Impact of the 13-Valent Conjugated Pneumococcal Vaccine on the Direct Costs of Invasive Pneumococcal Disease Requiring Hospital Admission in Children Aged < 5 Years: A Prospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Case Selection
2.3. Demographic, Clinical and Epidemiological Variables
2.4. Statistical Analysis
2.5. Economic Analysis
2.6. Data Confidentiality and Ethical Aspects
3. Results
3.1. Health Resources Associated with IPD
3.2. Distribution of the Cost of IPD According to Clinical form and Health Resources
3.3. Annual Evolution of the Costs Associated with IPD
3.4. Variation in the Mean Cost of IPD
3.5. Variation in the Proportion of Costs Associated with Serotypes
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- O’Brien, K.L.; Wolfson, L.J.; Watt, J.P.; Henkle, E.; Deloria Knoll, M.; McCall, N.; Lee, E.; Mulholland, K.; Levine, O.S.; Cherian, T.; et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: Global estimates. Lancet 2009, 893–902. [Google Scholar] [CrossRef]
- Wahl, B.; O’Brien, K.L.; Greenbaum, A.; Majumder, A.; Liu, L.; Chu, Y.; Lukšić, I.; Nair, H.; McAllister, D.A.; Campbell, H.; et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: Global, regional, and national estimates for 2000–15. Lancet Glob. Health 2018, 6, e744–e757. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper—February 2019. Wkly. Epidemiol. Rec. 2019, 94, 85–104. [Google Scholar]
- Summary of Prevenar 7 Product Characteristics. European Medicines Agency. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000323/WC500041558.pdf (accessed on 6 July 2020).
- Summary of Synflorix Product Characteristics. European Medicines Agency. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Pro-duct_Information/human/000973/WC500054346.pdf (accessed on 6 July 2020).
- Summary of Prevenar 13 Product Characteristics. European Medicines Agency. Available online: https://www.ema.europa.eu/en/documents/overview/prevenar-epar-summary-public_en.pdf (accessed on 6 July 2020).
- European Centre for Disease Prevention and Control. Invasive Pneumococcal Disease; Annual Epidemiological Report for 2017; ECDC: Solna, Sweden, 2019; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2017-invasive-pneumococcal-disease.pdf (accessed on 6 July 2020).
- Ben-Shimol, S.; Greenberg, D.; Givon-Lavi, N.; Schlesinger, Y.; Somekh, E.; Aviner, S.; Miron, D.; Dagan, R. Early impact of sequential introduction of 7-valent and 13-valent pneumococcal conjugate vaccine on IPD in Israeli children <5 years: An active prospective nationwide surveillance. Vaccine 2014, 32, 3452–3459. [Google Scholar] [CrossRef]
- Fortunato, F.; Martinelli, D.; Cappelli, M.G.; Cozza, V.; Prato, R. Impact of pneumococcal conjugate universal routine vaccination on pneumococcal disease in Italian children. J. Immunol. Res. 2015. [Google Scholar] [CrossRef] [Green Version]
- Griffin, M.R.; Mitchel, E.; Moore, M.R.; Whitney, C.G.; Grijalva, C.G. Declines in pneumonia hospitalizations of children aged <2 years associated with the use of pneumococcal conjugate vaccines—Tennessee, 1998–2012. MWR Morb. Mortal. Wkly Rep. 2014, 63, 995–998. [Google Scholar]
- Lepoutre, A.; Varon, E.; Georges, S.; Dorléans, F.; Janoir, C.; Gutmann, L.; Lévy-Bruhl, D. Impact of the pneumococcal conjugate vaccines on invasive pneumococcal disease in France, 2001-2012. Vaccine 2015, 33, 359–366. [Google Scholar] [CrossRef]
- Guevara, M.; Ezpeleta, C.; Gil-Setas, A.; Torroba, L.; Beristain, X.; Aguinaga, A.; García-Irure, J.J.; Navascués, A.; García-Cenoz, M.; Castilla, J. Reduced incidence of invasive pneumococcal disease after introduction of the 13-valent conjugate vaccine in Navarre, Spain, 2001-2013. Vaccine 2014, 32, 2553–2562. [Google Scholar] [CrossRef]
- Moore, M.R.; Link-Gelles, R.; Schaffner, W.; Lynfield, R.; Lexau, C.; Bennett, N.M.; Petit, S.; Zansky, S.M.; Harrison, L.H.; Reingold, A.; et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: Analysis of multisite, population-based surveillance. Lancet Infect. Dis. 2015, 15, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Picazo, J.; Ruiz-Contreras, J.; Casado-Flores, J.; Negreira, S.; García-de-Miguel, M.J.; Hernández-Sampelayo, T.; Otheo, E.; Méndez, C. Expansion of serotype coverage in the universal pediatric vaccination calendar: Short-term effects on age- and serotype-dependent incidence of invasive pneumococcal clinical presentations in Madrid, Spain. Clin. Vaccine Immunol. 2013, 20, 1524–1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picazo, J.; Ruiz-Contreras, J.; Casado-Flores, J.; Giangaspro, E.; García-de-Miguel, M.J.; Hernández-Sampelayo, T.; Otheo, E.; Méndez, C. Impact of introduction of conjugate vaccines in the vaccination schedule on the incidence of pediatric invasive pneumococcal disease requiring hospitalization in Madrid 2007 to 2011. Pediatr. Infect Dis. J. 2013, 32, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Waight, P.A.; Andrews, N.J.; Ladhani, N.J.; Sheppard, C.L.; Slack, M.P.; Miller, E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: An observational cohort study. Lancet Infect. Dis. 2015, 15, 629. [Google Scholar] [CrossRef] [Green Version]
- van de Vooren, K.; Duranti, S.; Curto, A.; Garattini, L. Cost effectiveness of the new pneumococcal vaccines: A systematic review of European studies. Pharmacoeconomics 2014, 32, 29–45. [Google Scholar] [CrossRef]
- Zhang, S.; Sammon, P.M.; King, I.; Andrade, A.L.; Toscano, C.M.; Araujo, S.N.; Sinha, A.; Madhi, S.A.; Khandaker, G.; Yin, J.K.; et al. Cost of management of severe pneumonia in young children: Systematic analysis. J. Glob. Health 2016, 6, 010408. [Google Scholar] [CrossRef] [PubMed]
- Brotons, P.; Gelabert, G.; Launes, C.; Sicuri, E.; Pallares, R.; Muñoz-Almagro, C. Cost of hospitalizing children with invasive pneumococcal pneumonia. Vaccine 2013, 31, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Baldo, V.; Cocchio, S.; Baldovin, T.; Buja, A.; Furlan, P.; Bertoncello, C.; Russo, F.; Saia, M. A population-based study on the impact of hospitalization for pneumonia in different age groups. BMC Infect Dis. 2014, 14, 485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spoorenberg, S.M.; Bos, W.J.; Heijligenberg, R.; Voorn, P.G.; Grutters, J.C.; Rijkers, G.T.; van de Garde, E.M. Microbial aetiology, outcomes, and costs of hospitalisation for community-acquired pneumonia; an observational analysis. BMC Infect. Dis. 2014, 14, 335. [Google Scholar] [CrossRef] [Green Version]
- Song, J.Y.; Choi, J.Y.; Lee, J.S.; Bae, I.G.; Kim, Y.K.; Sohn, J.W.; Jo, Y.M.; Choi, W.S.; Lee, J.; Park, K.H.; et al. Clinical and economic burden of invasive pneumococcal disease in adults: A multicenter hospital-based study. BMC Infect Dis. 2013, 13, 202. [Google Scholar] [CrossRef] [Green Version]
- Calderón, C.; Dennis, R. Economic cost of Streptococcus pneumoniae community-acquired pneumonia, meningitis and bacteremia in an adult population that required hospitalization in Bogotá, Colombia. Biomedica 2014, 34, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Registre del Conjunt Mínim Bàsic de Dades (CMBD) dels Hospital D’aguts. Servei Català de la Salut. 2016. Available online: http://catsalut.gencat.cat/ca/proveidors-professionals/registres-catalegs/registres/cmbd/informes-anuals/ (accessed on 6 July 2020).
- Tarragó, D.; Fenoll, A.; Sánchez-Tatay, D.; Arroyo, L.A.; Muñoz-Almagro, C.; Esteva, C.; Hausdorff, W.P.; Casal, J.; Obando, I. Identification of pneumococcal serotypes from culture-negative clinical specimens by novel real-time PCR. Clin. Microbiol. Infect. 2008, 14, 828–834. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Neisseria Meningitidis, Streptococcus Pneumoniae, Diagnosis of Meningitis Caused by Laboratory Methods for the and Haemophilus Influenzae: WHO Manual, 2nd ed.; World Health Organization: Geneva, Switzerland, 2019; Available online: http://apps.who.int/iris/bitstream/handle/10665/70765/WHO_IVB_11.09_eng.pdf?sequence=1 (accessed on 6 July 2020).
- Fenoll, A.; Jado, I.; Vicioso, D.; Casal, J. Dot blot assay for the serotyping of pneumococci. J. Clin. Microbiol. 1997, 35, 764–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selva, L.; Berger, C.; Garcia-Garcia, J.J.; de Paz, H.; Nadal, D.; Muñoz-Almagro, C. Direct identification of Streptococcus pneumoniae capsular types in pleural fluids by using multiplex PCR combined with automated fluorescence-based capillary electrophoresis. J. Clin. Microbiol. 2014, 52, 2736–2737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DECRET 170/2010, de 16 de Novembre, de Regulació del Sistema de Pagament dels Convenis i Contractes de Gestió de Serveis Assistencials en l’àmbit del Servei Català de la Salut. Available online: https://portaljuridic.gencat.cat/eli/es-ct/d/2010/11/16/170 (accessed on 6 July 2020).
- ORDRE SLT/79/2014, de 12 de Març, per la Qual es Determinen per a L’any 2014 els Preus Unitaris i la Resta de Valors a Què es Refereix L’article 5 del Decret 170/2010, de 16 de Novembre, de Regulació del Sistema de Pagament de Serveis Sanitaris en L’àmbit del Servei Català de la Salut. Available online: https://portaljuridic.gencat.cat/eli/es-ct/o/2014/03/12/slt79 (accessed on 6 July 2020).
- Navas, E.; Torner, N.; Broner, S.; Godoy, P.; Martínez, A.; Bartolomé, R.; Domínguez, A.; Working Group for the Study of Outbreaks of Acute Gastroenteritis in Catalonia (Spain). Economic costs of outbreaks of acute viral gastroenteritis due to norovirus in Catalonia (Spain), 2010–2011. BMC Public Health 2015, 15, 999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ordre SLT/99/2013, de 24 de Maig, per la Qual S’estableixen per a L’any 2013 les Tarifes Màximes dels Serveis de Transport Sanitari no Urgent que Convingui o Contracti el Servei Català de la Salut. Available online: https://portaljuridic.gencat.cat/eli/es-ct/o/2013/05/24/slt99 (accessed on 6 July 2020).
- ORDRE SLT/78/2014, de 12 de Març, per la qual es Prorroguen per a L’any 2014 les Tarifes Màximes Corresponents a la Prestació i Concertació de Determinats Serveis Sanitaris. Available online: https://portaljuridic.gencat.cat/eli/es-ct/o/2014/03/12/slt78 (accessed on 6 July 2020).
- Ceyhan, M.; Ozsurekci, Y.; Aykac, K.; Hacibedel, B.; Ozbilgili, E. Economic burden of pneumococcal infections in children under 5 years of age. Hum. Vaccin Immunother. 2018, 14, 106–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández, S.; Muñoz-Almagro, C.; Ciruela, P.; Soldevila, N.; Izquierdo, C.; Codina, M.G.; Díaz, A.; Moraga-Llop, F.; García-García, J.J.; Domínguez, Á. Invasive pneumococcal disease and influenza activity in a pediatric population: Impact of PCV13 vaccination in pandemic and nonpandemic influenza periods. J. Clin. Microbiol. 2019, 57, e00363-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagos, R.; Muñoz, A.; Espinoza, A.; Dowes, A.; Ruttimann, R.; Colindres, R.; Levine, M.M. Direct medical costs of invasive pneumococcal disease and radiologically-diagnosed pneumonia among Chilean children. Rev. Panam Salud Pública 2009, 26, 101–111. [Google Scholar] [CrossRef] [Green Version]
- Ricketson, L.J.; Conradi, N.G.; Vanderkooi, O.G.; Kellner, J.D. Changes in the nature and severity of invasive pneumococcal disease in children before and after the seven-valent and thirteen-valent pneumococcal conjugate vaccine programs in Calgary, Canada. Pediatr. Infect Dis. J. 2018, 37, 22–27. [Google Scholar] [CrossRef]
- Ciruela, P.; Izquierdo, C.; Broner, S.; Muñoz-Almagro, C.; Hernández, S.; Ardanuy, C.; Pallarés, R.; Domínguez, A.; Jané, M.; Catalan Working Group on Invasive Pneumococcal Disease. The changing epidemiology of invasive pneumococcal disease after PCV13 vaccination in a country with intermediate vaccination coverage. Vaccine 2018, 36, 7744–7752. [Google Scholar] [CrossRef]
- Muñoz-Almagro, C.; Jordan, I.; Gene, A.; Latorre, C.; Garcia-Garcia, J.J.; Pallares, R. Emergence of invasive pneumococcal disease caused by nonvaccine serotypes in the era of 7-valent conjugate vaccine. Clin. Infect. Dis. 2008, 46, 174–182. [Google Scholar] [CrossRef] [Green Version]
- Feikin, D.R.; Kagucia, E.W.; Loo, J.D.; Link-Gelles, R.; Puhan, M.A.; Cherian, T.; Levine, O.S.; Whitney, C.G.; O’Brien, K.L.; Moore, M.R.; et al. Serotype-specific changes in invasive pneumococcal disease after pneumococcal conjugate vaccine introduction: A pooled analysis of multiple surveillance sites. PLoS Med. 2013, 10, e1001517. [Google Scholar] [CrossRef] [Green Version]
- Goettler, D.; Streng, A.; Kemmling, D.; Schoen, C.; von Kries, R.; Rose, M.A.; van der Linden MLiese, J.G. Increase in Streptococcus pneumoniae serotype 3 associated parapneumonic pleural effusion/empyema after the introduction of PCV13 in Germany. Vaccine 2020, 38, 570–577. [Google Scholar] [CrossRef]
- Domínguez, Á.; Ciruela, P.; Hernández, S.; García-García, J.J.; Soldevila, N.; Izquierdo, C.; Moraga-Llop, F.; Díaz, A.; F de Sevilla, M.; González-Peris, S.; et al. Effectiveness of the 13-valent pneumococcal conjugate vaccine in preventing invasive pneumococcal disease in children aged 7-59 months. A matched case-control study. PLoS ONE 2017, 12, e0183191. [Google Scholar] [CrossRef] [PubMed]
- Hernández, S.; Moraga-Llop, F.; Díaz, A.; de Sevilla, M.F.; Ciruela, P.; Muñoz-Almagro, C.; Codina, G.; Campins, M.; García-García, J.J.; Esteva, C.; et al. Failures of 13-valent conjugated pneumococcal vaccine in age-appropriately vaccinated children 2–59 months of age, Spain. Emerg. Infect. Dis. 2020, 26, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Johnson, K.M.; Ray, G.T.; Wroe, P.; Lieu, T.A.; Moore, M.R.; Zell, E.R.; Linder, J.A.; Grijalva, C.G.; Metlay, J.P.; et al. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine 2011, 29, 3398–3412. [Google Scholar] [CrossRef] [Green Version]
- Savulescu, C.; Krizova, P.; Lepoutre, A.; Mereckiene, J.; Vestrheim, D.F.; Ciruela, P.; Ordobas, M.; Guevara, M.; McDonald, E.; Morfeldt, E.; et al. Effect of high-valency pneumococcal conjugate vaccines on invasive pneumococcal disease in children in SpIDnet countries: An observational multicentre study. Lancet Respir. Med. 2017, 5, 648–656. [Google Scholar] [CrossRef] [Green Version]
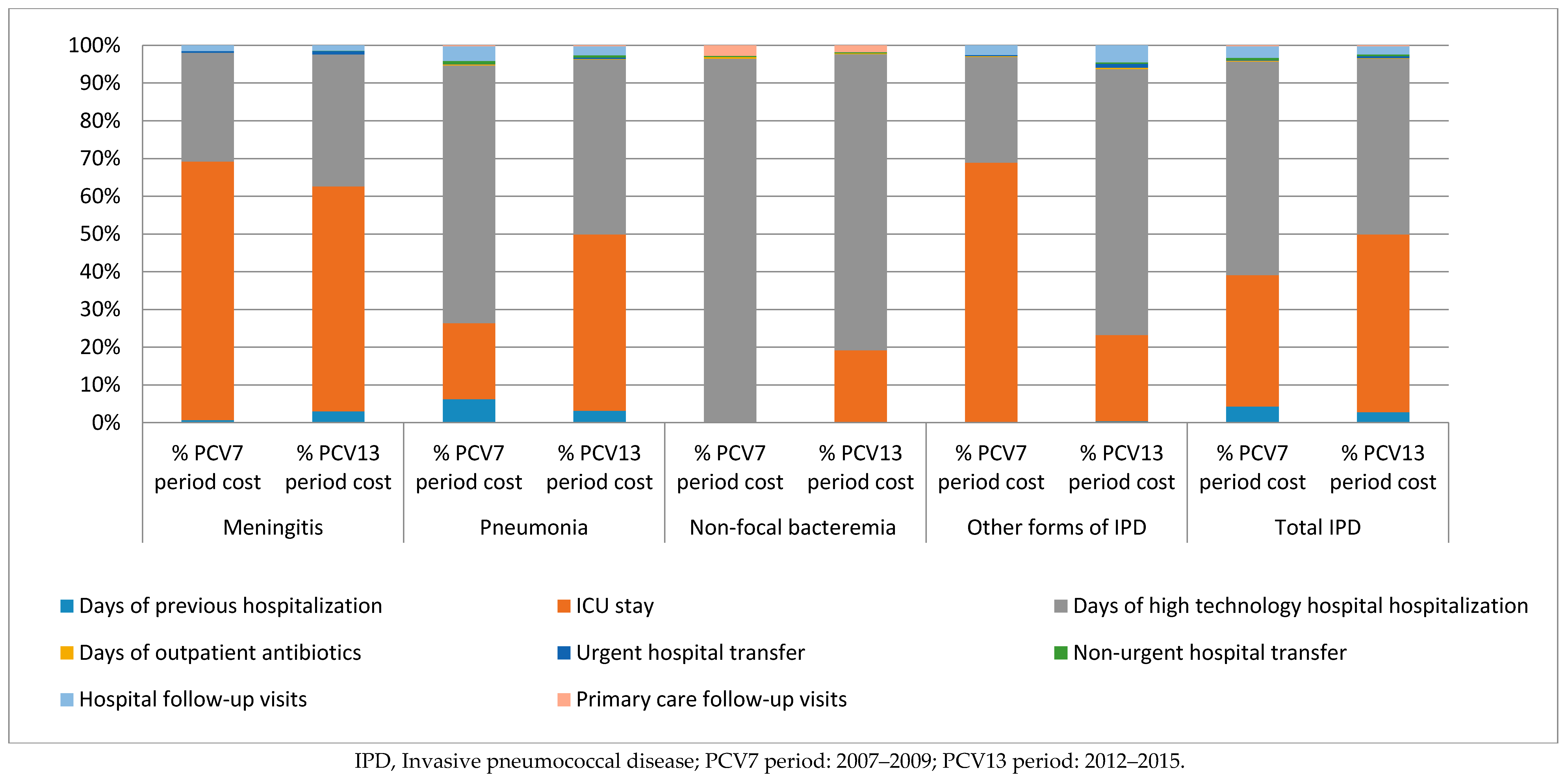


| Variable | PCV7 Period 1 (319 Cases) | PCV13 Period 2 (154 Cases) | p Value |
|---|---|---|---|
| Sex | |||
| Female | 148 (46.4%) | 58 (37.7%) | 0.073 |
| Male | 171 (53.6%) | 96 (62.3%) | |
| Mean age (SD) | 29.6 (15.7) | 26.7 (16.0) | 0.057 |
| Clinical presentation | |||
| Meningitis | 29 (9.1%) | 18 (11.7%) | 0.273 |
| Septic shock | 3 (0.3%) | 4 (2.6%) | 0.222 |
| Pneumonia | 254 (79.6%) | 104 (67.5%) | 0.004 |
| Non-focal bacteremia | 25 (7.8%) | 16 (10.4%) | 0.355 |
| Osteoarticular infection | 6 (1.9%) | 7 (4.5%) | 0.097 |
| Mastoiditis | - | 5 (3.2%) | - |
| Orbital cellulitis | 2 (0.6%) | - | - |
| ICU admission | 44 (13.8%) | 37 (24.0%) | 0.006 |
| Median ICU stay (SD) | 0.8 (3.1) | 1.6 (5.5) | 0.086 |
| Hospital transfer | 77 (24.1%) | 52 (33.8%) | 0.028 |
| Urgent transfer | 11 (3.4%) | 20 (13.0%) | >0.001 |
| Non-urgent transfer | 66 (20.7%) | 32 (20.8%) | 0.982 |
| Mean days of previous hospitalization (SD) | 0.8 (2.0) | 0.7 (2.2) | 0.587 |
| Mean days of high-tech hospital hospitalization (SD) | 9.8 (6.3) | 10.6 (7.5) | 0.226 |
| Outpatient treatment | 265 (83.1%) | 124 (80.5%) | 0.496 |
| Follow up visits | 0.263 | ||
| Primary care | 72 (22.6%) | 42 (27.3%) | |
| Specialist | 247 (77.4%) | 112 (72.7%) |
| Year (Cases) | 2007 (88) | 2008 (98) | 2009 (133) | 2012 (45) | 2013 (34) | 2014 (35) | 2015 (40) |
|---|---|---|---|---|---|---|---|
| Days of previous hospitalization | €16,750.4 | €12,723.8 | €11,596.4 | €5959.3 | €4026.5 | €805.3 | €6281.4 |
| ICU stay | €58,500.0 | €67,600.0 | €209,300.0 | €75,400.0 | €93,600.0 | €35,100.0 | €122,200.0 |
| Days of high technology hospital hospitalization | €160,566.3 | €176,936.4 | €207,238.5 | €80,109.0 | €87,597.5 | €54,334.8 | €62,345.7 |
| Days of outpatient antibiotics | €493.1 | €621.5 | €809.3 | €328.7 | €171.9 | €190.2 | €218.6 |
| Urgent hospital transfer | €327.0 | €490.6 | €981.1 | €654.1 | €981.1 | €817.6 | €817.6 |
| Non-urgent hospital transfer | €1635.2 | €1880.5 | €1880.5 | €899.4 | €735.8 | €327.0 | €654.1 |
| Hospital follow-up visits | €7830.0 | €10,461.3 | €11,359.9 | €4043.3 | €3209.0 | €2374.7 | €3979.2 |
| Primary care follow-up visits | €660.0 | €390.0 | €1110.0 | €330.0 | €270.0 | €420.0 | €240.0 |
| Total | €246,761.9 | €271,104.2 | €444,275.6 | €167,723.8 | €190,591.8 | €94,369.6 | €196,736.5 |
| Total per patient | €2804.1 | €2766.4 | €3340.4 | €3728.0 | €5605.6 | €2696.3 | €4981.4 |
| Health Resources | Meningitis | Pneumonia | Non-Focal Bacteremia | Other Focal IPD | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCV7 Period | PCV13 Period | Percent | PCV7 Period | PCV13 Period | % | PCV7 Period | PCV13 Period | Percent | PCV7 Period | PCV13 Period | Percent | PCV7 Period | PCV13 Period | Percent | |
| Days of previous hospitalization (SD) | €61.1 (174.4) | €268.4 (665.2) | 339.4% | €154.7 (356.9) | €116.2 (312.9) | −24.9% | €0.0 (0.0) | €0.0 (0.0) | - | €0.0 (0.0) | €10.1 (40.3) | - | €128.7 (329.5) | €110.9 (347.2) | −13.9% |
| ICU stay (SD) | €5872.4 (7766.0) | €5272.2 (4356.8) | −10.2% | €501.6 (2915.1) | €1700.0 (8335.9) | 238.9% | €0.0 (0.0) | €325.0 (1300.0) | - | €3427.3 (7674.9) | €650.0 (1424.1) | −81.0% | €1051.4 (4070.2) | €1865.6 (7140.4) | 77.4% |
| Days of high-tech hospital hospitalization (SD) | €2474.1 (1871.2) | €3096.0 (1882.8) | 25.1% | €1699.7 (976.8) | €1686.2 (935.9) | −0.8% | €1037.9 (644.7) | €1327.9 (1656.9) | 27.9% | €1393.2 (678.9) | €2002.7 (1500.8) | 43.7% | €1707.6 (1098.0) | €1846.7 (1305.9) | 8.1% |
| Days of outpatient antibiotics (SD) | €0.7 (2.3) | €0.7 (2.4) | 3.1% | €6.6 (3.8) | €6.5 (4.9) | −0.5% | €4.9 (3.9) | €4.9 (2.7) | −0.7% | €9.8 (9.8) | €10.1 (6.7) | 3.4% | €6.0 (4.4) | €5.9 (5.3) | 0.6% |
| Urgent hospital transfer (SD) | €39.5 (71.2) | €81.8 (84.1) | 107.1% | €1.9 (17.7) | €12.6 (43.8) | 551.3% | €0.0 (0.0) | €0.0 (0.0) | - | €14.9 (49.3) | €30.7 (65.9) | 106.2% | €5.6 (29.9) | €21.2 (55.1) | 276.6% |
| Non-urgent hospital transfer (SD) | €0.0 (0.0) | €4.5 (19.3) | - | €20.9 (35.7) | €22.0 (36.4) | 5.2% | €3.3 (16.3) | €5.1 (20.4) | 56.2% | €0.0 (0.0) | €10.2 (27.9) | - | €16.9 (33.2) | €17.0 (33.3) | 0.4% |
| Hospital follow-up visits (SD) | €128.4 (0.0) | €128.4 (0.0) | 0.0% | €96.5 (50.6) | €88.9 (55.3) | −7.9% | €0.0 (0.0) | €0.0 (0.0) | - | €128.4 (0.0) | €128.4 (0.0) | 0.0% | €92.9 (53.7) | €88.3 (56.9) | −4.9% |
| Primary care follow-up visits (SD) | €0.00 (0.0) | €0.0 (0.0) | - | €5.6 (11.7) | €7.5 (13.0) | 35.1% | €30.0 (0.0) | €30.0 (0.0) | 0.0% | €0.0 (0.0) | €0.0 (0.0) | - | €6.8 (12.6) | €8.2 (13.4) | 20.8% |
| Total (SD) | €8576.5 (9114,1) | €8852.0 (5135,7) | 3.2% | €2487.5 (3118.6) | €3639.9 (8522.3) | 46.3% | €1076.1 (648.9) | €1692.9 (2043.7) | 57.3% | €4973.5 (7540.3) | €2842.1 (2256.6) | −42.8% | €3016.1 (4510.2) | €3963.9 (7493.6) | 31.4% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, S.; Navas, E.; Aznar-Lou, I.; Ciruela, P.; García-García, J.J.; Moraga-Llop, F.; Muñoz-Almagro, C.; Codina, G.; F. de Sevilla, M.; González-Peris, S.; et al. Impact of the 13-Valent Conjugated Pneumococcal Vaccine on the Direct Costs of Invasive Pneumococcal Disease Requiring Hospital Admission in Children Aged < 5 Years: A Prospective Study. Vaccines 2020, 8, 387. https://doi.org/10.3390/vaccines8030387
Hernández S, Navas E, Aznar-Lou I, Ciruela P, García-García JJ, Moraga-Llop F, Muñoz-Almagro C, Codina G, F. de Sevilla M, González-Peris S, et al. Impact of the 13-Valent Conjugated Pneumococcal Vaccine on the Direct Costs of Invasive Pneumococcal Disease Requiring Hospital Admission in Children Aged < 5 Years: A Prospective Study. Vaccines. 2020; 8(3):387. https://doi.org/10.3390/vaccines8030387
Chicago/Turabian StyleHernández, Sergi, Encarna Navas, Ignacio Aznar-Lou, Pilar Ciruela, Juan José García-García, Fernando Moraga-Llop, Carmen Muñoz-Almagro, Gemma Codina, Mariona F. de Sevilla, Sebastià González-Peris, and et al. 2020. "Impact of the 13-Valent Conjugated Pneumococcal Vaccine on the Direct Costs of Invasive Pneumococcal Disease Requiring Hospital Admission in Children Aged < 5 Years: A Prospective Study" Vaccines 8, no. 3: 387. https://doi.org/10.3390/vaccines8030387
APA StyleHernández, S., Navas, E., Aznar-Lou, I., Ciruela, P., García-García, J. J., Moraga-Llop, F., Muñoz-Almagro, C., Codina, G., F. de Sevilla, M., González-Peris, S., Esteva, C., Planes, A. M., Izquierdo, C., Martínez-Osorio, J., Campins, M., Uriona, S., Salleras, L., Serrano-Blanco, A., Jané, M., & Domínguez, Á. (2020). Impact of the 13-Valent Conjugated Pneumococcal Vaccine on the Direct Costs of Invasive Pneumococcal Disease Requiring Hospital Admission in Children Aged < 5 Years: A Prospective Study. Vaccines, 8(3), 387. https://doi.org/10.3390/vaccines8030387







