197. [Google Scholar] [CrossRef]
- Nguyen, N.; Olsen, A.W.; Lorenzen, E.; Andersen, P.; Hvid, M.; Follmann, F.; Dietrich, J. Parenteral vaccination protects against transcervical infection with Chlamydia trachomatis and generate tissue-resident T cells post-challenge. NPJ Vaccines 2020, 5, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scidmore, M.A. Cultivation and Laboratory Maintenance of Chlamydia trachomatis. Curr. Protoc. Microbiol. 2005. [Google Scholar] [CrossRef]
- Käser, T.; Cnudde, T.; Hamonic, G.; Rieder, M.; Pasternak, J.A.; Lai, K.; Tikoo, S.K.; Wilson, H.L.; Meurens, F. Porcine retinal cell line VIDO R1 and Chlamydia suis to modelize ocular chlamydiosis. Vet. Immunol. Immunopathol. 2015, 166, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Käser, T.; Pasternak, J.A.; Hamonic, G.; Rieder, M.; Lai, K.; Delgado-Ortega, M.; Gerdts, V.; Meurens, F. Flow cytometry as an improved method for the titration of Chlamydiaceae and other intracellular bacteria. Cytom. A 2016, 89, 451–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzen, E.; Follmann, F.; Secher, J.O.; Goericke-Pesch, S.; Hansen, M.S.; Zakariassen, H.; Olsen, A.W.; Andersen, P.; Jungersen, G.; Agerholm, J.S. Intrauterine inoculation of minipigs with Chlamydia trachomatis during diestrus establishes a longer lasting infection compared to vaginal inoculation during estrus. Microbes Infect. 2017, 19, 334–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez, A.; Karriker, L.A. Herd Evaluation. In Diseases of Swine, 10th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; Wiley-Blackwell: Ames, IA, USA, 2012; p. 10. [Google Scholar]
- Rahman, K.S.; Darville, T.; Wiesenfeld, H.C.; Hillier, S.L.; Kaltenboeck, B. Mixed Chlamydia trachomatis Peptide Antigens Provide a Specific and Sensitive Single-Well Colorimetric Enzyme-Linked Immunosorbent Assay for Detection of Human Anti-C. trachomatis Antibodies. mSphere 2018, 3, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 24 May 2020).
- Aronesty, E. Comparison of Sequencing Utility Programs. Open Bioinform. J. 2013, 7, 1–8. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Navarro, M.N.; Feijoo-Carnero, C.; Arandilla, A.G.; Trost, M.; Cantrell, D.A. Protein kinase D2 is a digital amplifier of T cell receptor-stimulated diacylglycerol signaling in naive CD8(+) T cells. Sci. Signal. 2014, 7, ra99. [Google Scholar] [CrossRef] [Green Version]
- Spitaler, M.; Emslie, E.; Wood, C.D.; Cantrell, D. Diacylglycerol and protein kinase D localization during T lymphocyte activation. Immunity 2006, 24, 535–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Essen, M.R.; Kongsbak, M.; Schjerling, P.; Olgaard, K.; Odum, N.; Geisler, C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat. Immunol. 2010, 11, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Carlesso, G.; Osipovich, A.B.; Llanes, J.; Lin, Q.; Hoek, K.L.; Khan, W.N.; Ruley, H.E. Subunit 1 of the prefoldin chaperone complex is required for lymphocyte development and function. J. Immunol. 2008, 181, 476–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, P.; Kraus, Z.J.; Stunz, L.L.; Liu, Y.; Bishop, G.A. TNF receptor-associated factor 3 is required for T cell-mediated immunity and TCR/CD28 signaling. J. Immunol. 2011, 186, 143–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douglas, R.S.; Gianoukakis, A.G.; Kamat, S.; Smith, T.J. Aberrant expression of the insulin-like growth factor-1 receptor by T cells from patients with Graves’ disease may carry functional consequences for disease pathogenesis. J. Immunol. 2007, 178, 3281–3287. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Chung, W.J.; Kwak, H.B.; Chung, C.H.; Kwack, K.B.; Lee, Z.H.; Kim, H.H. Tumor necrosis factor-alpha supports the survival of osteoclasts through the activation of Akt and ERK. J. Biol. Chem. 2001, 276, 49343–49349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Zhang, L.; Zhang, X.; Moreno, J.; Celluzzi, C.; Tondravi, M.; Shi, Y. Regulation of activation-induced receptor activator of NF- k B ligand (RANKL) expression in T cells. Eur. J. Immunol. 2002, 32, 1090–1098. [Google Scholar] [CrossRef]
- Ellis, G.I.; Zhi, L.; Akundi, R.; Bueler, H.; Marti, F. Mitochondrial and cytosolic roles of PINK1 shape induced regulatory T-cell development and function. Eur. J. Immunol. 2013, 43, 3355–3360. [Google Scholar] [CrossRef] [Green Version]
- Cohen, C.J.; Crome, S.Q.; MacDonald, K.G.; Dai, E.L.; Mager, D.L.; Levings, M.K. Human Th1 and Th17 cells exhibit epigenetic stability at signature cytokine and transcription factor loci. J. Immunol. 2011, 187, 5615–5626. [Google Scholar] [CrossRef] [Green Version]
- Miao, T.; Symonds, A.L.J.; Singh, R.; Symonds, J.D.; Ogbe, A.; Omodho, B.; Zhu, B.; Li, S.; Wang, P. Egr2 and 3 control adaptive immune responses by temporally uncoupling expansion from T cell differentiation. J. Exp. Med. 2017, 214, 1787–1808. [Google Scholar] [CrossRef]
- Solt, L.A.; Kumar, N.; Nuhant, P.; Wang, Y.; Lauer, J.L.; Liu, J.; Istrate, M.A.; Kamenecka, T.M.; Roush, W.R.; Vidovic, D.; et al. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature 2011, 472, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Li, P.; He, R.; Liu, H.; Liu, N.; Xia, Y.; Bi, G.; Du, Q.; Xia, M.; Pei, L.; et al. DAPK1 (death associated protein kinase 1) mediates mTORC1 activation and antiviral activities in CD8(+) T cells. Cell. Mol. Immunol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ohland, C.L.; Jobin, C. Microbial activities and intestinal homeostasis: A delicate balance between health and disease. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 28–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzen, E.; Follmann, F.; Boje, S.; Erneholm, K.; Olsen, A.W.; Agerholm, J.S.; Jungersen, G.; Andersen, P. Intramuscular Priming and Intranasal Boosting Induce Strong Genital Immunity Through Secretory IgA in Minipigs Infected with Chlamydia trachomatis. Front. Immunol. 2015, 6, 628. [Google Scholar] [CrossRef]
- Abraham, S.; Juel, H.B.; Bang, P.; Cheeseman, H.M.; Dohn, R.B.; Cole, T.; Kristiansen, M.P.; Korsholm, K.S.; Lewis, D.; Olsen, A.W.; et al. Safety and immunogenicity of the chlamydia vaccine candidate CTH522 adjuvanted with CAF01 liposomes or aluminium hydroxide: A first-in-human, randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infect. Dis. 2019, 19, 1091–1100. [Google Scholar] [CrossRef]
- Barral, R.; Desai, R.; Zheng, X.; Frazer, L.C.; Sucato, G.S.; Haggerty, C.L.; O’Connell, C.M.; Zurenski, M.A.; Darville, T. Frequency of Chlamydia trachomatis-specific T cell interferon-gamma and interleukin-17 responses in CD4-enriched peripheral blood mononuclear cells of sexually active adolescent females. J. Reprod. Immunol. 2014, 103, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Karunakaran, K.P.; Kelly, I.; Shen, C.; Jiang, X.; Foster, L.J.; Brunham, R.C. Immunization with live and dead Chlamydia muridarum induces different levels of protective immunity in a murine genital tract model: Correlation with MHC class II peptide presentation and multifunctional Th1 cells. J. Immunol. 2011, 186, 3615–3621. [Google Scholar] [CrossRef]
- Darville, T.; Hiltke, T.J. Pathogenesis of genital tract disease due to Chlamydia trachomatis. J. Infect. Dis. 2010, 201 (Suppl. 2), S114–S125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rey-Ladino, J.; Ross, A.G.; Cripps, A.W. Immunity, immunopathology, and human vaccine development against sexually transmitted Chlamydia trachomatis. Hum. Vaccines Immunother. 2014, 10, 2664–2673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olive, A.J.; Gondek, D.C.; Starnbach, M.N. CXCR3 and CCR5 are both required for T cell-mediated protection against C. trachomatis infection in the murine genital mucosa. Mucosal Immunol. 2011, 4, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Roan, N.R.; Gierahn, T.M.; Higgins, D.E.; Starnbach, M.N. Monitoring the T cell response to genital tract infection. Proc. Natl. Acad. Sci. USA 2006, 103, 12069–12074. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Antigen | Clone | Isotype | Fluorochrome | Labeling Strategy | Primary Ab Source | 2nd Ab Source |
|---|---|---|---|---|---|---|
| Peripheral blood mononuclear cell (PBMC) proliferation staining panel | ||||||
| CD3 | PPT3 | IgG1 | FITC | Directly conjugated | Southern Biotech | - |
| CD4 | 74-12-4 | IgG2b | Brilliant Violet 480 | Secondary antibody | BEI Resources | Jackson Immunoresearch |
| CD8α | 76-2-11 | IgG2a | Brilliant Violet 605 | Biotin-streptavidin | Southern Biotech | Biolegend |
| CCR7 | 3D12 | rIgG2a | Brilliant Blue 700 | Directly conjugated | BD Biosciences | - |
| Live/Dead | - | - | Near Infra-red | - | Invitrogen | - |
| Proliferation | - | - | CellTraceTM Violet | - | Invitrogen | - |
| PBMC Intracellular cytokine staining panel | ||||||
| CD3 | PPT3 | IgG1 | FITC | Directly conjugated | Southern Biotech | - |
| CD4 | 74-12-4 | IgG2b | Brilliant Violet 421 | Secondary antibody | BEI Resources | Jackson Immunoresearch |
| CD8α | 76-2-11 | IgG2a | PE-Cy5.5 | Biotin-streptavidin | Southern Biotech | Southern Biotech |
| CCR7 | 3D12 | rIgG2a | Brilliant Violet 480 | Directly conjugated | BD Biosciences | - |
| IFN-γ | P2G10 | IgG1 | PE | Directly conjugated | BD Biosciences | - |
| Live/Dead | - | - | Near-Infrared | - | Invitrogen | - |
| PBMC MACS reanalysis staining panel | ||||||
| CD4 | 74-12-4 | IgG2b | PE | Secondary antibody | BEI Resources | Southern Biotech |
| CD172a | 74-22-15 | IgG1 | Alexa Flour 647 | Secondary antibody | BEI Resources | Southern Biotech |
| Live/Dead | - | - | Near Infra-red | - | Invitrogen | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaral, A.F.; Rahman, K.S.; Kick, A.R.; Cortes, L.M.; Robertson, J.; Kaltenboeck, B.; Gerdts, V.; O’Connell, C.M.; Poston, T.B.; Zheng, X.; et al. Mucosal Vaccination with UV-Inactivated Chlamydia suis in Pre-Exposed Outbred Pigs Decreases Pathogen Load and Induces CD4 T-Cell Maturation into IFN-?+ Effector Memory Cells. Vaccines 2020, 8, 353. https://doi.org/10.3390/vaccines8030353
Amaral AF, Rahman KS, Kick AR, Cortes LM, Robertson J, Kaltenboeck B, Gerdts V, O’Connell CM, Poston TB, Zheng X, et al. Mucosal Vaccination with UV-Inactivated Chlamydia suis in Pre-Exposed Outbred Pigs Decreases Pathogen Load and Induces CD4 T-Cell Maturation into IFN-?+ Effector Memory Cells. Vaccines. 2020; 8(3):353. https://doi.org/10.3390/vaccines8030353
Chicago/Turabian StyleAmaral, Amanda F., Khondaker S. Rahman, Andrew R. Kick, Lizette M. Cortes, James Robertson, Bernhard Kaltenboeck, Volker Gerdts, Catherine M. O’Connell, Taylor B. Poston, Xiaojing Zheng, and et al. 2020. "Mucosal Vaccination with UV-Inactivated Chlamydia suis in Pre-Exposed Outbred Pigs Decreases Pathogen Load and Induces CD4 T-Cell Maturation into IFN-?+ Effector Memory Cells" Vaccines 8, no. 3: 353. https://doi.org/10.3390/vaccines8030353
APA StyleAmaral, A. F., Rahman, K. S., Kick, A. R., Cortes, L. M., Robertson, J., Kaltenboeck, B., Gerdts, V., O’Connell, C. M., Poston, T. B., Zheng, X., Liu, C., Omesi, S. Y., Darville, T., & Käser, T. (2020). Mucosal Vaccination with UV-Inactivated Chlamydia suis in Pre-Exposed Outbred Pigs Decreases Pathogen Load and Induces CD4 T-Cell Maturation into IFN-?+ Effector Memory Cells. Vaccines, 8(3), 353. https://doi.org/10.3390/vaccines8030353






