Tick-Borne Encephalitis Virus: A Quest for Better Vaccines against a Virus on the Rise
Abstract
:1. Tick-Borne Encephalitis Virus
2. Currently Available Vaccines
3. Immune Response to TBEV Infection and Vaccination
3.1. Innate Immunity Against TBEV
3.2. Adaptive Immunity Against TBEV
3.2.1. Antibody Response
Impact of Pre-Existing TBEV-Specific Immunity
Antibody-Dependent Enhancement in TBEV Infection?
3.2.2. CD4+ T Cell Response
3.2.3. CD8+ T Cell Response
3.2.4. Vaccine Failures
4. Novel Approaches and TBEV Target Antigens for the Development of Improved TBEV Vaccines
- Highly immunogenic in all age and risk groups, rapid and high seroconversion rates.
- Induction of long-lasting immunity without the need for booster vaccinations.
- No vaccine failures.
- Protection against all TBEV subtypes.
- Cost-effective and safe.
4.1. Novel TBEV Vaccine Strategies Aiming at the Induction of Humoral Immunity
4.2. Novel TBEV Vaccine Approaches Aiming at the Induction of Cellular Immunity
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Simmonds, P.; Becher, P.; Bukh, J.; Gould, E.A.; Meyers, G.; Monath, T.; Muerhoff, S.; Pletnev, A.; Rico-Hesse, R.; Smith, D.B.; et al. ICTV Virus Taxonomy Profile: Flaviviridae. J. Gen. Virol. 2017, 98, 2–3. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Vaccines against tick-borne encephalitis: WHO Position Paper. Wkly. Epidemiol. Rec. = Relev. Épidémiologique Hebd. 2011, 86, 241–256. [Google Scholar]
- Ruzek, D.; Avšič Županc, T.; Borde, J.; Chrdle, A.; Eyer, L.; Karganova, G.; Kholodilov, I.; Knap, N.; Kozlovskaya, L.; Matveev, A.; et al. Tick-borne encephalitis in Europe and Russia: Review of pathogenesis, clinical features, therapy, and vaccines. Antivir. Res. 2019, 164, 23–51. [Google Scholar] [CrossRef]
- Pulkkinen, L.I.A.; Butcher, S.J.; Anastasina, M. Tick-Borne Encephalitis Virus: A Structural View. Viruses 2018, 10, 350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Füzik, T.; Formanová, P.; Růžek, D.; Yoshii, K.; Niedrig, M.; Plevka, P. Structure of tick-borne encephalitis virus and its neutralization by a monoclonal antibody. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Růžek, D.; Yoshii, K.; Bloom, M.E.; Gould, E.A. Virology. In The TBE Book; Dobler, G., Erber, W., Bröker, M., Schmitt, H.-J., Eds.; Global Health Press: Singapore, 2020; pp. 16–31. [Google Scholar]
- Heinz, F.X.; Stiasny, K.; Püschner-Auer, G.; Holzmann, H.; Allison, S.L.; Mandl, C.W.; Kunz, C. Structural Changes and Functional Control of the Tick-Borne Encephalitis Virus Glycoprotein E by the Heterodimeric Association with Protein prM. Virology 1994, 198, 109–117. [Google Scholar] [CrossRef]
- Elshuber, S.; Allison, S.L.; Heinz, F.X.; Mandl, C.W. Cleavage of protein prM is necessary for infection of BHK-21 cells by tick-borne encephalitis virus. J. Gen. Virol. 2003, 84, 183–191. [Google Scholar] [CrossRef]
- Stadler, K.; Allison, S.L.; Schalich, J.; Heinz, F.X. Proteolytic Activation of Tick-Borne Encephalitis Virus by Furin. J. Virol. 1997, 71, 8475–8481. [Google Scholar] [CrossRef] [Green Version]
- Heinz, F.X.; Allison, S.L. Flavivirus Structure and Membrane Fusion. Adv. Virus Res. 2003, 59, 63–97. [Google Scholar] [CrossRef]
- Crooks, A.J.; Lee, J.M.; Easterbrook, L.M.; Timofeev, A.V.; Stephenson, J.R. The NS1 protein of tick-borne encephalitis virus forms multimeric species upon secretion from the host cell. J. Gen. Virol. 1994, 75, 3453–3460. [Google Scholar] [CrossRef]
- Bogovic, P.; Strle, F. Tick-borne encephalitis: A review of epidemiology, clinical characteristics, and management. World J. Clin. Cases 2015, 3, 430–441. [Google Scholar] [CrossRef]
- Balogh, Z.; Ferenczi, E.; Szeles, K.; Stefanoff, P.; Gut, W.; Szomor, K.N.; Takacs, M.; Berencsi, G. Tick-borne encephalitis outbreak in Hungary due to consumption of raw goat milk. J. Virol. Methods 2010, 163, 481–485. [Google Scholar] [CrossRef]
- Caini, S.; Szomor, K.; Ferenczi, E.; Székelyné Gáspár, Á.; Csohán, Á.; Krisztalovics, K.; Molnár, Z.; Horváth, J.K. Tick-borne encephalitis transmitted by unpasteurised cow milk in western Hungary, September to October 2011. Eurosurveillance 2012, 17, 20128. [Google Scholar] [PubMed]
- Dorko, E.; Hockicko, J.; Rimárová, K.; Bušová, A.; Popaďák, P.; Popaďáková, J.; Schréter, I. Milk outbreaks of tick-borne encephalitis in Slovakia, 2012–2016. Cent. Eur. J. Public Health 2018, 26, S47–S50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holzmann, H.; Aberle, S.W.; Stiasny, K.; Werner, P.; Mischak, A.; Zainer, B.; Netzer, M.; Koppi, S.; Bechter, E.; Heinz, F.X. Tick-borne Encephalitis from Eating Goat Cheese in a Mountain Region of Austria. Emerg. Infect. Dis. 2009, 15, 1671–1673. [Google Scholar] [CrossRef]
- Hudopisk, N.; Korva, M.; Janet, E.; Simetinger, M.; Grgič-Vitek, M.; Gubenšek, J.; Natek, V.; Kraigher, A.; Strle, F.; Avšič-Županc, T. Tick-borne Encephalitis Associated with Consumption of Raw Goat Milk, Slovenia, 2012. Emerg. Infect. Dis. 2013, 19, 806–808. [Google Scholar] [CrossRef]
- Lindquist, L.; Vapalahti, O. Tick-borne encephalitis. Lancet 2008, 371, 1861–1871. [Google Scholar] [CrossRef]
- Dai, X.; Shang, G.; Lu, S.; Yang, J.; Xu, J. A new subtype of eastern tick-borne encephalitis virus discovered in Qinghai-Tibet Plateau, China. Emerg. Microbes Infect. 2018, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Kovalev, S.Y.; Mukhacheva, T.A. Reconsidering the classification of tick-borne encephalitis virus within the Siberian subtype gives new insights into its evolutionary history. Infect. Genet. Evol. 2017, 55, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Erber, W.; Schmitt, H.-J.; Janković, T.V. TBE-epidemiology by country-An overview. In The TBE Book; Dobler, G., Erber, W., Bröker, M., Schmitt, H.-J., Eds.; Global Health Press: Singapore, 2020; pp. 159–170. [Google Scholar]
- Boelke, M.; Bestehorn, M.; Marchwald, B.; Kubinski, M.; Liebig, K.; Glanz, J.; Schulz, C.; Dobler, G.; Monazahian, M.; Becker, S.C. First Isolation and Phylogenetic Analyses of Tick-Borne Encephalitis Virus in Lower Saxony, Germany. Viruses 2019, 11, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Graaf, J.A.; Reimerink, J.H.J.; Voorn, G.P.; De Vaate, E.A.L.B.; De Vries, A.; Rockx, B.; Schuitemaker, A.; Hira, V. First human case of tick-borne encephalitis virus infection acquired in the Netherlands, July 2016. Eurosurveillance 2016, 21, 30318. [Google Scholar] [CrossRef] [PubMed]
- Holding, M.; Dowall, S.D.; Medlock, J.M.; Carter, D.P.; McGinley, L.; Curran-French, M.; Pullan, S.T.; Chamberlain, J.; Hansford, K.M.; Baylis, M.; et al. Detection of new endemic focus of tick-borne encephalitis virus (TBEV), Hampshire/Dorset border, England, September 2019. Eurosurveillance 2019, 24, 1900658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holding, M.; Dowall, S.D.; Medlock, J.M.; Carter, D.P.; Pullan, S.T.; Lewis, J.; Vipond, R.; Rocchi, M.S.; Baylis, M.; Hewson, R. Tick-Borne Encephalitis Virus, United Kingdom. Emerg. Infect. Dis. 2020, 26, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Wallenhammar, A.; Lindqvist, R.; Asghar, N.; Gunaltay, S.; Fredlund, H.; Davidsson, Å.; Andersson, S.; Överby, A.K.; Johansson, M. Revealing new tick-borne encephalitis virus foci by screening antibodies in sheep milk. Parasit. Vectors 2020, 13, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randolph, S.E. To what extent has climate change contributed to the recent epidemiology of tick-borne diseases? Vet. Parasitol. 2010, 167, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Randolph, S.E.; Asokliene, L.; Avsic-Zupanc, T.; Bormane, A.; Burri, C.; Gern, L.; Golovljova, I.; Hubalek, Z.; Knap, N.; Kondrusik, M.; et al. Variable spikes in tick-borne encephalitis incidence in 2006 independent of variable tick abundance but related to weather. Parasit. Vectors 2008, 1, 44. [Google Scholar] [CrossRef] [Green Version]
- Agergaard, C.N.; Rosenstierne, M.W.; Bødker, R.; Rasmussen, M.; Andersen, P.H.S.; Fomsgaard, A. New tick-borne encephalitis virus hot spot in Northern Zealand, Denmark, October 2019. Eurosurveillance 2019, 24, 1900639. [Google Scholar] [CrossRef]
- Mikryukova, T.P.; Moskvitina, N.S.; Kononova, Y.V.; Korobitsyn, I.G.; Kartashov, M.Y.; Tyuten’kov, O.Y.; Protopopova, E.V.; Romanenko, V.N.; Chausov, E.V.; Gashkov, S.I.; et al. Surveillance of tick-borne encephalitis virus in wild birds and ticks in Tomsk city and its suburbs (Western Siberia). Ticks Tick-Borne Dis. 2014, 5, 145–151. [Google Scholar] [CrossRef]
- Růžek, D.; Dobler, G.; Mantke, O.D. Tick-borne encephalitis: Pathogenesis and clinical implications. Travel Med. Infect. Dis. 2010, 8, 223–232. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Tick-Borne Encephalitis among U.S. Travelers to Europe and Asia-2000–2009. Morb. Mortal. Wkly. Rep. 2010, 59, 335–338. [Google Scholar]
- Dobler, G.; Gniel, D.; Petermann, R.; Pfeffer, M. Epidemiology and distribution of tick-borne encephalitis. Wien. Med. Wochenschr. 2012, 162, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Kollaritsch, H.; Paulke-Korinek, M.; Holzmann, H.; Hombach, J.; Bjorvatn, B.; Barrett, A. Vaccines and vaccination against tick-borne encephalitis. Expert Rev. Vaccines 2012, 11, 1103–1119. [Google Scholar] [CrossRef] [PubMed]
- Dobler, G. Zoonotic tick-borne flaviviruses. Vet. Microbiol. 2010, 140, 221–228. [Google Scholar] [CrossRef]
- Holzmann, H. Diagnosis of tick-borne encephalitis. Vaccine 2003, 21, S1/36–S1/40. [Google Scholar] [CrossRef]
- Xing, Y.; Schmitt, H.-J.; Arguedas, A.; Junfeng, Y. Tick-borne encephalitis in China: A review of epidemiology and vaccines. Vaccine 2017, 35, 1227–1237. [Google Scholar] [CrossRef]
- Lu, Z.; Broeker, M.; Liang, G. Tick-Borne Encephalitis in Mainland China. Vector-Borne Zoonotic Dis. 2008, 8, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Barrett, P.N.; Schober-Bendixen, S.; Ehrlich, H.J. History of TBE vaccines. Vaccine 2003, 21, S1/41–S1/49. [Google Scholar] [CrossRef]
- Klockmann, U.; Bock, H.L.; Franke, V.; Hein, B.; Reiner, G.; Hilfenhaus, J. Preclinical investigations of the safety, immunogenicity and efficacy of a purified, inactivated tick-borne encephalitis vaccine. J. Biol. Stand. 1989, 17, 331–342. [Google Scholar] [CrossRef]
- Vorovitch, M.F.; Grishina, K.G.; Volok, V.P.; Chernokhaeva, L.L.; Grishin, K.V.; Karganova, G.G.; Ishmukhametov, A.A. Evervac: Phase I/II study of immunogenicity and safety of a new adjuvant-free TBE vaccine cultivated in Vero cell culture. Hum. Vaccin. Immunother. 2020. [Google Scholar] [CrossRef]
- Pöllabauer, E.M.; Kollaritsch, H. Prevention: Vaccines and immunoglobulins. In The TBE Book; Dobler, G., Erber, W., Bröker, M., Schmitt, H.-J., Eds.; Global Health Press: Singapore, 2020; pp. 363–380. [Google Scholar]
- Wittermann, C.; Schöndorf, I.; Gniel, D. Antibody response following administration of two paediatric tick-borne encephalitis vaccines using two different vaccination schedules. Vaccine 2009, 27, 1661–1666. [Google Scholar] [CrossRef]
- Prymula, R.; Pöllabauer, E.M.; Pavlova, B.G.; Löw-Baselli, A.; Fritsch, S.; Angermayr, R.; Geisberger, A.; Barrett, P.N.; Ehrlich, H.J. Antibody persistence after two vaccinations with either FSME-IMMUN® Junior or ENCEPUR® Children followed by third vaccination with FSME-IMMUN® Junior. Hum. Vaccin. Immunother. 2012, 8, 736–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demicheli, V.; Debalini, M.G.; Rivetti, A. Vaccines for preventing tick-borne encephalitis (Review). Cochrane Database Syst. Rev. 2009. [Google Scholar] [CrossRef]
- Beran, J.; Xie, F.; Zent, O. Five year follow-up after a first booster vaccination against tick-borne encephalitis following different primary vaccination schedules demonstrates long-term antibody persistence and safety. Vaccine 2014, 32, 4275–4280. [Google Scholar] [CrossRef] [PubMed]
- Schöndorf, I.; Beran, J.; Cizkova, D.; Lesna, V.; Banzhoff, A.; Zent, O. Tick-borne encephalitis (TBE) vaccination: Applying the most suitable vaccination schedule. Vaccine 2007, 25, 1470–1475. [Google Scholar] [CrossRef] [PubMed]
- Zent, O.; Beran, J.; Jilg, W.; Mach, T.; Banzhoff, A. Clinical evaluation of a polygeline-free tick-borne encephalitis vaccine for adolescents and adults. Vaccine 2003, 21, 738–741. [Google Scholar] [CrossRef]
- Loew-Baselli, A.; Poellabauer, E.-M.; Pavlova, B.G.; Koska, M.; Bobrovsky, R.; Konior, R.; Ehrlich, H.J. Seropersistence of tick-borne encephalitis antibodies, safety and booster response to FSME-IMMUN® 0.5 ml in adults aged 18–67 years. Hum. Vaccin. 2009, 5, 551–556. [Google Scholar] [CrossRef] [Green Version]
- Pavlova, B.G.; Loew-Baselli, A.; Fritsch, S.; Poellabauer, E.-M.; Vartian, N.; Rinke, I.; Ehrlich, H.J. Tolerability of modified tick-borne encephalitis vaccine FSME-IMMUN “NEW” in children: Results of post-marketing surveillance. Vaccine 2003, 21, 742–745. [Google Scholar] [CrossRef]
- Vorovitch, M.F.; Maikova, G.B.; Chernokhaeva, L.L.; Romanenko, V.V.; Karganova, G.G.; Ishmukhametov, A.A. Comparison of the Immunogenicity and Safety of Two Pediatric TBE Vaccines Based on the Far Eastern and European Virus Subtypes. Adv. Virol. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Maikova, G.B.; Chernokhaeva, L.L.; Rogova, Y.V.; Kozlovskaya, L.I.; Kholodilov, I.S.; Romanenko, V.V.; Esyunina, M.S.; Ankudinova, A.A.; Kilyachina, A.S.; Vorovitch, M.F.; et al. Ability of inactivated vaccines based on far-eastern tick-borne encephalitis virus strains to induce humoral immune response in originally seropositive and seronegative recipients. J. Med. Virol. 2019, 91, 190–200. [Google Scholar] [CrossRef]
- Yoshii, K.; Song, J.Y.; Park, S.-B.; Yang, J.; Schmitt, H.-J. Tick-borne encephalitis in Japan, Republic of Korea and China. Emerg. Microbes Infect. 2017, 6, 1–10. [Google Scholar] [CrossRef]
- Wittermann, C.; Petri, E.; Zent, O. Long-term persistence of tick-borne encephalitis antibodies in children 5 years after first booster vaccination with Encepur® Children. Vaccine 2009, 27, 1585–1588. [Google Scholar] [CrossRef] [PubMed]
- Rendi-Wagner, P.; Kundi, M.; Zent, O.; Dvorak, G.; Jaehnig, P.; Holzmann, H.; Mikolasek, A.; Kollaritsch, H. Persistence of protective immunity following vaccination against tick-borne encephalitis-Longer than expected? Vaccine 2004, 22, 2743–2749. [Google Scholar] [CrossRef] [PubMed]
- Hainz, U.; Jenewein, B.; Asch, E.; Pfeiffer, K.-P.; Berger, P.; Grubeck-Loebenstein, B. Insufficient protection for healthy elderly adults by tetanus and TBE vaccines. Vaccine 2005, 23, 3232–3235. [Google Scholar] [CrossRef]
- Weinberger, B.; Keller, M.; Fischer, K.-H.; Stiasny, K.; Neuner, C.; Heinz, F.X.; Grubeck-Loebenstein, B. Decreased antibody titers and booster responses in tick-borne encephalitis vaccinees aged 50–90 years. Vaccine 2010, 28, 3511–3515. [Google Scholar] [CrossRef]
- Zavadska, D.; Anca, I.; André, F.; Bakir, M.; Chlibek, R.; Čižman, M.; Ivaskeviciene, I.; Mangarov, A.; Mészner, Z.; Pokorn, M.; et al. Recommendations for tick-borne encephalitis vaccination from the Central European Vaccination Awareness Group (CEVAG). Hum. Vaccin. Immunother. 2013, 9, 362–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinz, F.X.; Stiasny, K.; Holzmann, H.; Grgic-Vitek, M.; Kriz, B.; Essl, A.; Kundi, M. Vaccination and Tick-borne Encephalitis, Central Europe. Emerg. Infect. Dis. 2013, 19, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Zenz, W.; Pansi, H.; Zoehrer, B.; Mutz, I.; Holzmann, H.; Kraigher, A.; Berghold, A.; Spork, D. Tick-Borne Encephalitis in Children in Styria and Slovenia Between 1980 and 2003. Pediatr. Infect. Dis. J. 2005, 24, 892–896. [Google Scholar] [CrossRef]
- Andersson, C.R.; Vene, S.; Insulander, M.; Lindquist, L.; Lundkvist, Å.; Günther, G. Vaccine failures after active immunisation against tick-borne encephalitis. Vaccine 2010, 28, 2827–2831. [Google Scholar] [CrossRef]
- Hansson, K.E.; Rosdahl, A.; Insulander, M.; Vene, S.; Lindquist, L.; Gredmark-Russ, S.; Askling, H.H. Tick-borne Encephalitis Vaccine Failures: A 10-year Retrospective Study Supporting the Rationale for Adding an Extra Priming Dose in Individuals Starting at Age 50 Years. Clin. Infect. Dis. 2020, 70, 245–251. [Google Scholar] [CrossRef]
- Lotrič-Furlan, S.; Bogovič, P.; Avšič-Županc, T.; Jelovšek, M.; Lusa, L.; Strle, F. Tick-borne encephalitis in patients vaccinated against this disease. J. Intern. Med. 2017, 282, 142–155. [Google Scholar] [CrossRef] [Green Version]
- Bender, A.; Jäger, G.; Scheuerer, W.; Feddersen, B.; Kaiser, R.; Pfister, H.-W. Two severe cases of tick-borne encephalitis despite complete active vaccination-the significance of neutralizing antibodies. J. Neurol. 2004, 251, 353–354. [Google Scholar] [CrossRef] [PubMed]
- Grgic-Vitek, M.; Avsic-Zupanc, T.; Klavs, I. Tick-borne encephalitis after vaccination: Vaccine failure or misdiagnosis. Vaccine 2010, 28, 7396–7400. [Google Scholar] [CrossRef] [PubMed]
- Kleiter, I.; Jilg, W.; Bogdahn, U.; Steinbrecher, A. Delayed Humoral Immunity in a Patient with Severe Tick-borne Encephalitis after Complete Active Vaccination. Infection 2007, 35, 26–29. [Google Scholar] [CrossRef]
- Sendi, P.; Hirzel, C.; Pfister, S.; Ackermann-Gäumann, R.; Grandgirard, D.; Hewer, E.; Nirkko, A.C. Fatal Outcome of European Tick-borne Encephalitis after Vaccine Failure. Front. Neurol. 2017, 8, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindqvist, R.; Upadhyay, A.; Överby, A.K. Tick-Borne Flaviviruses and the Type I Interferon Response. Viruses 2018, 10, 340. [Google Scholar] [CrossRef] [Green Version]
- Carletti, T.; Zakaria, M.K.; Marcello, A. The host cell response to tick-borne encephalitis virus. Biochem. Biophys. Res. Commun. 2017, 492, 533–540. [Google Scholar] [CrossRef]
- Dörrbecker, B.; Dobler, G.; Spiegel, M.; Hufert, F.T. Tick-borne encephalitis virus and the immune response of the mammalian host. Travel Med. Infect. Dis. 2010, 8, 213–222. [Google Scholar] [CrossRef]
- Blom, K.; Braun, M.; Pakalniene, J.; Lunemann, S.; Enqvist, M.; Dailidyte, L.; Schaffer, M.; Lindquist, L.; Mickiene, A.; Michaëlsson, J.; et al. NK Cell Responses to Human Tick-Borne Encephalitis Virus Infection. J. Immunol. 2016, 197, 2762–2771. [Google Scholar] [CrossRef]
- Best, S.M.; Morris, K.L.; Shannon, J.G.; Robertson, S.J.; Mitzel, D.N.; Park, G.S.; Boer, E.; Wolfinbarger, J.B.; Bloom, M.E. Inhibition of Interferon-Stimulated JAK-STAT Signaling by a Tick-Borne Flavivirus and Identification of NS5 as an Interferon Antagonist. J. Virol. 2005, 79, 12828–12839. [Google Scholar] [CrossRef] [Green Version]
- Fares, M.; Cochet-Bernoin, M.; Gonzalez, G.; Montero-Menei, C.N.; Blanchet, O.; Benchoua, A.; Boissart, C.; Lecollinet, S.; Richardson, J.; Haddad, N.; et al. Pathological modeling of TBEV infection reveals differential innate immune responses in human neurons and astrocytes that correlate with their susceptibility to infection. J. Neuroinflammation 2020, 17, 76. [Google Scholar] [CrossRef] [Green Version]
- Lieskovská, J.; Páleníková, J.; Langhansová, H.; Chmelař, J.; Kopecký, J. Saliva of Ixodes ricinus enhances TBE virus replication in dendritic cells by modulation of pro-survival Akt pathway. Virology 2018, 514, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Kotál, J.; Langhansová, H.; Lieskovská, J.; Andersen, J.F.; Francischetti, I.M.B.; Chavakis, T.; Kopecký, J.; Pedra, J.H.F.; Kotsyfakis, M.; Chmelař, J. Modulation of host immunity by tick saliva. J. Proteomics 2015, 128, 58–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Růžek, D.; Salát, J.; Palus, M.; Gritsun, T.S.; Gould, E.A.; Dyková, I.; Skallová, A.; Jelínek, J.; Kopecký, J.; Grubhoffer, L. CD8+ T-cells mediate immunopathology in tick-borne encephalitis. Virology 2009, 384, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pierson, T.C.; Diamond, M.S. Molecular mechanisms of antibody-mediated neutralisation of flavivirus infection. Expert Rev. Mol. Med. 2008, 10, e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hangartner, L.; Zinkernagel, R.M.; Hengartner, H. Antiviral antibody responses: The two extremes of a wide spectrum. Nat. Rev. Immunol. 2006, 6, 231–243. [Google Scholar] [CrossRef]
- Pierson, T.C.; Fremont, D.H.; Kuhn, R.J.; Diamond, M.S. Structural Insights into the Mechanisms of Antibody-Mediated Neutralization of Flavivirus Infection: Implications for Vaccine Development. Cell Host Microbe 2008, 4, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Günther, G.; Haglund, M.; Lindquist, L.; Skoldenberg, B.; Forsgren, M. Intrathecal IgM, IgA and IgG antibody response in tick-borne encephalitis. Long-term follow-up related to clinical course and outcome. Clin. Diagn. Virol. 1997, 8, 17–29. [Google Scholar] [CrossRef]
- Remoli, M.E.; Marchi, A.; Fortuna, C.; Benedetti, E.; Minelli, G.; Fiorentini, C.; Mel, R.; Venturi, G.; Ciufolini, M.G. Anti-tick-borne encephalitis (TBE) virus neutralizing antibodies dynamics in natural infections versus vaccination. Pathog. Dis. 2014, 73, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, R.; Holzmann, H. Laboratory Findings in Tick-Borne Encephalitis-Correlation with Clinical Outcome. Infection 2000, 28, 78–84. [Google Scholar] [CrossRef]
- Bradt, V.; Malafa, S.; von Braun, A.; Jarmer, J.; Tsouchnikas, G.; Medits, I.; Wanke, K.; Karrer, U.; Stiasny, K.; Heinz, F.X. Pre-existing yellow fever immunity impairs and modulates the antibody response to tick-borne encephalitis vaccination. npj Vaccines 2019, 4, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beran, J.; Lattanzi, M.; Xie, F.; Moraschini, L.; Galgani, I. Second five-year follow-up after a booster vaccination against tick-borne encephalitis following different primary vaccination schedules demonstrates at least 10 years antibody persistence. Vaccine 2019, 37, 4623–4629. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, B.; Grubeck-Loebenstein, B. Vaccines for the elderly. Clin. Microbiol. Infect. 2012, 18, 100–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stiasny, K.; Aberle, J.H.; Keller, M.; Grubeck-Loebenstein, B.; Heinz, F.X. Age Affects Quantity but not Quality of Antibody Responses after Vaccination with an Inactivated Flavivirus Vaccine against Tick-Borne Encephalitis. PLoS ONE 2012, 7, e34145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuivanen, S.; Hepojoki, J.; Vene, S.; Vaheri, A.; Vapalahti, O. Identification of linear human B-cell epitopes of tick-borne encephalitis virus. Virol. J. 2014, 11, 115. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, R.J.; Dowd, K.A.; Beth Post, C.; Pierson, T.C. Shake, rattle, and roll: Impact of the dynamics of flavivirus particles on their interactions with the host. Virology 2015, 479, 508–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, S.; Jost, C.A.; Xu, Q.; Ess, J.; Martin, J.E.; Oliphant, T.; Whitehead, S.S.; Durbin, A.P.; Graham, B.S.; Diamond, M.S.; et al. Maturation of West Nile Virus Modulates Sensitivity to Antibody-Mediated Neutralization. PLoS Pathog. 2008, 4, e1000060. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.-C.; Chiu, H.-C.; Chen, L.-K.; Chang, G.-J.J.; Chiou, S.-S. Formalin Inactivation of Japanese Encephalitis Virus Vaccine Alters the Antigenicity and Immunogenicity of a Neutralization Epitope in Envelope Protein Domain III. PLoS Negl. Trop. Dis. 2015, 9, e0004167. [Google Scholar] [CrossRef] [Green Version]
- Zlatkovic, J.; Tsouchnikas, G.; Jarmer, J.; Koessl, C.; Stiasny, K.; Heinz, F.X. Aluminum Hydroxide Influences Not Only the Extent but Also the Fine Specificity and Functional Activity of Antibody Responses to Tick-Borne Encephalitis Virus in Mice. J. Virol. 2013, 87, 12187–12195. [Google Scholar] [CrossRef] [Green Version]
- Jarmer, J.; Zlatkovic, J.; Tsouchnikas, G.; Vratskikh, O.; Strauss, J.; Aberle, J.H.; Chmelik, V.; Kundi, M.; Stiasny, K.; Heinz, F.X. Variation of the Specificity of the Human Antibody Responses after Tick-Borne Encephalitis Virus Infection and Vaccination. J. Virol. 2014, 88, 13845–13857. [Google Scholar] [CrossRef] [Green Version]
- Albinsson, B.; Vene, S.; Rombo, L.; Blomberg, J.; Lundkvist, Å.; Rönnberg, B. Distinction between serological responses following tick-borne encephalitis virus (TBEV) infection vs vaccination, Sweden 2017. Eurosurveillance 2018, 23, 17-00838. [Google Scholar] [CrossRef] [PubMed]
- Girl, P.; Bestehorn-Willmann, M.; Zange, S.; Borde, J.P.; Dobler, G.; von Buttlar, H. Tick-Borne Encephalitis Virus (TBEV): Nonstructural Protein 1 IgG Enzyme-Linked Immunosorbent Assay for Differentiating Infection versus Vaccination Antibody Responses. J. Clin. Microbiol. 2020, 58, e01783-19. [Google Scholar] [CrossRef] [PubMed]
- Mora-Cárdenas, E.; Aloise, C.; Faoro, V.; Knap Gašper, N.; Korva, M.; Caracciolo, I.; D’Agaro, P.; Avšič-Županc, T.; Marcello, A. Comparative specificity and sensitivity of NS1-based serological assays for the detection of flavivirus immune response. PLoS Negl. Trop. Dis. 2020, 14, e0008039. [Google Scholar] [CrossRef] [PubMed]
- Salat, J.; Mikulasek, K.; Larralde, O.; Formanova, P.P.; Chrdle, A.; Haviernik, J.; Elsterova, J.; Teislerova, D.; Palus, M.; Eyer, L.; et al. Tick-Borne Encephalitis Virus Vaccines Contain Non-Structural Protein 1 Antigen and May Elicit NS1-Specific Antibody Responses in Vaccinated Individuals. Vaccines 2020, 8, 81. [Google Scholar] [CrossRef] [Green Version]
- Aberle, J.H.; Schwaiger, J.; Aberle, S.W.; Stiasny, K.; Scheinost, O.; Kundi, M.; Chmelik, V.; Heinz, F.X. Human CD4+ T Helper Cell Responses after Tick-Borne Encephalitis Vaccination and Infection. PLoS ONE 2015, 10, e0140545. [Google Scholar] [CrossRef] [Green Version]
- Schwaiger, J.; Aberle, J.H.; Stiasny, K.; Knapp, B.; Schreiner, W.; Fae, I.; Fischer, G.; Scheinost, O.; Chmelik, V.; Heinz, F.X. Specificities of Human CD4+ T Cell Responses to an Inactivated Flavivirus Vaccine and Infection: Correlation with Structure and Epitope Prediction. J. Virol. 2014, 88, 7828–7842. [Google Scholar] [CrossRef] [Green Version]
- Varnaitė, R.; Blom, K.; Lampen, M.H.; Vene, S.; Thunberg, S.; Lindquist, L.; Ljunggren, H.-G.; Rombo, L.; Askling, H.H.; Gredmark-Russ, S. Magnitude and Functional Profile of the Human CD4+ T Cell Response throughout Primary Immunization with Tick-Borne Encephalitis Virus Vaccine. J. Immunol. 2020, 204, 914–922. [Google Scholar] [CrossRef]
- Volkova, T.D.; Koroev, D.O.; Titova, M.A.; Oboznaya, M.B.; Filatova, M.P.; Vorovich, M.F.; Ozherelkov, S.V.; Timofeev, A.V.; Volpina, O.M. Synthetic Fragments of the NS1 Protein of the Tick-Borne Encephalitis Eirus Exhibiting a Protective Effect. Russ. J. Bioorganic Chem. 2007, 33, 213–217. [Google Scholar] [CrossRef]
- Aberle, J.H.; Stiasny, K.; Kundi, M.; Heinz, F.X. Mechanistic insights into the impairment of memory B cells and antibody production in the elderly. Age 2013, 35, 371–381. [Google Scholar] [CrossRef] [Green Version]
- Lampen, M.H.; Uchtenhagen, H.; Blom, K.; Varnaitė, R.; Pakalniene, J.; Dailidyte, L.; Wälchli, S.; Lindquist, L.; Mickiene, A.; Michaëlsson, J.; et al. Breadth and Dynamics of HLA-A2–and HLA-B7–Restricted CD8+ T Cell Responses against Nonstructural Viral Proteins in Acute Human Tick-Borne Encephalitis Virus Infection. ImmunoHorizons 2018, 2, 172–184. [Google Scholar] [CrossRef] [Green Version]
- Blom, K.; Braun, M.; Pakalniene, J.; Dailidyte, L.; Béziat, V.; Lampen, M.H.; Klingström, J.; Lagerqvist, N.; Kjerstadius, T.; Michaëlsson, J.; et al. Specificity and Dynamics of Effector and Memory CD8 T Cell Responses in Human Tick-Borne Encephalitis Virus Infection. PLoS Pathog. 2015, 11, e1004622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, S.C. A novel recombinant adenovirus vector expressing a flavivirus non-structural protein protects against lethal flavivirus challenge. Clin. Sci. 1993, 85, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.C.; Stephenson, J.R.; Wilkinson, G.W. High-Level Expression of the Tick-Borne Encephalitis Virus NS1 Protein by Using an Adenovirus-Based Vector: Protection Elicited in a Murine Model. J. Virol. 1992, 66, 2086–2095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, S.C.; Stephenson, J.R.; Wilkinson, G.W.G. Protection elicited by a replication-defective adenovirus vector expressing the tick-borne encephalitis virus non-structural glycoprotein NS1. J. Gen. Virol. 1994, 75, 2399–2402. [Google Scholar] [CrossRef] [PubMed]
- Volpina, O.M.; Volkova, T.D.; Koroev, D.O.; Ivanov, V.T.; Ozherelkov, S.V.; Khoretonenko, M.V.; Vorovitch, M.F.; Stephenson, J.R.; Timofeev, A.V. A synthetic peptide based on the NS1 non-structural protein of tick-borne encephalitis virus induces a protective immune response against fatal encephalitis in an experimental animal model. Virus Res. 2005, 112, 95–99. [Google Scholar] [CrossRef]
- Rey, F.A.; Stiasny, K.; Vaney, M.; Dellarole, M.; Heinz, F.X. The bright and the dark side of human antibody responses to flaviviruses: Lessons for vaccine design. EMBO Rep. 2018, 19, 206–224. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera-A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Rey, F.A.; Heinz, F.X.; Mandl, C.; Kunz, C.; Harrison, S.C. The envelope glycoprotein from tick-borne encephalitis virus at 2 Å resolution. Nature 1995, 375, 291–298. [Google Scholar] [CrossRef]
- Roehrig, J.T. Antigenic Structure of Flavivirus Proteins. Adv. Virus Res. 2003, 59, 141–175. [Google Scholar] [CrossRef]
- Kiermayr, S.; Stiasny, K.; Heinz, F.X. Impact of Quaternary Organization on the Antigenic Structure of the Tick-Borne Encephalitis Virus Envelope Glycoprotein E. J. Virol. 2009, 83, 8482–8491. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.-Y.; Tsai, W.-Y.; Lin, S.-R.; Kao, C.-L.; Hu, H.-P.; King, C.-C.; Wu, H.-C.; Chang, G.-J.; Wang, W.-K. Antibodies to Envelope Glycoprotein of Dengue Virus during the Natural Course of Infection Are Predominantly Cross-Reactive and Recognize Epitopes Containing Highly Conserved Residues at the Fusion Loop of Domain II. J. Virol. 2008, 82, 6631–6643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliphant, T.; Nybakken, G.E.; Engle, M.; Xu, Q.; Nelson, C.A.; Sukupolvi-Petty, S.; Marri, A.; Lachmi, B.-E.; Olshevsky, U.; Fremont, D.H.; et al. Antibody Recognition and Neutralization Determinants on Domains I and II of West Nile Virus Envelope Protein. J. Virol. 2006, 80, 12149–12159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stiasny, K.; Kiermayr, S.; Holzmann, H.; Heinz, F.X. Cryptic Properties of a Cluster of Dominant Flavivirus Cross-Reactive Antigenic Sites. J. Virol. 2006, 80, 9557–9568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, K.M.; Nybakken, G.E.; Thompson, B.S.; Engle, M.J.; Marri, A.; Fremont, D.H.; Diamond, M.S. Antibodies against West Nile Virus Nonstructural Protein NS1 Prevent Lethal Infection through Fc γ Receptor-Dependent and -Independent Mechanisms. J. Virol. 2006, 80, 1340–1351. [Google Scholar] [CrossRef] [Green Version]
- Falgout, B.; Bray, M.; Schlesinger, J.J.; Lai, C.-J. Immunization of Mice with Recombinant Vaccinia Virus Expressing Authentic Dengue Virus Nonstructural Protein NS1 Protects against Lethal Dengue Virus Encephalitis. J. Virol. 1990, 64, 4356–4363. [Google Scholar] [CrossRef] [Green Version]
- Gould, E.A.; Buckley, A.; Barrett, A.D.T.; Cammack, N. Neutralizing (54K) and Non-neutralizing (54K and 48K) Monoclonal Antibodies against Structural and Non-structural Yellow Fever Virus Proteins Confer Immunity in Mice. J. Gen. Virol. 1986, 67, 591–595. [Google Scholar] [CrossRef]
- Henchal, E.A.; Henchal, L.S.; Schlesinger, J.J. Synergistic Interactions of Anti-NS1 Monoclonal Antibodies Protect Passively Immunized Mice from Lethal Challenge with Dengue 2 Virus. J. Gen. Virol. 1988, 69, 2101–2107. [Google Scholar] [CrossRef]
- Schlesinger, J.J.; Brandriss, M.W.; Cropp, C.B.; Monath, T.P. Protection against Yellow Fever in Monkeys by Immunization with Yellow Fever Virus Nonstructural Protein NS1. J. Virol. 1986, 60, 1153–1155. [Google Scholar] [CrossRef] [Green Version]
- Schlesinger, J.J.; Brandriss, M.W.; Walsh, E.E. Protection of Mice against Dengue 2 Virus Encephalitis by Immunization with the Dengue 2 Virus Non-structural Glycoprotein NS1. J. Gen. Virol. 1987, 68, 853–857. [Google Scholar] [CrossRef]
- Wan, S.-W.; Chen, P.-W.; Chen, C.-Y.; Lai, Y.-C.; Chu, Y.-T.; Hung, C.-Y.; Lee, H.; Wu, H.F.; Chuang, Y.-C.; Lin, J.; et al. Therapeutic Effects of Monoclonal Antibody against Dengue Virus NS1 in a STAT1 Knockout Mouse Model of Dengue Infection. J. Immunol. 2017, 199, 2834–2844. [Google Scholar] [CrossRef] [Green Version]
- Kreil, T.R.; Maier, E.; Fraiss, S.; Eibl, M.M. Neutralizing Antibodies Protect against Lethal Flavivirus Challenge but Allow for the Development of Active Humoral Immunity to a Nonstructural Virus Protein. J. Virol. 1998, 72, 3076–3081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufmann, B.M.; Summers, P.L.; Dubois, R.D.; Houston Cohen, W.; Gentry, M.K.; Timchack, R.L.; Burke, D.S.; Eckels, K.H. Monoclonal Antibodies for Dengue Virus prM Glycoprotein Protect Mice against Lethal Dengue Infection. Am. J. Trop. Med. Hyg. 1989, 41, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Bray, M.; Lai, C.-J. Dengue Virus Premembrane and Membrane Proteins Elicit a Protective Immune Response. Virology 1991, 185, 505–508. [Google Scholar] [CrossRef]
- Beltramello, M.; Williams, K.L.; Simmons, C.P.; MacAgno, A.; Simonelli, L.; Quyen, N.T.H.; Sukupolvi-Petty, S.; Navarro-Sanchez, E.; Young, P.R.; De Silva, A.M.; et al. The Human Immune Response to Dengue Virus is Dominated by Highly Cross-Reactive Antibodies Endowed with Neutralizing and Enhancing Activity. Cell Host Microbe 2010, 8, 271–283. [Google Scholar] [CrossRef] [Green Version]
- Dejnirattisai, W.; Jumnainsong, A.; Onsirisakul, N.; Fitton, P.; Vasanawathana, S.; Limpitikul, W.; Puttikhunt, C.; Edwards, C.; Duangchinda, T.; Supasa, S.; et al. Cross-Reacting Antibodies Enhance Dengue Virus Infection in Humans. Science 2010, 328, 745–748. [Google Scholar] [CrossRef] [Green Version]
- Tsouchnikas, G.; Zlatkovic, J.; Jarmer, J.; Strauß, J.; Vratskikh, O.; Kundi, M.; Stiasny, K.; Heinz, F.X. Immunization with Immune Complexes Modulates the Fine Specificity of Antibody Responses to a Flavivirus Antigen. J. Virol. 2015, 89, 7970–7978. [Google Scholar] [CrossRef] [Green Version]
- Chernokhaeva, L.L.; Rogova, Y.V.; Vorovitch, M.F.; Romanova, L.I.; Kozlovskaya, L.I.; Maikova, G.B.; Kholodilov, I.S.; Karganova, G.G. Protective immunity spectrum induced by immunization with a vaccine from the TBEV strain Sofjin. Vaccine 2016, 34, 2354–2361. [Google Scholar] [CrossRef]
- Domnich, A.; Panatto, D.; Klementievna Arbuzova, E.; Signori, A.; Avio, U.; Gasparini, R.; Amicizia, D. Immunogenicity against Far Eastern and Siberian subtypes of tick-borne encephalitis (TBE) virus elicited by the currently available vaccines based on the European subtype: Systematic review and meta-analysis. Hum. Vaccin. Immunother. 2014, 10, 2819–2833. [Google Scholar] [CrossRef] [Green Version]
- Fritz, R.; Orlinger, K.K.; Hofmeister, Y.; Janecki, K.; Traweger, A.; Perez-Burgos, L.; Barrett, P.N.; Kreil, T.R. Quantitative comparison of the cross-protection induced by tick-borne encephalitis virus vaccines based on European and Far Eastern virus subtypes. Vaccine 2012, 30, 1165–1169. [Google Scholar] [CrossRef]
- McAuley, A.J.; Sawatsky, B.; Ksiazek, T.; Torres, M.; Korva, M.; Lotrič-Furlan, S.; Avšič-Županc, T.; von Messling, V.; Holbrook, M.R.; Freiberg, A.N.; et al. Cross-neutralisation of viruses of the tick-borne encephalitis complex following tick-borne encephalitis vaccination and/or infection. npj Vaccines 2017, 2, 1–10. [Google Scholar] [CrossRef]
- Morozova, O.V.; Bakhvalova, V.N.; Potapova, O.F.; Grishechkin, A.E.; Isaeva, E.I.; Aldarov, K.V.; Klinov, D.V.; Vorovich, M.F. Evaluation of immune response and protective effect of four vaccines against the tick-borne encephalitis virus. Vaccine 2014, 32, 3101–3106. [Google Scholar] [CrossRef] [PubMed]
- Chernokhaeva, L.L.; Rogova, Y.V.; Kozlovskaya, L.I.; Romanova, L.I.; Osolodkin, D.I.; Vorovitch, M.F.; Karganova, G.G. Experimental Evaluation of the Protective Efficacy of Tick-Borne Encephalitis (TBE) Vaccines Based on European and Far-Eastern TBEV Strains in Mice and in Vitro. Front. Microbiol. 2018, 9, 1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, Y.; Fritz, R.; Orlinger, K.; Kiermayr, S.; Ilk, R.; Portsmouth, D.; Pöllabauer, E.-M.; Löw-Baselli, A.; Hessel, A.; Kölch, D.; et al. Molecular Basis of the Divergent Immunogenicity of Two Pediatric Tick-Borne Encephalitis Virus Vaccines. J. Virol. 2016, 90, 1964–1972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chidumayo, N.N.; Yoshii, K.; Kariwa, H. Evaluation of the European tick-borne encephalitis vaccine against Omsk hemorrhagic fever virus. Microbiol. Immunol. 2014, 58, 112–118. [Google Scholar] [CrossRef]
- Mansfield, K.L.; Horton, D.L.; Johnson, N.; Li, L.; Barrett, A.D.T.; Smith, D.J.; Galbraith, S.E.; Solomon, T.; Fooks, A.R. Flavivirus-induced antibody cross-reactivity. J. Gen. Virol. 2011, 92, 2821–2829. [Google Scholar] [CrossRef]
- Schuller, E.; Klade, C.S.; Heinz, F.X.; Kollaritsch, H.; Rendi-Wagner, P.; Jilma, B.; Tauber, E. Effect of pre-existing anti-tick-borne encephalitis virus immunity on neutralising antibody response to the Vero cell-derived, inactivated Japanese encephalitis virus vaccine candidate IC51. Vaccine 2008, 26, 6151–6156. [Google Scholar] [CrossRef]
- Koraka, P.; Zeller, H.; Niedrig, M.; Osterhaus, A.D.M.E.; Groen, J. Reactivity of serum samples from patients with a flavivirus infection measured by immunofluorescence assay and ELISA. Microbes Infect. 2002, 4, 1209–1215. [Google Scholar] [CrossRef]
- Takada, A.; Kawaoka, Y. Antibody-dependent enhancement of viral infection: Molecular mechanisms and in vivo implications. Rev. Med. Virol. 2003, 13, 387–398. [Google Scholar] [CrossRef]
- Haslwanter, D.; Blaas, D.; Heinz, F.X.; Stiasny, K. A novel mechanism of antibody-mediated enhancement of flavivirus infection. PLoS Pathog. 2017, 13, e1006643. [Google Scholar] [CrossRef] [Green Version]
- St. John, A.L.; Rathore, A.P.S. Adaptive immune responses to primary and secondary dengue virus infections. Nat. Rev. Immunol. 2019, 19, 218–230. [Google Scholar] [CrossRef]
- Wilken, L.; Rimmelzwaan, G.F. Adaptive Immunity to Dengue Virus: Slippery Slope or Solid Ground for Rational Vaccine Design? Pathogens 2020, 9, 470. [Google Scholar] [CrossRef] [PubMed]
- Phillpotts, R.J.; Stephenson, J.R.; Porterfield, J.S. Antibody-dependent Enhancement of Tick-borne Encephalitis Virus Infectivity. J. Gen. Virol. 1985, 66, 1831–1837. [Google Scholar] [CrossRef] [PubMed]
- Kopecký, J.; Grubhoffer, L.; Tomková, E. Interaction of tick-borne encephalitis virus with mouse peritoneal macrophages. The effect of antiviral antibody and lectin. Acta Virol. 1991, 35, 218–225. [Google Scholar] [PubMed]
- Kreil, T.R.; Eibl, M.M. Pre-and Postexposure Protection by Passive Immunoglobulin but no Enhancement of Infection with a Flavivirus in a Mouse Model. J. Virol. 1997, 71, 2921–2927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsterova, J.; Palus, M.; Sirmarova, J.; Kopecky, J.; Niller, H.H.; Ruzek, D. Tick-borne encephalitis virus neutralization by high dose intravenous immunoglobulin. Ticks Tick-Borne Dis. 2017, 8, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Matveev, A.L.; Kozlova, I.V.; Stronin, O.V.; Khlusevich, Y.A.; Doroshchenko, E.K.; Baykov, I.K.; Lisak, O.V.; Emelyanova, L.A.; Suntsova, O.V.; Matveeva, V.A.; et al. Post-exposure administration of chimeric antibody protects mice against European, Siberian, and Far-Eastern subtypes of tick-borne encephalitis virus. PLoS ONE 2019, 14, e0215075. [Google Scholar] [CrossRef]
- Duehr, J.; Lee, S.; Singh, G.; Foster, G.A.; Krysztof, D.; Stramer, S.L.; González, M.C.B.; Menichetti, E.; Geretschläger, R.; Gabriel, C.; et al. Tick-Borne Encephalitis Virus Vaccine-Induced Human Antibodies Mediate Negligible Enhancement of Zika Virus Infection In Vitro and in a Mouse Model. MSphere 2018, 3, e00011-18. [Google Scholar] [CrossRef] [Green Version]
- Wieczorek, M.; Abualrous, E.T.; Sticht, J.; Álvaro-Benito, M.; Stolzenberg, S.; Noé, F.; Freund, C. Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation. Front. Immunol. 2017, 8, 292. [Google Scholar] [CrossRef] [Green Version]
- Sant, A.J.; McMichael, A. Revealing the role of CD4+ T cells in viral immunity. J. Exp. Med. 2012, 209, 1391–1395. [Google Scholar] [CrossRef] [Green Version]
- Elong Ngono, A.; Young, M.P.; Bunz, M.; Xu, Z.; Hattakam, S.; Vizcarra, E.; Regla-Nava, J.A.; Tang, W.W.; Yamabhai, M.; Wen, J.; et al. CD4+ T cells promote humoral immunity and viral control during Zika virus infection. PLoS Pathog. 2019, 15, e1007474. [Google Scholar] [CrossRef] [Green Version]
- Sitati, E.M.; Diamond, M.S. CD4+ T-Cell Responses Are Required for Clearance of West Nile Virus from the Central Nervous System. J. Virol. 2006, 80, 12060–12069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turtle, L.; Bali, T.; Buxton, G.; Chib, S.; Chan, S.; Soni, M.; Hussain, M.; Isenman, H.; Fadnis, P.; Venkataswamy, M.M.; et al. Human T cell responses to Japanese encephalitis virus in health and disease. J. Exp. Med. 2016, 213, 1331–1352. [Google Scholar] [CrossRef] [PubMed]
- Koblischke, M.; Stiasny, K.; Aberle, S.W.; Malafa, S.; Tsouchnikas, G.; Schwaiger, J.; Kundi, M.; Heinz, F.X.; Aberle, J.H. Structural Influence on the Dominance of Virus-Specific CD4 T Cell Epitopes in Zika Virus Infection. Front. Immunol. 2018, 9, 1196. [Google Scholar] [CrossRef] [PubMed]
- Brien, J.D.; Uhrlaub, J.L.; Nikolich-Žugich, J. West Nile Virus-Specific CD4 T Cells Exhibit Direct Antiviral Cytokine Secretion and Cytotoxicity and Are Sufficient for Antiviral Protection. J. Immunol. 2008, 181, 8568–8575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurane, I.; Meager, A.; Ennis, F.A. Dengue Virus-Specific Human T Cell Clones. Serotype Crossreactive Proliferation, Interferon γ Production, and Cytotoxic Activity. J. Exp. Med. 1989, 170, 763–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Chambers, T.J. Yellow Fever Virus Encephalitis: Properties of the Brain-Associated T-Cell Response during Virus Clearance in Normal and Gamma Interferon-Deficient Mice and Requirement for CD4+ Lymphocytes. J. Virol. 2001, 75, 2107–2118. [Google Scholar] [CrossRef] [Green Version]
- Mangada, M.M.; Rothman, A.L. Altered Cytokine Responses of Dengue-Specific CD4+ T Cells to Heterologous Serotypes. J. Immunol. 2005, 175, 2676–2683. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.L.; Martins, M.A.; Espírito-Santo, L.R.; Campi-Azevedo, A.C.; Silveira-Lemos, D.; Ribeiro, J.G.L.; Homma, A.; Kroon, E.G.; Teixeira-Carvalho, A.; Elói-Santos, S.M.; et al. Characterization of main cytokine sources from the innate and adaptive immune responses following primary 17DD yellow fever vaccination in adults. Vaccine 2011, 29, 583–592. [Google Scholar] [CrossRef]
- Yauch, L.E.; Prestwood, T.R.; May, M.M.; Morar, M.M.; Zellweger, R.M.; Peters, B.; Sette, A.; Shresta, S. CD4+ T Cells Are Not Required for the Induction of Dengue Virus-Specific CD8+ T Cell or Antibody Responses but Contribute to Protection after Vaccination. J. Immunol. 2010, 185, 5405–5416. [Google Scholar] [CrossRef] [Green Version]
- Xiang, J.; McLinden, J.H.; Rydze, R.A.; Chang, Q.; Kaufman, T.M.; Klinzman, D.; Stapleton, J.T. Viruses within the Flaviviridae Decrease CD4 Expression and Inhibit HIV Replication in Human CD4+ Cells. J. Immunol. 2009, 183, 7860–7869. [Google Scholar] [CrossRef] [Green Version]
- Rathore, A.P.S.; St John, A.L. Cross-Reactive Immunity among Flaviviruses. Front. Immunol. 2020, 11, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aihara, H.; Takasaki, T.; Matsutani, T.; Suzuki, R.; Kurane, I. Establishment and Characterization of Japanese Encephalitis Virus-Specific, Human CD4+ T-Cell Clones: Flavivirus Cross-Reactivity, Protein Recognition, and Cytotoxic Activity. J. Virol. 1998, 72, 8032–8036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saron, W.A.A.; Rathore, A.P.S.; Ting, L.; Ooi, E.E.; Low, J.; Abraham, S.N.; St John, A.L. Flavivirus serocomplex cross-reactive immunity is protective by activating heterologous memory CD4 T cells. Sci. Adv. 2018, 4, eaar4297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elong Ngono, A.; Shresta, S. Cross-Reactive T Cell Immunity to Dengue and Zika Viruses: New Insights Into Vaccine Development. Front. Immunol. 2019, 10, 1316. [Google Scholar] [CrossRef] [Green Version]
- Gelpi, E.; Preusser, M.; Laggner, U.; Garzuly, F.; Holzmann, H.; Heinz, F.X.; Budka, H. Inflammatory response in human tick-borne encephalitis: Analysis of postmortem brain tissue. J. Neurovirol. 2006, 12, 322–327. [Google Scholar] [CrossRef]
- Gelpi, E.; Preusser, M.; Garzuly, F.; Holzmann, H.; Heinz, F.X.; Budka, H. Visualization of Central European Tick-Borne Encephalitis Infection in Fatal Human Cases. J. Neuropathol. Exp. Neurol. 2005, 64, 506–512. [Google Scholar] [CrossRef] [Green Version]
- Fujii, Y.; Hayasaka, D.; Kitaura, K.; Takasaki, T.; Suzuki, R.; Kurane, I. T-Cell Clones Expressing Different T-Cell Receptors Accumulate in the Brains of Dying and Surviving Mice After Peripheral Infection with Far Eastern Strain of Tick-Borne Encephalitis Virus. Viral Immunol. 2011, 24, 291–302. [Google Scholar] [CrossRef]
- Johnson, R.T.; Burke, D.S.; Elwell, M.; Leake, C.J.; Nisalak, A.; Hoke, C.H.; Lorsomrudee, W. Japanese Encephalitis: Immunocytochemical Studies of Viral Antigen and Inflammatory Cells in Fatal Cases. Ann. Neurol. 1985, 18, 567–573. [Google Scholar] [CrossRef]
- Liu, Y.; Blanden, R.V.; Müllbacher, A. Identification of Cytolytic Lymphocytes in West Nile Virus-infected Murine Central Nervous System. J. Gen. Virol. 1989, 70, 565–573. [Google Scholar] [CrossRef]
- Wang, Y.; Lobigs, M.; Lee, E.; Müllbacher, A. CD8+ T Cells Mediate Recovery and Immunopathology in West Nile Virus Encephalitis. J. Virol. 2003, 77, 13323–13334. [Google Scholar] [CrossRef] [Green Version]
- Aberle, J.H.; Aberle, S.W.; Kofler, R.M.; Mandl, C.W. Humoral and Cellular Immune Response to RNA Immunization with Flavivirus Replicons Derived from Tick-Borne Encephalitis Virus. J. Virol. 2005, 79, 15107–15113. [Google Scholar] [CrossRef] [Green Version]
- Gomez, I.; Marx, F.; Saurwein-Teissl, M.; Gould, E.A.; Grubeck-Loebenstein, B. Characterization of Tick-Borne Encephalitis Virus-Specific Human T Lymphocyte Responses by Stimulation with Structural TBEV Proteins Expressed in a Recombinant Baculovirus. Viral Immunol. 2003, 16, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Dobler, G.; Kaier, K.; Hehn, P.; Böhmer, M.M.; Kreusch, T.M.; Borde, J.P. Tick-borne encephalitis virus vaccination breakthrough infections in Germany-A retrospective analysis from 2001–2018. Clin. Microbiol. Infect. 2019. [Google Scholar] [CrossRef]
- Rendi-Wagner, P.; Zent, O.; Jilg, W.; Plentz, A.; Beran, J.; Kollaritsch, H. Persistence of antibodies after vaccination against tick-borne encephalitis. Int. J. Med. Microbiol. 2006, 296, 202–207. [Google Scholar] [CrossRef]
- Lotric-Furlan, S.; Avšič-Županc, T.; Strle, F. Tick-borne encephalitis after active immunization. Int. J. Med. Microbiol. 2008, 298, 309–313. [Google Scholar] [CrossRef]
- Stiasny, K.; Holzmann, H.; Heinz, F.X. Characteristics of antibody responses in tick-borne encephalitis vaccination breakthroughs. Vaccine 2009, 27, 7021–7026. [Google Scholar] [CrossRef] [PubMed]
- Garner-Spitzer, E.; Wagner, A.; Paulke-Korinek, M.; Kollaritsch, H.; Heinz, F.X.; Redlberger-Fritz, M.; Stiasny, K.; Fischer, G.F.; Kundi, M.; Wiedermann, U. Tick-Borne Encephalitis (TBE) and Hepatitis B Nonresponders Feature Different Immunologic Mechanisms in Response to TBE and Influenza Vaccination with Involvement of Regulatory T and B Cells and IL-10. J. Immunol. 2013, 191, 2426–2436. [Google Scholar] [CrossRef] [PubMed]
- Heinz, F.X.; Allison, S.L.; Stiasny, K.; Schalich, J.; Holzmann, H.; Mandl, C.W.; Kunz, C. Recombinant and virion-derived soluble and particulate immunogens for vaccination against tick-borne encephalitis. Vaccine 1995, 13, 1636–1642. [Google Scholar] [CrossRef]
- Aberle, J.H.; Aberle, S.W.; Allison, S.L.; Stiasny, K.; Ecker, M.; Mandl, C.W.; Berger, R.; Heinz, F.X. A DNA Immunization Model Study with Constructs Expressing the Tick-Borne Encephalitis Virus Envelope Protein E in Different Physical Forms. J. Immunol. 1999, 163, 6756–6761. [Google Scholar]
- Slon Campos, J.L.; Poggianella, M.; Marchese, S.; Mossenta, M.; Rana, J.; Arnoldi, F.; Bestagno, M.; Burrone, O.R. DNA-immunisation with dengue virus E protein domains I/II, but not domain III, enhances Zika, West Nile and Yellow Fever virus infection. PLoS ONE 2017, 12, e0181734. [Google Scholar] [CrossRef] [Green Version]
- Martina, B.E.E.; van den Doel, P.; Koraka, P.; van Amerongen, G.; Spohn, G.; Haagmans, B.L.; Provacia, L.B.V.; Osterhaus, A.D.M.E.; Rimmelzwaan, G.F. A Recombinant Influenza A Virus Expressing Domain III of West Nile Virus Induces Protective Immune Responses against Influenza and West Nile Virus. PLoS ONE 2011, 6, e18995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Camacho, C.; De Lorenzo, G.; Slon-Campos, J.L.; Dowall, S.; Abbink, P.; Larocca, R.A.; Kim, Y.C.; Poggianella, M.; Graham, V.; Findlay-Wilson, S.; et al. Immunogenicity and Efficacy of Zika Virus Envelope Domain III in DNA, Protein, and ChAdOx1 Adenoviral-Vectored Vaccines. Vaccines 2020, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.; Jassey, A.; Wang, J.Y.; Wang, W.-C.; Liu, C.-H.; Lin, L.-T. Virus-Like Particle Systems for Vaccine Development against Viruses in the Flaviviridae Family. Vaccines 2019, 7, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allison, S.L.; Stadler, K.; Mandl, C.W.; Kunz, C.; Heinz, F.X. Synthesis and Secretion of Recombinant Tick-Borne Encephalitis Virus Protein E in Soluble and Particulate Form. J. Virol. 1995, 69, 5816–5820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehrke, R.; Ecker, M.; Aberle, S.W.; Allison, S.L.; Heinz, F.X.; Mandl, C.W. Incorporation of Tick-Borne Encephalitis Virus Replicons into Virus-Like Particles by a Packaging Cell Line. J. Virol. 2003, 77, 8924–8933. [Google Scholar] [CrossRef] [Green Version]
- Yun, S.-M.; Jeong, Y.E.; Wang, E.; Lee, Y.-J.; Han, M.G.; Park, C.; Lee, W.-J.; Choi, W. Cloning and Expression of Recombinant Tick-Borne Encephalitis Virus-like Particles in Pichia pastoris. Osong Public Heal. Res. Perspect. 2014, 5, 274–278. [Google Scholar] [CrossRef] [Green Version]
- Schalich, J.; Allison, S.L.; Stiasny, K.; Mandl, C.W.; Kunz, C.; Heinz, F.X. Recombinant Subviral Particles from Tick-Borne Encephalitis Virus Are Fusogenic and Provide a Model System for Studying Flavivirus Envelope Glycoprotein Functions. J. Virol. 1996, 70, 4549–4557. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Limas, W.A.; Sekar, K.; Tyo, K.E.J. Virus-like particles: The future of microbial factories and cell-free systems as platforms for vaccine development. Curr. Opin. Biotechnol. 2013, 24, 1089–1093. [Google Scholar] [CrossRef]
- Garg, H.; Mehmetoglu-Gurbuz, T.; Ruddy, G.M.; Joshi, A. Capsid containing virus like particle vaccine against Zika virus made from a stable cell line. Vaccine 2019, 37, 7123–7131. [Google Scholar] [CrossRef]
- Schmaljohn, C.; VanderZanden, L.; Bray, M.; Custer, D.; Meyer, B.; Li, D.; Rossi, C.; Fuller, D.; Fuller, J.; Haynes, J.; et al. Naked DNA Vaccines Expressing the prM and E Genes of Russian Spring Summer Encephalitis Virus and Central European Encephalitis Virus Protect Mice from Homologous and Heterologous Challenge. J. Virol. 1997, 71, 9563–9569. [Google Scholar] [CrossRef] [Green Version]
- Schmaljohn, C.; Custer, D.; VanderZanden, L.; Spik, K.; Rossi, C.; Bray, M. Evaluation of Tick-Borne Encephalitis DNA Vaccines in Monkeys. Virology 1999, 263, 166–174. [Google Scholar] [CrossRef] [Green Version]
- Holzer, G.W.; Remp, G.; Antoine, G.; Pfleiderer, M.; Enzersberger, O.M.; Emsenhuber, W.; Hämmerle, T.; Gruber, F.; Urban, C.; Falkner, F.G.; et al. Highly Efficient Induction of Protective Immunity by a Vaccinia Virus Vector Defective in Late Gene Expression. J. Virol. 1999, 73, 4536–4542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timofeev, A.V.; Ozherelkov, S.V.; Pronin, A.V.; Deeva, A.V.; Karganova, G.G.; Elbert, L.B.; Stephenson, J.R. Immunological basis for protection in a murine model of tick-borne encephalitis by a recombinant adenovirus carrying the gene encoding the NS1 non-structural protein. J. Gen. Virol. 1998, 79, 689–695. [Google Scholar] [CrossRef]
- Khoretonenko, M.V.; Vorovitch, M.F.; Zakharova, L.G.; Pashvykina, G.V.; Ovsyannikova, N.V.; Stephenson, J.R.; Timofeev, A.V.; Altstein, A.D.; Shneider, A.M. Vaccinia virus recombinant expressing gene of tick-borne encephalitis virus non-structural NS1 protein elicits protective activity in mice. Immunol. Lett. 2003, 90, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Yu, J.; Lu, M.; Ma, Y.; Attia, Z.; Shan, C.; Xue, M.; Liang, X.; Craig, K.; Makadiya, N.; et al. A Zika virus vaccine expressing premembrane-envelope-NS1 polyprotein. Nat. Commun. 2018, 9, 3067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandl, C.W.; Aberle, J.H.; Aberle, S.W.; Holzmann, H.; Allison, S.L.; Heinz, F.X. In vitro-synthesized infectious RNA as an attenuated live vaccine in a flavivirus model. Nat. Med. 1998, 4, 1438–1440. [Google Scholar] [CrossRef] [PubMed]
- Gritsun, T.S.; Lashkevich, V.A.; Gould, E.A. Tick-borne encephalitis. Antivir. Res. 2003, 57, 129–146. [Google Scholar] [CrossRef]
- VanBlargan, L.A.; Himansu, S.; Foreman, B.M.; Ebel, G.D.; Pierson, T.C.; Diamond, M.S. An mRNA Vaccine Protects Mice against Multiple Tick-Transmitted Flavivirus Infections. Cell Rep. 2018, 25, 3382–3392. [Google Scholar] [CrossRef] [Green Version]
- Aberle, J.H.; Koblischke, M.; Stiasny, K. CD4 T cell responses to flaviviruses. J. Clin. Virol. 2018, 108, 126–131. [Google Scholar] [CrossRef]
- Panagioti, E.; Klenerman, P.; Lee, L.N.; van der Burg, S.H.; Arens, R. Features of Effective T Cell-Inducing Vaccines against Chronic Viral Infections. Front. Immunol. 2018, 9, 276. [Google Scholar] [CrossRef] [Green Version]
- Volz, A.; Lim, S.; Kaserer, M.; Lülf, A.; Marr, L.; Jany, S.; Deeg, C.A.; Pijlman, G.P.; Koraka, P.; Osterhaus, A.D.M.E.; et al. Immunogenicity and protective efficacy of recombinant Modified Vaccinia virus Ankara candidate vaccines delivering West Nile virus envelope antigens. Vaccine 2016, 34, 1915–1926. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Zhang, T.; Zhang, Y.; Wang, H.; Deng, F. Zika Virus Baculovirus-Expressed Virus-Like Particles Induce Neutralizing Antibodies in Mice. Virol. Sin. 2018, 33, 213–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardi, N.; Hogan, M.J.; Pelc, R.S.; Muramatsu, H.; Andersen, H.; DeMaso, C.R.; Dowd, K.A.; Sutherland, L.L.; Scearce, R.M.; Parks, R.; et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 2017, 543, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Omori-Urabe, Y.; Yoshii, K.; Ikawa-Yoshida, A.; Kariwa, H.; Takashima, I. Needle-free jet injection of DNA and protein vaccine of the Far-Eastern subtype of tick-borne encephalitis virus induces protective immunity in mice. Microbiol. Immunol. 2011, 55, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Suschak, J.J.; Williams, J.A.; Schmaljohn, C.S. Advancements in DNA vaccine vectors, non-mechanical delivery methods, and molecular adjuvants to increase immunogenicity. Hum. Vaccin. Immunother. 2017, 13, 2837–2848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [Green Version]
- Mastelic Gavillet, B.; Eberhardt, C.S.; Auderset, F.; Castellino, F.; Seubert, A.; Tregoning, J.S.; Lambert, P.-H.; de Gregorio, E.; Del Giudice, G.; Siegrist, C.-A. MF59 Mediates Its B Cell Adjuvanticity by Promoting T Follicular Helper Cells and Thus Germinal Center Responses in Adult and Early Life. J. Immunol. 2015, 194, 4836–4845. [Google Scholar] [CrossRef] [Green Version]
- Dmitriev, I.P.; Khromykh, A.A.; Ignatyev, G.M.; Gainullina, M.N.; Ageenko, V.A.; Dryga, S.A.; Vorobyeva, M.S.; Sandakhchiev, L.S. Immunization with recombinant vaccinia viruses expressing structural and part of the nonstructural region of tick-borne encephalitis virus cDNA protect mice against lethal encephalitis. J. Biotechnol. 1996, 44, 97–103. [Google Scholar] [CrossRef]
- Korenkov, D.; Isakova-Sivak, I.; Rudenko, L. Basics of CD8 T-cell immune responses after influenza infection and vaccination with inactivated or live attenuated influenza vaccine. Expert Rev. Vaccines 2018, 17, 977–987. [Google Scholar] [CrossRef]
- Collins, N.D.; Barrett, A.D.T. Live Attenuated Yellow Fever 17D Vaccine: A Legacy Vaccine Still Controlling Outbreaks in Modern Day. Curr. Infect. Dis. Rep. 2017, 19, 14. [Google Scholar] [CrossRef]
- De Fabritus, L.; Nougairède, A.; Aubry, F.; Gould, E.A.; De Lamballerie, X. Attenuation of Tick-Borne Encephalitis Virus Using Large-Scale Random Codon Re-encoding. PLoS Pathog. 2015, 11, e1004738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gritsun, T.S.; Desai, A.; Gould, E.A. The degree of attenuation of tick-borne encephalitis virus depends on the cumulative effects of point mutations. J. Gen. Virol. 2001, 82, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Kofler, R.M.; Heinz, F.X.; Mandl, C.W. Capsid Protein C of Tick-Borne Encephalitis Virus Tolerates Large Internal Deletions and Is a Favorable Target for Attenuation of Virulence. J. Virol. 2002, 76, 3534–3543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandl, C.W.; Allison, S.L.; Holzmann, H.; Meixner, T.; Heinz, F.X. Attenuation of Tick-Borne Encephalitis Virus by Structure-Based Site-Specific Mutagenesis of a Putative Flavivirus Receptor Binding Site. J. Virol. 2000, 74, 9601–9609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandl, C.W.; Holzmann, H.; Meixner, T.; Rauscher, S.; Stadler, P.F.; Allison, S.L.; Heinz, F.X. Spontaneous and Engineered Deletions in the 3’ Noncoding Region of Tick-Borne Encephalitis Virus: Construction of Highly Attenuated Mutants of a Flavivirus. J. Virol. 1998, 72, 2132–2140. [Google Scholar] [CrossRef] [Green Version]
- Mandl, C.W.; Kroschewski, H.; Allison, S.L.; Kofler, R.; Holzmann, H.; Meixner, T.; Heinz, F.X. Adaptation of Tick-Borne Encephalitis Virus to BHK-21 Cells Results in the Formation of Multiple Heparan Sulfate Binding Sites in the Envelope Protein and Attenuation in Vivo. J. Virol. 2001, 75, 5627–5637. [Google Scholar] [CrossRef] [Green Version]
- Kofler, R.M.; Leitner, A.; O’Riordain, G.; Heinz, F.X.; Mandl, C.W. Spontaneous Mutations Restore the Viability of Tick-Borne Encephalitis Virus Mutants with Large Deletions in Protein C. J. Virol. 2003, 77, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Guy, B.; Noriega, F.; Ochiai, R.L.; L’azou, M.; Delore, V.; Skipetrova, A.; Verdier, F.; Coudeville, L.; Savarino, S.; Jackson, N. A recombinant live attenuated tetravalent vaccine for the prevention of dengue. Expert Rev. Vaccines 2017, 16, 1–13. [Google Scholar] [CrossRef]
- Appaiahgari, M.B.; Vrati, S. IMOJEV®: A Yellow fever virus-based novel Japanese encephalitis vaccine. Expert Rev. Vaccines 2010, 9, 1371–1384. [Google Scholar] [CrossRef]
- Rumyantsev, A.A.; Goncalvez, A.P.; Giel-Moloney, M.; Catalan, J.; Liu, Y.; Gao, Q.; Almond, J.; Kleanthous, H.; Pugachev, K.V. Single-dose vaccine against tick-borne encephalitis. Proc. Natl. Acad. Sci. USA 2013, 110, 13103–13108. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-J.; Li, X.-F.; Ye, Q.; Li, S.-H.; Deng, Y.-Q.; Zhao, H.; Xu, Y.-P.; Ma, J.; Qin, E.-D.; Qin, C.-F. Recombinant chimeric Japanese encephalitis virus/tick-borne encephalitis virus is attenuated and protective in mice. Vaccine 2014, 32, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Pletnev, A.G.; Bray, M.; Huggins, J.; Lai, C.-J. Construction and characterization of chimeric tick-borne encephalitis/dengue type 4 viruses. Proc. Natl. Acad. Sci. USA 1992, 89, 10532–10536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pletnev, A.G.; Bray, M.; Lai, C.-J. Chimeric Tick-Borne Encephalitis and Dengue Type 4 Viruses: Effects of Mutations on Neurovirulence in Mice. J. Virol. 1993, 67, 4956–4963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pletnev, A.G.; Men, R. Attenuation of the Langat tick-borne flavivirus by chimerization with mosquito-borne flavivirus dengue type 4. Proc. Natl. Acad. Sci. USA 1998, 95, 1746–1751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiss, B.L.; Maximova, O.A.; Pletnev, A.G. Insertion of MicroRNA Targets into the Flavivirus Genome Alters Its Highly Neurovirulent Phenotype. J. Virol. 2011, 85, 1464–1472. [Google Scholar] [CrossRef] [Green Version]
- Heiss, B.L.; Maximova, O.A.; Thach, D.C.; Speicher, J.M.; Pletnev, A.G. MicroRNA Targeting of Neurotropic Flavivirus: Effective Control of Virus Escape and Reversion to Neurovirulent Phenotype. J. Virol. 2012, 86, 5647–5659. [Google Scholar] [CrossRef] [Green Version]
- Teterina, N.L.; Liu, G.; Maximova, O.A.; Pletnev, A.G. Silencing of neurotropic flavivirus replication in the central nervous system by combining multiple microRNA target insertions in two distinct viral genome regions. Virology 2014, 456, 247–258. [Google Scholar] [CrossRef] [Green Version]
- Tsetsarkin, K.A.; Maximova, O.A.; Liu, G.; Kenney, H.; Teterina, N.L.; Pletnev, A.G. Stable and Highly Immunogenic MicroRNA-Targeted Single-Dose Live Attenuated Vaccine Candidate against Tick-Borne Encephalitis Constructed Using Genetic Backbone of Langat Virus. MBio 2019, 10, e02904-18. [Google Scholar] [CrossRef] [Green Version]
- Engel, A.R.; Rumyantsev, A.A.; Maximova, O.A.; Speicher, J.M.; Heiss, B.; Murphy, B.R.; Pletnev, A.G. The neurovirulence and neuroinvasiveness of chimeric tick-borne encephalitis/dengue virus can be attenuated by introducing defined mutations into the envelope and NS5 protein genes and the 3’ non-coding region of the genome. Virology 2010, 405, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Rumyantsev, A.A.; Chanock, R.M.; Murphy, B.R.; Pletnev, A.G. Comparison of live and inactivated tick-borne encephalitis virus vaccines for safety, immunogenicity and efficacy in rhesus monkeys. Vaccine 2006, 24, 133–143. [Google Scholar] [CrossRef]
- Maximova, O.A.; Ward, J.M.; Asher, D.M.; Claire, M.S.; Finneyfrock, B.W.; Speicher, J.M.; Murphy, B.R.; Pletnev, A.G. Comparative Neuropathogenesis and Neurovirulence of Attenuated Flaviviruses in Nonhuman Primates. J. Virol. 2008, 82, 5255–5268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pletnev, A.G.; Bray, M.; Hanley, K.A.; Speicher, J.; Elkins, R. Tick-Borne Langat/Mosquito-Borne Dengue Favivirus Chimera, a Candidate Live Attenuated Vaccine for Protection against Disease Caused by Members of the Tick-Borne Encephalitis Virus Complex: Evaluation in Rhesus Monkeys and in Mosquitos. J. Virol. 2001, 75, 8259–8267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pletnev, A.G.; Karganova, G.G.; Dzhivanyan, T.I.; Lashkevich, V.A.; Bray, M. Chimeric Langat/Dengue Viruses Protect Mice from Heterologous Challenge with the Highly Virulent Strains of Tick-Borne Encephalitis Virus. Virology 2000, 274, 26–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, P.F.; Ankrah, S.; Henderson, S.E.; Durbin, A.P.; Speicher, J.; Whitehead, S.S.; Murphy, B.R.; Pletnev, A.G. Evaluation of the Langat/dengue 4 chimeric virus as a live attenuated tick-borne encephalitis vaccine for safety and immunogenicity in healthy adult volunteers. Vaccine 2008, 26, 882–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grifoni, A.; Voic, H.; Dhanda, S.K.; Kidd, C.K.; Brien, J.D.; Buus, S.; Stryhn, A.; Durbin, A.P.; Whitehead, S.; Diehl, S.A.; et al. T Cell Responses Induced by Attenuated Flavivirus Vaccination Are Specific and Show Limited Cross-Reactivity with Other Flavivirus Species. J. Virol. 2020, 94. [Google Scholar] [CrossRef]
- Kofler, R.M.; Aberle, J.H.; Aberle, S.W.; Allison, S.L.; Heinz, F.X.; Mandl, C.W. Mimicking live flavivirus immunization with a noninfectious RNA vaccine. Proc. Natl. Acad. Sci. USA 2004, 101, 1951–1956. [Google Scholar] [CrossRef] [Green Version]
- Mok, D.Z.L.; Chan, K.R. The Effects of Pre-Existing Antibodies on Live-Attenuated Viral Vaccines. Viruses 2020, 12, 520. [Google Scholar] [CrossRef]
- Woodland, D.L. Jump-starting the immune system: Prime-boosting comes of age. Trends Immunol. 2004, 25, 98–104. [Google Scholar] [CrossRef]
- Kardani, K.; Bolhassani, A.; Shahbazi, S. Prime-boost vaccine strategy against viral infections: Mechanisms and benefits. Vaccine 2016, 34, 413–423. [Google Scholar] [CrossRef]
- Guimarães-Walker, A.; Mackie, N.; McCormack, S.; Hanke, T.; Schmidt, C.; Gilmour, J.; Barin, B.; McMichael, A.; Weber, J.; Legg, K.; et al. Lessons from IAVI-006, a Phase I clinical trial to evaluate the safety and immunogenicity of the pTHr.HIVA DNA and MVA.HIVA vaccines in a prime-boost strategy to induce HIV-1 specific T-cell responses in healthy volunteers. Vaccine 2008, 26, 6671–6677. [Google Scholar] [CrossRef]
- Churchyard, G.J.; Morgan, C.; Adams, E.; Hural, J.; Graham, B.S.; Moodie, Z.; Grove, D.; Gray, G.; Bekker, L.-G.; McElrath, M.J.; et al. A Phase IIA Randomized Clinical Trial of a Multiclade HIV-1 DNA Prime Followed by a Multiclade rAd5 HIV-1 Vaccine Boost in Healthy Adults (HVTN204). PLoS ONE 2011, 6, e21225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaoko, W.; Karita, E.; Kayitenkore, K.; Omosa-Manyonyi, G.; Allen, S.; Than, S.; Adams, E.M.; Graham, B.S.; Koup, R.A.; Bailer, R.T.; et al. Safety and Immunogenicity Study of Multiclade HIV-1 Adenoviral Vector Vaccine Alone or as Boost following a Multiclade HIV-1 DNA Vaccine in Africa. PLoS ONE 2010, 5, e12873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aleshin, S.E.; Timofeev, A.V.; Khoretonenko, M.V.; Zakharova, L.G.; Pashvykina, G.V.; Stephenson, J.R.; Shneider, A.M.; Altstein, A.D. Combined prime-boost vaccination against tick-borne encephalitis (TBE) using a recombinant vaccinia virus and a bacterial plasmid both expressing TBE virus non-structural NS1 protein. BMC Microbiol. 2005, 5, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
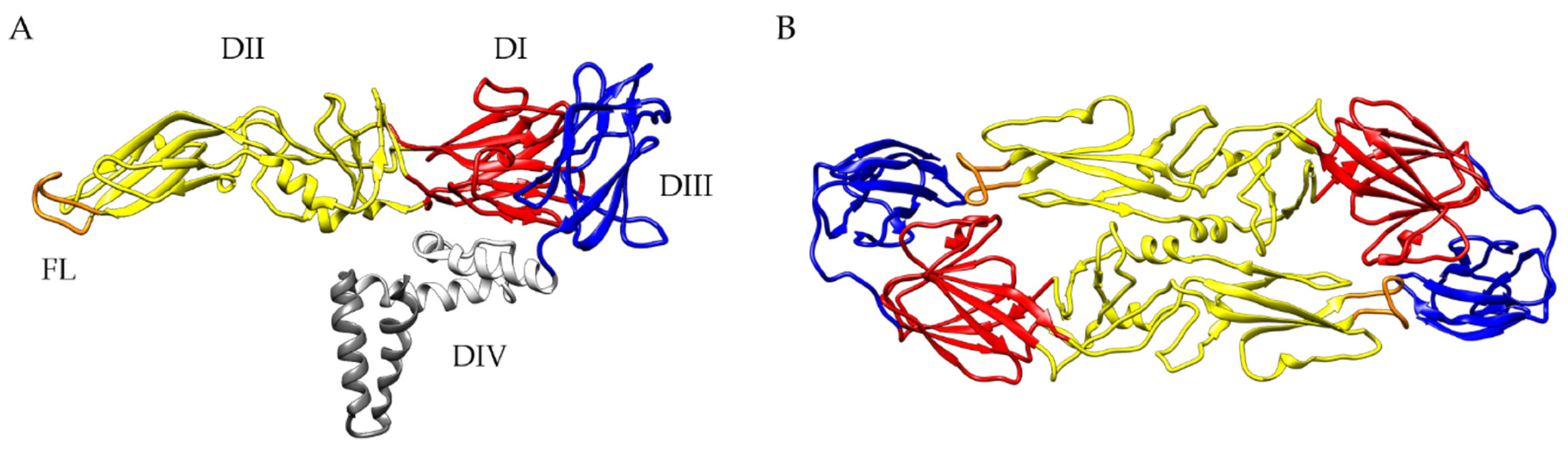

| Vaccine [3] | TBEV Strain (Subtype) [3] | Antigen Content [3] | Adjuvant [3] | Stabilizer [3] | Pediatric Vaccine Available [3] | Immunization Schedule [42] |
|---|---|---|---|---|---|---|
| FSME-IMMUN a | Neudoerfl (TBEV-Eu) | 2.4 µg | Al(OH)3 | HSA | Yes | 1st + 2nd dose: 1–3m, 3rd dose: 5–12m, 1st booster dose: after 3y, subsequent booster doses every 5y (<60 years) * or every 3y (≥60 years) |
| Encepur b | K23 (TBEV-Eu) | 1.5 µg | Al(OH)3 | Sucrose | Yes | 1st + 2nd dose: 2w–3m, 3rd dose: 9–12m, 1st booster dose: after 3y, subsequent booster doses every 5y (<60 years) * or every 3y (≥60 years) |
| TBE vaccine Moscow c | Sofjin (TBEV-FE) | 1.0 ± 0.5 µg/mL | Al(OH)3 | Sucrose, HSA, gelatose | No, used for ≥3 years | 1st + 2nd dose: 1–7m, 1st booster dose: after 1y, subsequent booster doses every 3y |
| Tick-E-Vac c | Sofjin (TBEV-FE) | 1.0 ± 0.5 µg/mL | Al(OH)3 | Sucrose, HSA | Yes | 1st + 2nd dose: 1–7m, 1st booster dose: after 1y, subsequent booster doses every 3y [3] |
| EnceVir d | 205 (TBEV-FE) | 2.0–2.5 µg | Al(OH)3 | Sucrose, HSA | Yes | 1st + 2nd dose: 1–7m, 1st booster dose: after 1y, subsequent booster doses every 3y |
| SenTaiBao e, [37] | Sen-Zhang (TBEV-FE) [37,38] | n.k. | Al(OH)3 [37] | HSA [37] | No, used for ≥8 years [37] | 1st + 2nd dose: 1–2w, annual booster doses |
| Infection | Vaccination | |
| Protective antibodies | E [88,93] NS1 [94,95,96,97] | E [88,93] NS1 [97] #† |
| CD4+ T cells | C [98,99,100] E [98,99,100] NS1 [101] | C [98,99,100] E [98,99,100,102] |
| CD8+ T cells * | NS2A [103] NS3 [103,104] NS4B [103] NS5 [103] | - |
| Approach | Strategy | Included TBEV Target Antigens | TBEV-Specific Adaptive Immunity | Protection (Challenge Virus) | Comment | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Antibodies | CD4+ T Cells | CD8+ T cells | ||||||
| Vaccination with proteins | ||||||||
| Mammalian cell line-based expression system | Production of recombinant E protein (soluble dimeric E without membrane anchor) from plasmid (COS-1 cells), comparison to virus-derived E dimers (with/without membrane anchor) and E rosettes (multimeric aggregates)
| E (Dimers or rosettes) | +(VN-Ab) | n.d. | n.d. | ++/+(TBEV) |
| [181] |
| Virus-like particles (VLPs) | ||||||||
| Mammalian cell line-based expression system | Production of VLPs from recombinant plasmid (COS-1 cells), purified VLPs used for immunization
| prM-E | +(VN-Ab) | n.d. | n.d. | ++(TBEV) |
| [181] |
| DNA vaccines | ||||||||
| DNA encoding for VLPs | In vivo production of VLPs from plasmid DNA encoding prM-E
| prM-E | +(VN-Ab in mice + NHP) | + | n.d. | ++(TBEV: mice) | Mice:
| [182,193,194] |
| DNA encoding for E protein | Immunization with plasmid DNA encoding antigens (secreted terminally truncated soluble E dimer, non-secreted full-length E, inefficiently secreted truncated E)
| E | +(VN-Ab) | + | n.d. | +/−(TBEV) |
| [182] |
| RNA vaccines | ||||||||
| ‘Naked’ infectious RNA | Application of infectious in vitro synthesized RNA of an attenuated TBEV mutant (carrying a 470 nt deletion in the 3′NCR for attenuation), immunization with purified infectious RNA leading to replication of highly attenuated mutant virus in vivo
| Whole TBEV | + | n.d. | n.d. | ++(TBEV) |
| [199] |
| ‘Naked’ non-infectious RNA | Application of in vitro synthesized non-infectious, replication-competent TBEV RNA (carrying an in-frame deletion of aa28–89 in the C protein with or without three point mutations (Gly112Pro; Met113Gln and Leu115Gln))
| Whole TBEV | +(VN-Ab) | + | + | ++(TBEV) |
| [174,239] |
| Recombinant adenoviruses (rAds) | ||||||||
| Human rAd | Insertion of TBEV NS1 under control of CMV major immediate-early promoter into replication-deficient Rad51ΔE1
| NS1 | + | n.d. | n.d. | +(TBEV) | [106,107] | |
| Human rAd | Insertion of TBEV NS1 into Rad51
| NS1 | + | + | + | +(TBEV, OHFV) |
| [196] |
| Recombinant Vaccinia viruses (rVACV) | ||||||||
| VACV | Insertion of TBEV NS1 into thymidine kinase gene under control of early–late poxvirus P65 promoter into VACV
| NS1 | + | n.d. | n.d. | +(TBEV) |
| [197] |
| VACV | Insertion of prM-E into a non-replicating late defective VACV (Uracil DNA glycosylase deficient)
| prM-E | +(VN-Ab) | n.d. | n.d. | ++/+(TBEV) |
| [195] |
| VACV | Insertion of structural and non-structural TBEV genes into thymidine kinase gene under control of VACV 7.5k promoter into VACV (C-prM-E-NS1 (vC-NS1); 5‘NCR-C-prM-E-NS1-NS2A (v5‘C-NS2A); C-prM-E-NS1-NS2A-NS2B-NS3 (vC-NS3))
| C-prM-E-NS1/5‘NCR-C-prM-E-NS1-NS2A/C-prM-E-NS1-NS2A-NS2B-NS3 | +(VN-Ab) | n.d. | n.d. | ++/+(TBEV) | vC-NS3:
| [211] |
| VACV/DNA | Prime-boost vaccination with VACV and bacterial plasmid expressing TBEV NS1 (recombinant VACV: NS1 into thymidine kinase gene under control of synthetic early-late poxvirus promoter; bacterial plasmid: NS1 under control of CMV immediate-early promoter)
| NS1 | + | n.d. | n.d. | +(TBEV) |
| [246] |
| Live attenuated viruses | ||||||||
| LGTV | Administration of attenuated LGTV strain
| Whole LGTV | + | n.d. | n.d. | +(field study) |
| [200] |
| Attenuated TBEV | Attenuation of TBEV by introducing deletions in the variable 3′NCR region
| Whole TBEV | + | n.d. | n.d. | ++(TBEV) |
| [218] |
| Attenuated TBEV | Introduction of single or multiple mutations in EDIII (aa308–311), combination of mutations in the 3′NCR with mutations at EDIII aa310
| Whole TBEV | + | n.d. | n.d. | ++(TBEV) |
| [217] |
| Attenuated TBEV | Multiple passaging of TBEV in BHK-21 cells and selection of binding mutants with high heparin sulfate affinity
| Whole TBEV | + | n.d. | n.d. | ++(TBEV) |
| [219] |
| Attenuated TBEV | Introduction of deletions into the TBEV C protein (4–21 aa deletions starting at aa28 of C)
| Whole TBEV | + | n.d. | n.d. | ++(TBEV) |
| [216] |
| Attenuated TBEV | Introduction of deletions into the TBEV C protein (19, 21, 27 or 30 aa deletions starting at aa28 of C)
| Whole TBEV | + | n.d. | n.d. | ++(TBEV) |
| [220] |
| Attenuated TBEV | Large-scale random codon re-encoding, random introduction of 273 synonymous mutations into NS5
| Whole TBEV | +(VN-Ab) | n.d. | n.d. | ++(TBEV) |
| [214] |
| Flavivirus chimera | ||||||||
| JEV-based | Replacement of prM-E from JEV live vaccine strain SA14-14-2 by corresponding genes from TBEV (ChinTBEV)
| prM-E | +(VN-Ab) | n.d. | n.d. | ++/+(TBEV) |
| [224] |
| YFV 17D-, DENV-2- or LGTV-based | Replacement of prM-E from YFV 17D, DENV-2 or LGTV by corresponding genes from TBEV
| prM-E | +(VN-Ab) | + | n.d. | ++/+(TBEV) |
| [223] |
| RepliVax (RV) platform | Replacement of prM-E from different flaviviruses (WNV, LGTV, TBEV or YFV 17D) by corresponding genes from TBEV, attenuation due to deletion in C
| prM-E | +(VN-Ab in mice + NHP) | + | n.d. | ++/+(TBEV: mice, LGTV: NHP) |
| [223] |
| LGTV-based | Replacement of prM-E of DENV-4 with corresponding genes from LGTV (LGTV/DENV-4)
| prM-E | +(VN-Ab against LGTV + TBEV) | n.d. | n.d. | n.d. |
| [237] |
| DENV-4-based | Replacement of prM-E of DENV-4 with corresponding genes from TBEV (TBEV/DENV-4)
| prM-E | +(VN-Ab in NHP; n.d. for mice) | n.d. | n.d. | +(LGTV) | Mice:
| [233] |
| DENV-4-based | Replacement of prM-E of DENV-4 with corresponding genes from TBEV and introduction of a 30 nt deletion in the DENV-4 3′NCR (TBEV/DENV-4∆30)
| prM-E | +(VN-Ab in NHP; n.d. for mice) | n.d. | n.d. | +(LGTV) | Mice:
| [233,234] |
| DENV-4-based | Introduction of single or multiple mutations into TBEV/DENV-4∆30 (deletion of 30 nt in 3′NCR of DENV-4; mutations in TBEV E (aa315) and DENV-4 NS5 (aa654, aa655))
| prM-E | n.d. | n.d. | n.d. | n.d. |
| [232] |
| miRNA targeted flavivirus chimera | Replacement of DENV-4 prM-E by corresponding genes from TBEV and introduction of single or multiple miRNA targeting sequences for cellular CNS-specific miRNAs into the 3′NCR of TBEV/DENV-4
| prM-E | +(VN-Ab in mice + NHP) | n.d. | n.d. | ++/+(Parental TBEV/DENV-4: mice) |
| [228], [229] |
| miRNA targeted flavivirus chimera | Replacement of LGTV prM-E by corresponding genes from TBEV and introduction of multiple miRNA targeting sequences for cellular CNS-specific miRNAs into the C, NS1 and 3′NCR of TBEV/LGTV
| prM-E | +(VN-Ab in mice + NHP) | n.d. | n.d. | ++(Parental TBEV/LGTV: mice, NHP; TBEV: mice) |
| [231] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubinski, M.; Beicht, J.; Gerlach, T.; Volz, A.; Sutter, G.; Rimmelzwaan, G.F. Tick-Borne Encephalitis Virus: A Quest for Better Vaccines against a Virus on the Rise. Vaccines 2020, 8, 451. https://doi.org/10.3390/vaccines8030451
Kubinski M, Beicht J, Gerlach T, Volz A, Sutter G, Rimmelzwaan GF. Tick-Borne Encephalitis Virus: A Quest for Better Vaccines against a Virus on the Rise. Vaccines. 2020; 8(3):451. https://doi.org/10.3390/vaccines8030451
Chicago/Turabian StyleKubinski, Mareike, Jana Beicht, Thomas Gerlach, Asisa Volz, Gerd Sutter, and Guus F. Rimmelzwaan. 2020. "Tick-Borne Encephalitis Virus: A Quest for Better Vaccines against a Virus on the Rise" Vaccines 8, no. 3: 451. https://doi.org/10.3390/vaccines8030451





