Enhanced Immunogenicity of Engineered HER2 Antigens Potentiates Antitumor Immune Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Human Samples
2.3. Tumor Cell Lines
2.4. Reagent and Antibodies
2.5. Generation of Modified HER2 Antigens and Adenoviral Vectors
2.6. Preparation of BVAC
2.7. Titration of HER2-Specific Antibodies
2.8. In Vivo and In Vitro Cytotoxicity Assay
2.9. Immunospot Analysis
2.10. Statistics
3. Results
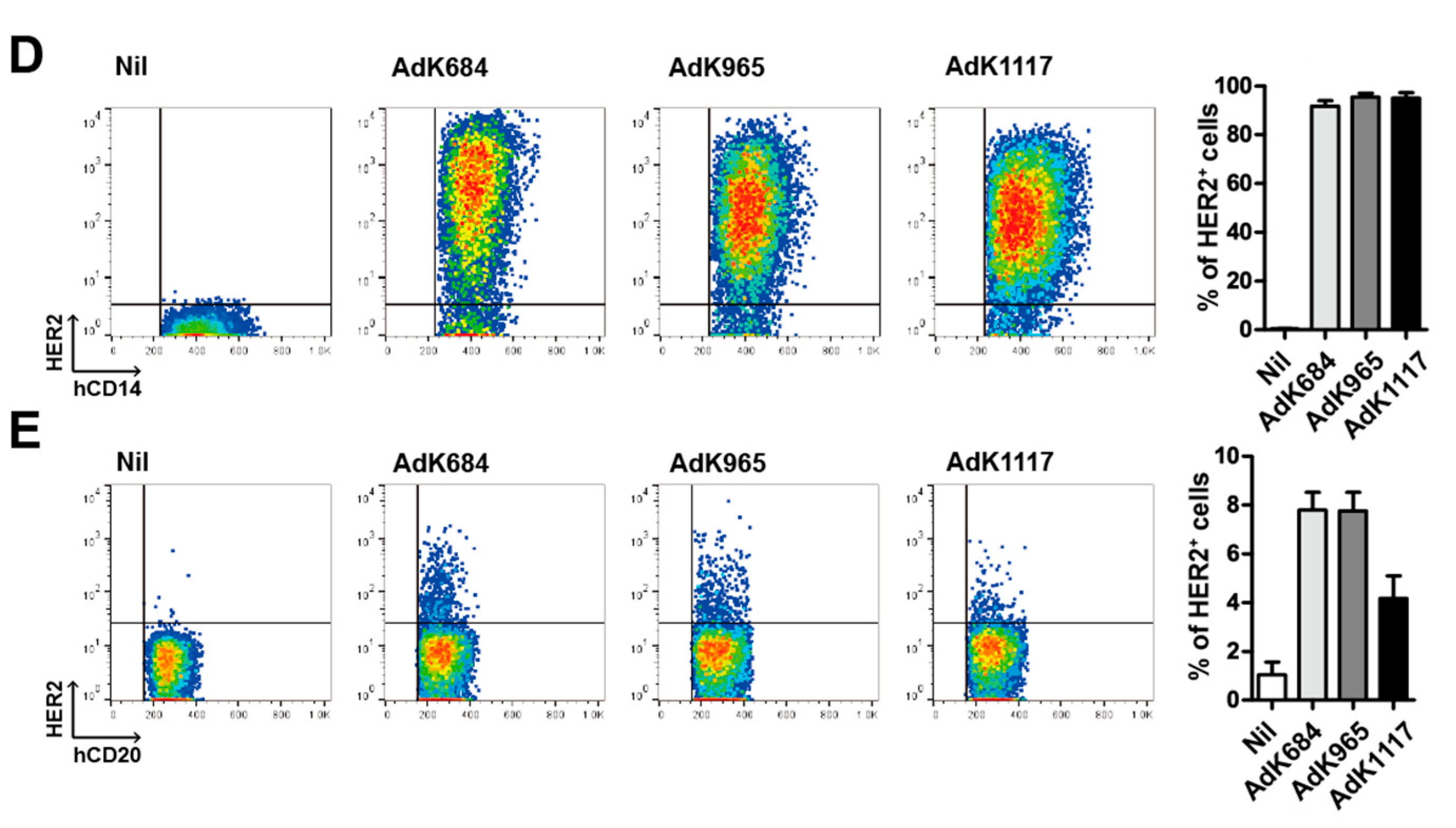
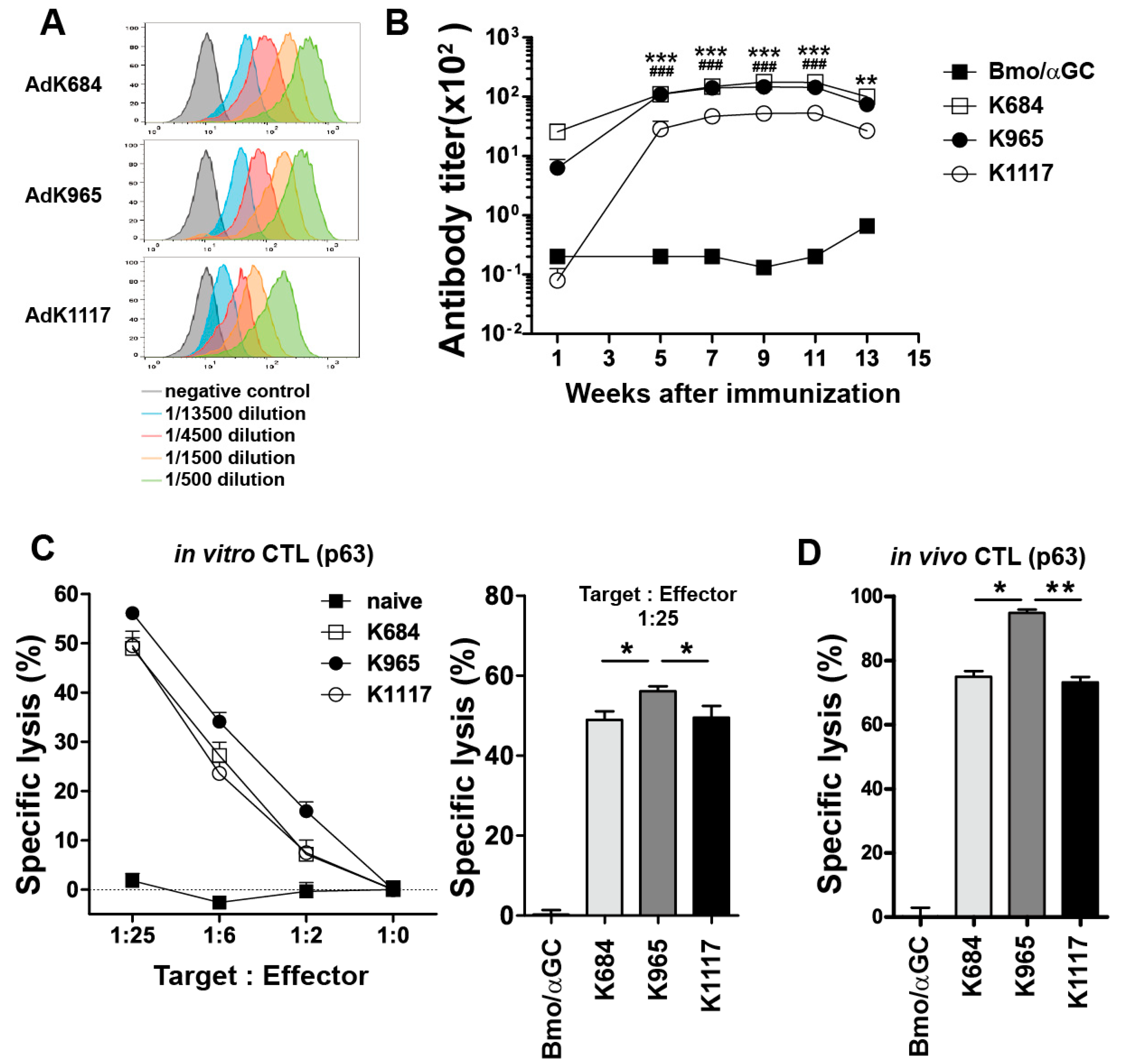
3.1. Development of Adenoviral Vectors Coding for Engineered HER2 Antigens
3.2. Immunization with the Engineered Antigens Elicits HER2-Specific Humoral and Cellular Immune Responses
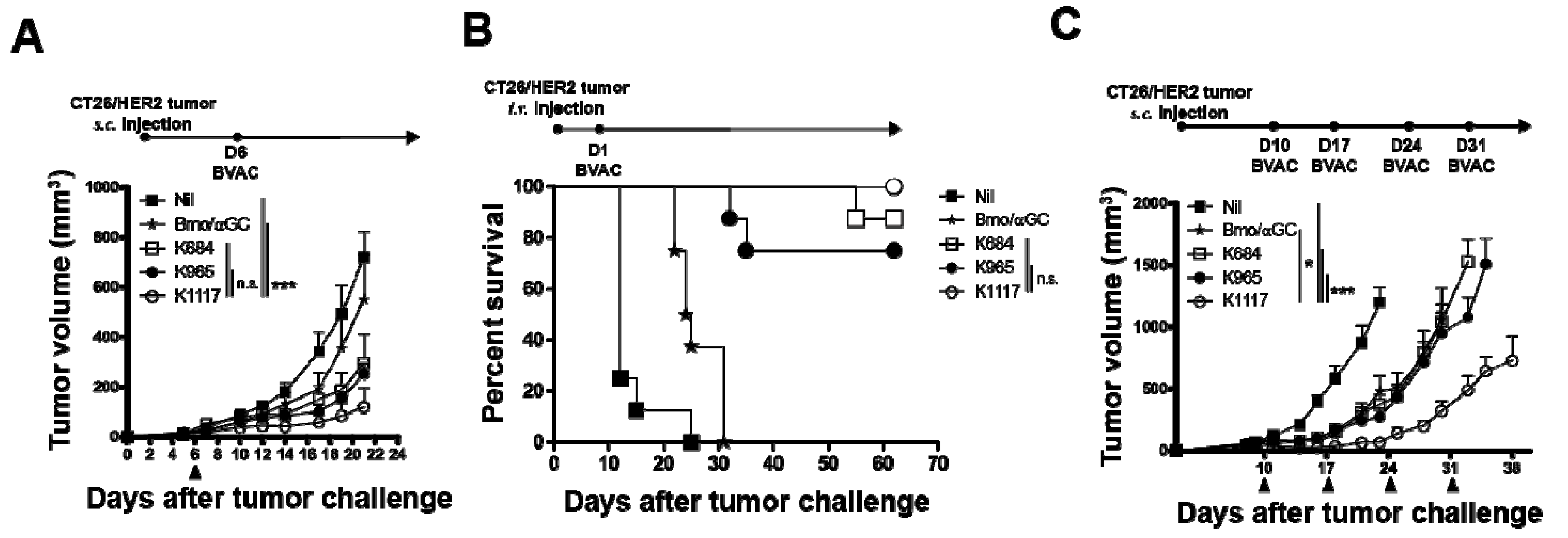
3.3. BVAC with the K1117 Antigen Efficiently Inhibits the Growth of HER2-Expressing Tumors
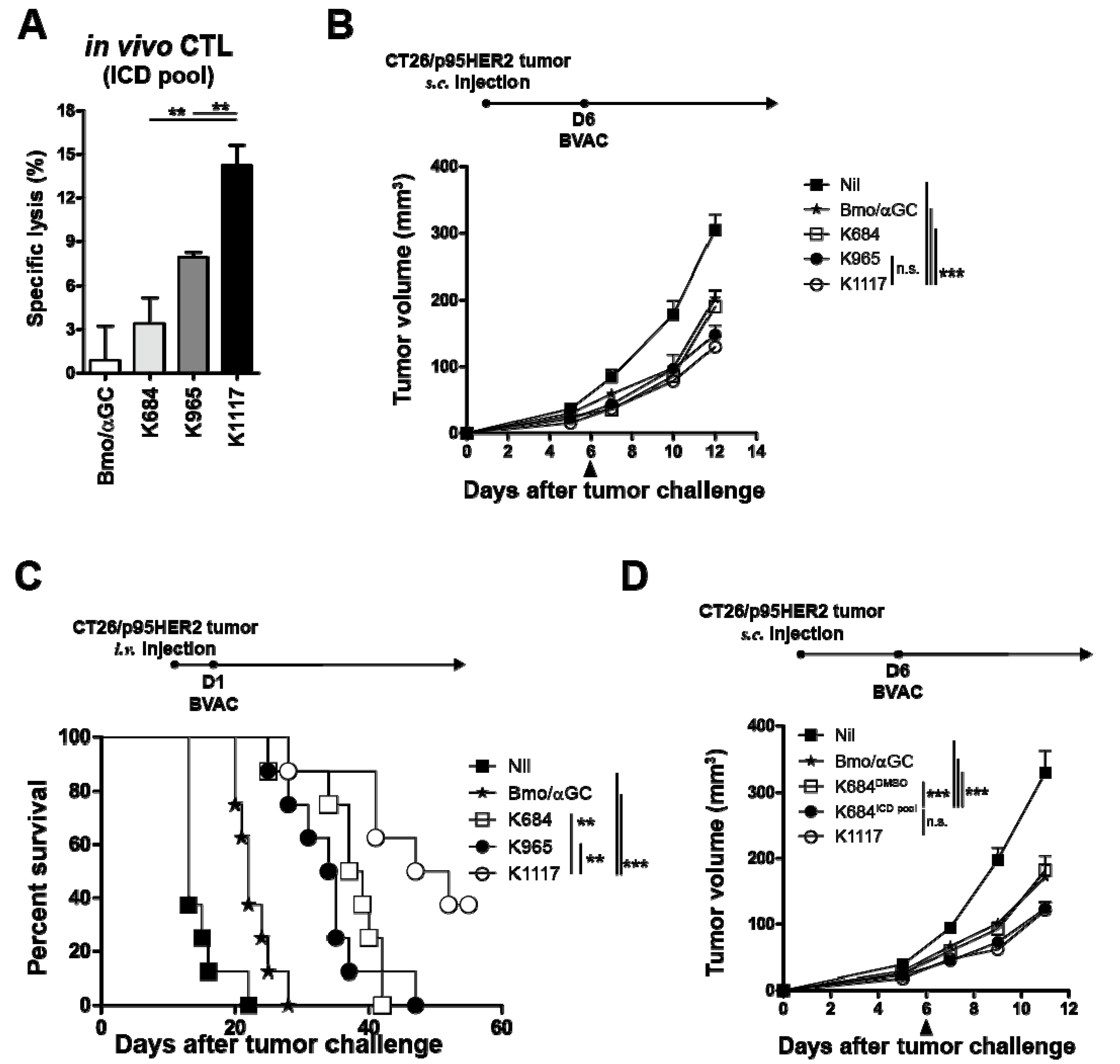
3.4. BVAC-K1117 Induces Antigen-Specific Cytotoxic Responses Against ICD of HER2
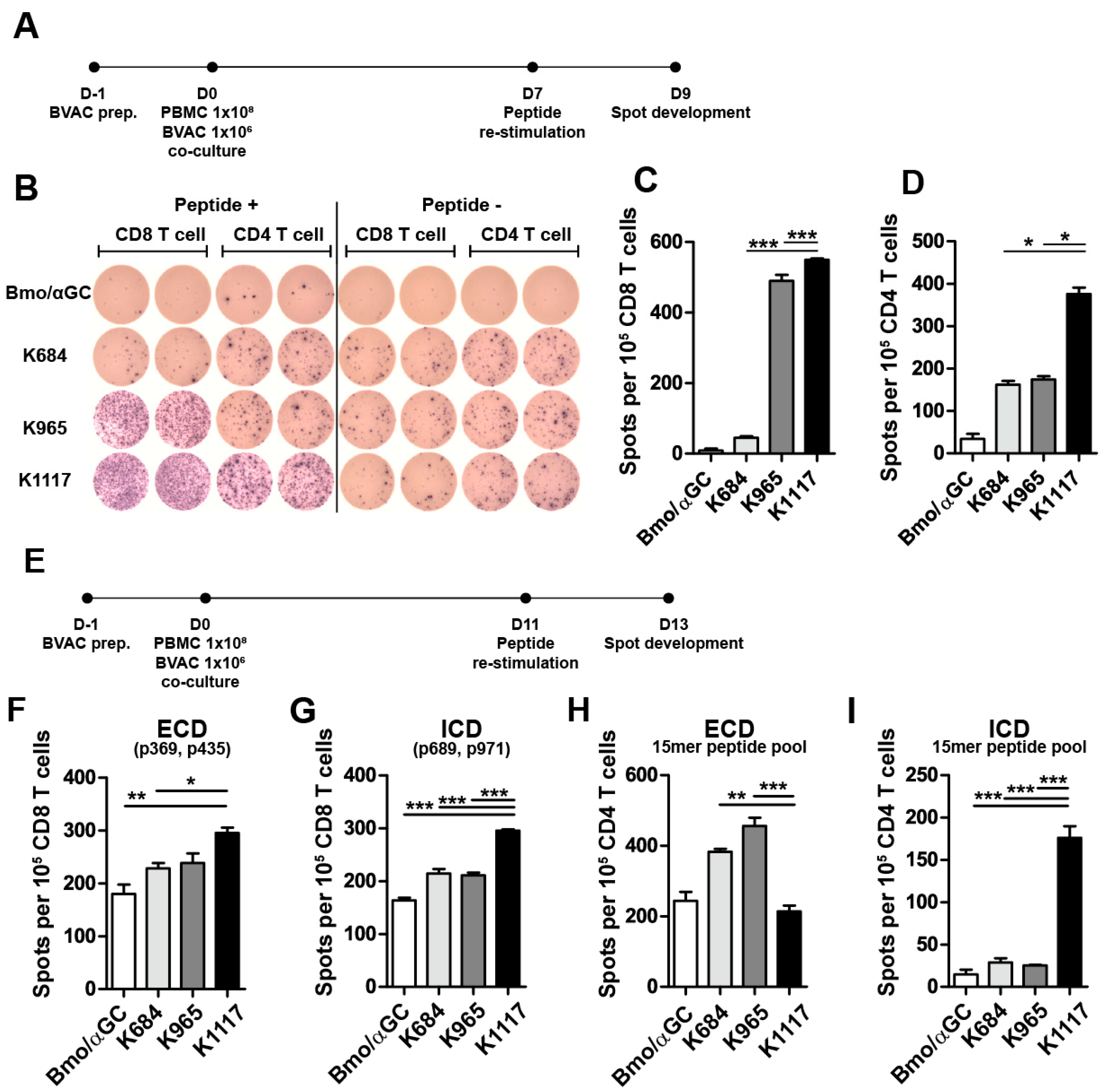
3.5. Engineered HER2 Antigens Enhance HER2-Specific T Cell Responses in Human PBMC Models
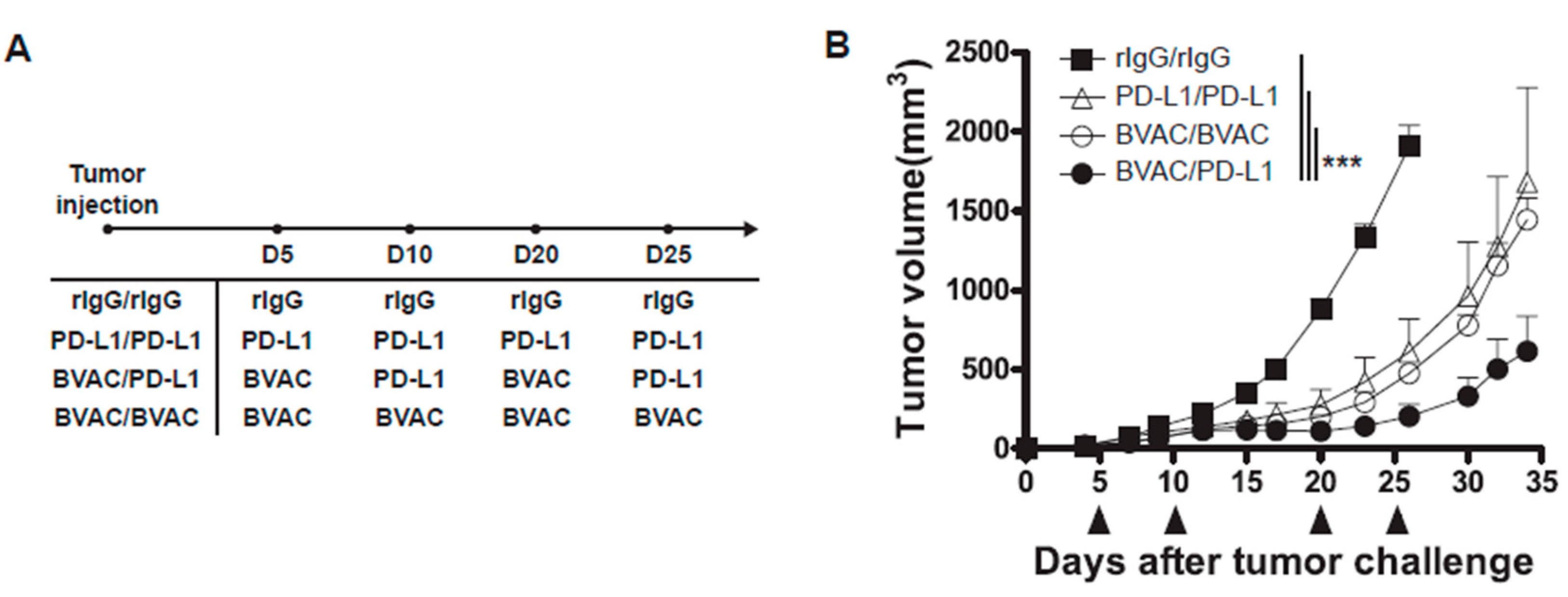
3.6. The Combination of Anti-PD-L1 Therapy with BVAC-K1117 Augments Antitumor Immunity in Murine Tumor Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neller, M.A.; Lopez, J.A.; Schmidt, C.W. Antigens for cancer immunotherapy. Semin. Immunol. 2008, 20, 286–295. [Google Scholar] [CrossRef] [PubMed]
- van der Burg, S.H.; Arens, R.; Ossendorp, F.; van Hall, T.; Melief, C.J. Vaccines for established cancer: Overcoming the challenges posed by immune evasion. Nat. Rev. Cancer 2016, 16, 219. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.M.; Butterfield, L.H. Dendritic cell–based cancer vaccines. J. Immunol. 2018, 200, 443–449. [Google Scholar] [CrossRef]
- Palucka, K.; Banchereau, J. Dendritic-cell-based therapeutic cancer vaccines. Immunity 2013, 39, 38–48. [Google Scholar] [CrossRef]
- Tagliamonte, M.; Petrizzo, A.; Tornesello, M.L.; Buonaguro, F.M.; Buonaguro, L. Antigen-specific vaccines for cancer treatment. Hum. Vaccines Immunother. 2014, 10, 3332–3346. [Google Scholar] [CrossRef] [PubMed]
- Schultze, J.L.; Grabbe, S.; von Bergwelt-Baildon, M.S. DCs and CD40-activated B cells: Current and future avenues to cellular cancer immunotherapy. Trends Immunol. 2004, 25, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Kim, B.S.; Kim, Y.J.; Ko, H.J.; Ko, S.Y.; Kim, D.H.; Kang, C.Y. CD1d-restricted T cells license B cells to generate long-lasting cytotoxic antitumor immunity in vivo. Cancer Res. 2006, 66, 6843–6850. [Google Scholar] [CrossRef]
- Kim, Y.J.; Ko, H.J.; Kim, Y.S.; Kim, D.H.; Kang, S.; Kim, J.M.; Chung, Y.; Kang, C.Y. Alpha-Galactosylceramide-loaded, antigen-expressing B cells prime a wide spectrum of antitumor immunity. Int. J. Cancer 2008, 122, 2774–2783. [Google Scholar] [CrossRef]
- Ko, H.J.; Lee, J.M.; Kim, Y.J.; Kim, Y.S.; Lee, K.A.; Kang, C.Y. Immunosuppressive myeloid-derived suppressor cells can be converted into immunogenic APCs with the help of activated NKT cells: An alternative cell-based antitumor vaccine. J. Immunol. 2009, 182, 1818–1828. [Google Scholar] [CrossRef]
- Kim, Y.J.; Han, S.H.; Kang, H.W.; Lee, J.M.; Kim, Y.S.; Seo, J.H.; Seong, Y.K.; Ko, H.J.; Choi, T.H.; Moon, C.; et al. NKT ligand-loaded, antigen-expressing B cells function as long-lasting antigen presenting cells in vivo. Cell. Immunol. 2011, 270, 135–144. [Google Scholar] [CrossRef]
- Kim, E.K.; Seo, H.S.; Chae, M.J.; Jeon, I.S.; Song, B.Y.; Park, Y.J.; Ahn, H.M.; Yun, C.O.; Kang, C.Y. Enhanced antitumor immunotherapeutic effect of B-cell-based vaccine transduced with modified adenoviral vector containing type 35 fiber structures. Gene Ther. 2014, 21, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Iwahashi, M.; Nakamori, M.; Ueda, K.; Ojima, T.; Naka, T.; Ishida, K.; Yamaue, H. Dendritic cells transduced with tumor-associated antigen gene elicit potent therapeutic antitumor immunity: Comparison with immunodominant peptide-pulsed DCs. Oncology 2005, 68, 163–170. [Google Scholar] [CrossRef]
- Yuan, J.; Latouche, J.-B.; Reagan, J.L.; Heller, G.; Riviere, I.; Sadelain, M.; Young, J.W. Langerhans cells derived from genetically modified human CD34+ hemopoietic progenitors are more potent than peptide-pulsed Langerhans cells for inducing antigen-specific CD8+ cytolytic T lymphocyte responses. J. Immunol. 2005, 174, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Van Nuffel, A.M.; Benteyn, D.; Wilgenhof, S.; Pierret, L.; Corthals, J.; Heirman, C.; Van Der Bruggen, P.; Coulie, P.G.; Neyns, B.; Thielemans, K. Dendritic cells loaded with mRNA encoding full-length tumor antigens prime CD4+ and CD8+ T cells in melanoma patients. Mol. Ther. 2012, 20, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Nezafat, N.; Sadraeian, M.; Rahbar, M.R.; Khoshnoud, M.J.; Mohkam, M.; Gholami, A.; Banihashemi, M.; Ghasemi, Y. Production of a novel multi-epitope peptide vaccine for cancer immunotherapy in TC-1 tumor-bearing mice. Biologicals 2015, 43, 11–17. [Google Scholar] [CrossRef]
- Jiang, P.; Cai, Y.; Chen, J.; Ye, X.; Mao, S.; Zhu, S.; Xue, X.; Chen, S.; Zhang, L. Evaluation of tandem Chlamydia trachomatis MOMP multi-epitopes vaccine in BALB/c mice model. Vaccine 2017, 35, 3096–3103. [Google Scholar] [CrossRef]
- Cheever, M.A.; Allison, J.P.; Ferris, A.S.; Finn, O.J.; Hastings, B.M.; Hecht, T.T.; Mellman, I.; Prindiville, S.A.; Viner, J.L.; Weiner, L.M.; et al. The prioritization of cancer antigens: A national cancer institute pilot project for the acceleration of translational research. Clin. Cancer Res. 2009, 15, 5323–5337. [Google Scholar] [CrossRef]
- Aurisicchio, L.; Peruzzi, D.; Koo, G.; Wei, W.-Z.; La Monica, N.; Ciliberto, G. Immunogenicity and therapeutic efficacy of a dual-component genetic cancer vaccine cotargeting carcinoembryonic antigen and HER2/neu in preclinical models. Hum. Gene Ther. 2014, 25, 121–131. [Google Scholar] [CrossRef]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef]
- Pedersen, K.; Angelini, P.D.; Laos, S.; Bach-Faig, A.; Cunningham, M.P.; Ferrer-Ramon, C.; Luque-Garcia, A.; Garcia-Castillo, J.; Parra-Palau, J.L.; Scaltriti, M.; et al. A naturally occurring HER2 carboxy-terminal fragment promotes mammary tumor growth and metastasis. Mol. Cell Biol. 2009, 29, 3319–3331. [Google Scholar] [CrossRef]
- Arribas, J.; Baselga, J.; Pedersen, K.; Parra-Palau, J.L. p95HER2 and breast cancer. Cancer Res. 2011, 71, 1515–1519. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.A.; Sáez, R.; Ramsey, E.E.; Garcia-Barchino, M.-J.; Rojo, F.; Evans, A.J.; Albanell, J.; Keenan, E.J.; Lluch, A.; García-Conde, J.; et al. NH2-terminal Truncated HER-2 Protein but not Full-Length Receptor Is Associated with Nodal Metastasis in Human Breast Cancer. Clin. Cancer Res. 2002, 8, 347–353. [Google Scholar] [PubMed]
- Parra-Palau, J.L.; Morancho, B.; Peg, V.; Escorihuela, M.; Scaltriti, M.; Vicario, R.; Zacarias-Fluck, M.; Pedersen, K.; Pandiella, A.; Nuciforo, P.; et al. Effect of p95HER2/611CTF on the response to trastuzumab and chemotherapy. J. Natl Cancer Inst. 2014, 106, dju291. [Google Scholar] [CrossRef] [PubMed]
- Parra-Palau, J.L.; Pedersen, K.; Peg, V.; Scaltriti, M.; Angelini, P.D.; Escorihuela, M.; Mancilla, S.; Pla, A.S.; y Cajal, S.R.; Baselga, J. A major role of p95/611-CTF, a carboxy-terminal fragment of HER2, in the down-modulation of the estrogen receptor in HER2-positive breast cancers. Cancer Res. 2010, 70, 8537–8546. [Google Scholar] [CrossRef]
- Sperinde, J.; Jin, X.; Banerjee, J.; Penuel, E.; Saha, A.; Diedrich, G.; Huang, W.; Leitzel, K.; Weidler, J.; Ali, S.M. Quantitation of p95HER2 in paraffin sections by using a p95-specific antibody and correlation with outcome in a cohort of trastuzumab-treated breast cancer patients. Clin. Cancer Res. 2010, 16, 4226–4235. [Google Scholar] [CrossRef]
- Ruiz, I.R.; Vicario, R.; Morancho, B.; Morales, C.B.; Arenas, E.J.; Herter, S.; Freimoser-Grundschober, A.; Somandin, J.; Sam, J.; Ast, O. p95HER2–T cell bispecific antibody for breast cancer treatment. Sci. Transl. Med. 2018, 10, eaat1445. [Google Scholar] [CrossRef]
- Hazan, R.; Margolis, B.; Dombalagian, M.; Ullrich, A.; Zilberstein, A.; Schlessinger, J. Identification of HER2 autophosphorylation site. Cell Growth Differ. 1990, 1, 3–7. [Google Scholar]
- Shi, Y.; Huang, W.; Tan, Y.; Jin, X.; Dua, R.; Penuel, E.; Mukherjee, A.; Sperinde, J.; Pannu, H.; Chenna, A. A novel proximity assay for the detection of proteins and protein complexes: Quantitation of HER1 and HER2 total protein expression and homodimerization in formalin-fixed, paraffin-embedded cell lines and breast cancer tissue. Diagn. Mol. Pathol. 2009, 18, 11–21. [Google Scholar] [CrossRef]
- Penichet, M.L.; Challita, P.-M.; Shin, S.-U.; Sampogna, S.L.; Rosenblatt, J.D.; Morrison, S.L. In vivo properties of three human HER2/neu-expressing murine cell lines in immunocompetent mice. Comp. Med. 1999, 49, 179–188. [Google Scholar]
- Kowarz, E.; Loscher, D.; Marschalek, R. Optimized Sleeping Beauty transposons rapidly generate stable transgenic cell lines. Biotechnol. J. 2015, 10, 647–653. [Google Scholar] [CrossRef]
- Tsushima, F.; Iwai, H.; Otsuki, N.; Abe, M.; Hirose, S.; Yamazaki, T.; Akiba, H.; Yagita, H.; Takahashi, Y.; Omura, K. Preferential contribution of B7-H1 to programmed death-1-mediated regulation of hapten-specific allergic inflammatory responses. Eur. J. Immunol. 2003, 33, 2773–2782. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Akiba, H.; Koyanagi, A.; Azuma, M.; Yagita, H.; Okumura, K. Blockade of B7-H1 on macrophages suppresses CD4+ T cell proliferation by augmenting IFN-γ-induced nitric oxide production. J. Immunol. 2005, 175, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Pan, Y.; Wei, Y.; Cheng, X.; Zhou, B.P.; Tan, M.; Zhou, X.; Xia, W.; Hortobagyi, G.N.; Yu, D.; et al. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell 2004, 6, 459–469. [Google Scholar] [CrossRef]
- Christgen, M.; Bartels, S.; Luft, A.; Persing, S.; Henkel, D.; Lehmann, U.; Kreipe, H. Activating human epidermal growth factor receptor 2 (HER2) gene mutation in bone metastases from breast cancer. Virchows Arch. 2018, 473, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Deng, Z.-L.; Luo, X.; Tang, N.; Song, W.-X.; Chen, J.; Sharff, K.A.; Luu, H.H.; Haydon, R.C.; Kinzler, K.W. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat. Protoc. 2007, 2, 1236. [Google Scholar] [CrossRef]
- Nagata, Y.; Furugen, R.; Hiasa, A.; Ikeda, H.; Ohta, N.; Furukawa, K.; Nakamura, H.; Kanematsu, T.; Shiku, H. Peptides derived from a wild-type murine proto-oncogene c-erbB-2/HER2/neu can induce CTL and tumor suppression in syngeneic hosts. J. Immunol. 1997, 159, 1336–1343. [Google Scholar] [PubMed]
- Fisk, B.; Blevins, T.L.; Wharton, J.T.; Ioannides, C.G. Identification of an immunodominant peptide of HER-2/neu protooncogene recognized by ovarian tumor-specific cytotoxic T lymphocyte lines. J. Exp. Med. 1995, 181, 2109–2117. [Google Scholar] [CrossRef]
- Knutson, K.L.; Schiffman, K.; Disis, M.L. Immunization with a HER-2/neu helper peptide vaccine generates HER-2/neu CD8 T-cell immunity in cancer patients. J. Clin. Investig. 2001, 107, 477–484. [Google Scholar] [CrossRef]
- Abiko, K.; Matsumura, N.; Hamanishi, J.; Horikawa, N.; Murakami, R.; Yamaguchi, K.; Yoshioka, Y.; Baba, T.; Konishi, I.; Mandai, M. IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br. J. Cancer 2015, 112, 1501. [Google Scholar] [CrossRef]
- Rubin, I.; Yarden, Y. The basic biology of HER2. Ann. Oncol. 2001, 12, S3–S8. [Google Scholar] [CrossRef]
- Wei, W.Z.; Shi, W.P.; Galy, A.; Lichlyter, D.; Hernandez, S.; Groner, B.; Heilbrun, L.; Jones, R.F. Protection against mammary tumor growth by vaccination with full-length, modified human ErbB-2 DNA. Int. J. Cancer 1999, 81, 748–754. [Google Scholar] [CrossRef]
- Hartman, Z.C.; Wei, J.; Osada, T.; Glass, O.; Lei, G.; Yang, X.Y.; Peplinski, S.; Kim, D.W.; Xia, W.; Spector, N.; et al. An adenoviral vaccine encoding full-length inactivated human Her2 exhibits potent immunogenicty and enhanced therapeutic efficacy without oncogenicity. Clin. Cancer Res. 2010, 16, 1466–1477. [Google Scholar] [CrossRef] [PubMed]
- Metharom, P.; Ellem, K.; Wei, M.Q. Gene transfer to dendritic cells induced a protective immunity against melanoma. Cell Mol. Immunol. 2005, 2, 281–288. [Google Scholar]
- Scott, A.M.; Wolchok, J.D.; Old, L.J. Antibody therapy of cancer. Nat. Rev. Cancer 2012, 12, 278–287. [Google Scholar] [CrossRef]
- Velmurugan, R.; Ramakrishnan, S.; Kim, M.; Ober, R.J.; Ward, E.S. Phagocytosis of antibody-opsonized tumor cells leads to the formation of a discrete vacuolar compartment in macrophages. Traffic 2018, 19, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Sakuishi, K.; Apetoh, L.; Sullivan, J.M.; Blazar, B.R.; Kuchroo, V.K.; Anderson, A.C. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 2010, 207, 2187–2194. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252. [Google Scholar] [CrossRef]
- Herbst, R.S.; Soria, J.-C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014, 515, 563. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568. [Google Scholar] [CrossRef]
- Karachaliou, N.; Gonzalez-Cao, M.; Crespo, G.; Drozdowskyj, A.; Aldeguer, E.; Gimenez-Capitan, A.; Teixido, C.; Molina-Vila, M.A.; Viteri, S.; De Los Llanos Gil, M. Interferon gamma, an important marker of response to immune checkpoint blockade in non-small cell lung cancer and melanoma patients. Ther. Adv. Med. Oncol. 2018, 10, 1758834017749748. [Google Scholar] [CrossRef]
- Massarelli, E.; William, W.; Johnson, F.; Kies, M.; Ferrarotto, R.; Guo, M.; Feng, L.; Lee, J.J.; Tran, H.; Kim, Y.U. Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16–related cancer: A phase 2 clinical trial. JAMA Oncol. 2019, 5, 67–73. [Google Scholar] [CrossRef]
- Han, J.; Duan, J.; Bai, H.; Wang, Y.; Wan, R.; Wang, X.; Chen, S.; Tian, Y.; Wang, D.; Fei, K. TCR Repertoire Diversity of Peripheral PD-1+ CD8+ T Cells Predicts Clinical Outcomes after Immunotherapy in Patients with Non–Small Cell Lung Cancer. Cancer Immunol. Res. 2020, 8, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Pressl, M.F.; Cordon-Cardo, C.; Slamon, D.J. Expression of the HER-Z/neu proto-oncogene in normal human adult and fetal tissues. Liver 1990, 7, 7. [Google Scholar]
- Valachis, A.; Nearchou, A.; Polyzos, N.P.; Lind, P. Cardiac toxicity in breast cancer patients treated with dual HER2 blockade. Int. J. Cancer 2013, 133, 2245–2252. [Google Scholar] [CrossRef]
- Bengala, C.; Zamagni, C.; Pedrazzoli, P.; Matteucci, P.; Ballestrero, A.; Da Prada, G.; Martino, M.; Rosti, G.; Danova, M.; Bregni, M. Cardiac toxicity of trastuzumab in metastatic breast cancer patients previously treated with high-dose chemotherapy: A retrospective study. Br. J. Cancer 2006, 94, 1016–1020. [Google Scholar] [CrossRef][Green Version]
- Morgan, R.A.; Yang, J.C.; Kitano, M.; Dudley, M.E.; Laurencot, C.M.; Rosenberg, S.A. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010, 18, 843–851. [Google Scholar] [CrossRef]
- Costa, R.; Zaman, S.; Sharpe, S.; Helenowski, I.; Shaw, C.; Han, H.; Soliman, H.; Czerniecki, B. A brief report of toxicity end points of HeR2 vaccines for the treatment of patients with HeR2+ breast cancer. Drug Des. Dev. Ther. 2019, 13, 309. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zheng, X.; Yang, H.; Moreira, J.M.A.; Brünner, N.; Christensen, H. Comparative analysis of evolutionarily conserved motifs of epidermal growth factor receptor 2 (HER2) predicts novel potential therapeutic epitopes. PLoS ONE 2014, 9, e106448. [Google Scholar] [CrossRef]
- Ko, H.-J.; Kim, Y.-J.; Kim, Y.-S.; Chang, W.-S.; Ko, S.-Y.; Chang, S.-Y.; Sakaguchi, S.; Kang, C.-Y. A combination of chemoimmunotherapies can efficiently break self-tolerance and induce antitumor immunity in a tolerogenic murine tumor model. Cancer Res. 2007, 67, 7477–7486. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, I.; Lee, J.-M.; Shin, K.-S.; Kang, T.; Park, M.H.; Seo, H.; Song, B.; Koh, C.-H.; Choi, J.; Shin, Y.K.; et al. Enhanced Immunogenicity of Engineered HER2 Antigens Potentiates Antitumor Immune Responses. Vaccines 2020, 8, 403. https://doi.org/10.3390/vaccines8030403
Jeon I, Lee J-M, Shin K-S, Kang T, Park MH, Seo H, Song B, Koh C-H, Choi J, Shin YK, et al. Enhanced Immunogenicity of Engineered HER2 Antigens Potentiates Antitumor Immune Responses. Vaccines. 2020; 8(3):403. https://doi.org/10.3390/vaccines8030403
Chicago/Turabian StyleJeon, Insu, Jeong-Mi Lee, Kwang-Soo Shin, Taeseung Kang, Myung Hwan Park, Hyungseok Seo, Boyeong Song, Choong-Hyun Koh, Jeongwon Choi, Young Kee Shin, and et al. 2020. "Enhanced Immunogenicity of Engineered HER2 Antigens Potentiates Antitumor Immune Responses" Vaccines 8, no. 3: 403. https://doi.org/10.3390/vaccines8030403
APA StyleJeon, I., Lee, J.-M., Shin, K.-S., Kang, T., Park, M. H., Seo, H., Song, B., Koh, C.-H., Choi, J., Shin, Y. K., Kim, B.-S., & Kang, C.-Y. (2020). Enhanced Immunogenicity of Engineered HER2 Antigens Potentiates Antitumor Immune Responses. Vaccines, 8(3), 403. https://doi.org/10.3390/vaccines8030403





