Cross-Protection of Hepatitis B Vaccination among Different Genotypes
Abstract
:1. Introduction
2. HBV Serotypes and Genotypes
2.1. Geographical Characteristics of HBV Genotype Distribution
2.2. Clinical Features Related to Differences in Genotype
| Genotype | Serotypes [48] | Geographical Distribution (Subgenotype) | Major Clinical Specificity | Major Biological Finding | References |
|---|---|---|---|---|---|
| A | adw | Asia, Africa (A1) | Sexual transmission (MSM), Chronic infection | BCP double mutation 1762T/1764A, 1888A, 1802–1803CG, 1858C | [28,31,32,49,50] |
| adw | Northwest Europe, North America (A2) | Sexual transmission (MSM) | BCP double mutation 1762T/1764A, 1802–1803CG | [29,30,50] | |
| B | adw, ayw | Tohoku, Hokkaido, Okinawa in Japan (Bj) | High rate of spontaneous HBeAg seroconversion | Precore stop codon mutations | [49,50,51] |
| adw, ayw | Asia (Ba) | Higher risk of developing HCC | Recombination with genotype C, 1802–1803TT, 1858T | [33,49,50] | |
| C | ayw, ayr, adr | Asia, Japan | Mother-to-infant transmission, Rapid progression to cirrhosis and HCC | 1802–1803TT, 1858C (genotype C1), 1858T (genotype C2) | [29,30,39,41,42,43,44,50] |
| D | ayw | Western Europe, Mediterranean | Histological inflammation, Early HBeAg seroconversion | BCP double mutation 1762T/1764A, 1802–1803CG, 1858T, T1764G1766 core promoter double mutants | [36,50,52] |
| E | ayw | West Africa | High viral loads, high frequency of HBeAg-positivity | 1802–1803CG, 1858T | [25,50] |
| F | adw | Alaska, Mexico, South America | HCC occurrence | 1802–1803TT, 1858C | [39] |
| G | adw | France, Germany, USA | Chronic infection, MSM | A 36-nucleotide insert, 3′ of position 1905, two translational stop codons at positions 2 and 28 of the precore/core region | [50] |
| H | adw | Central America | HCV coinfection and obesity are common cofactors. | 1858C | [26,27,50] |
| I | adw | Vietnam | (Few clinical features) | A recombinant of genotypes A/C/G | [17] |
| J | Japan | Isolated from a single Japanese man with HCC | A recombinant of genotype C and gibbon HBV in the S region | [18] |
2.3. Major Biological Features Related to Differences in Genotype
3. Transmission and Protection
3.1. Transmission
3.2. Protection
4. The Features and Effects of HB Vaccination
4.1. The Features of HB Vaccines
4.2. The Effects of HB Vaccination
5. Induction of Cross-Genotype Protection by HB Vaccination
5.1. Prevention of Genotype A Strain Infection and Vaccine Escape Mutant Infection by Genotype C-Derived HB Vaccines In Vitro and In Vivo
5.2. Prevention of Genotype C HBV Infection with a Genotype A-Derived Vaccine, and Genotype A HBV Infection with a Genotype C-Derived Vaccine In Vitro
6. Vaccine Escape Mutants
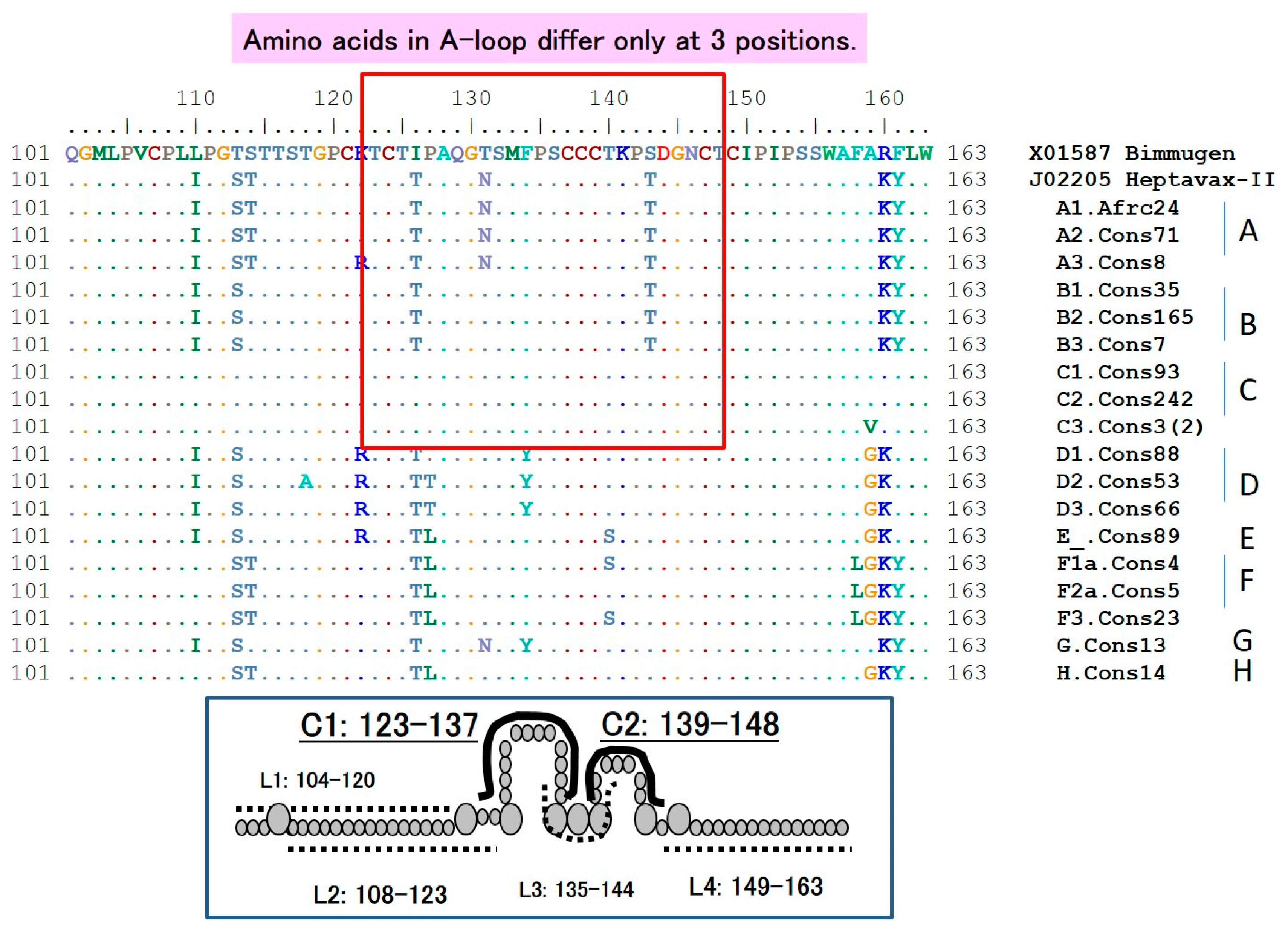
6.1. The HBsAg “a” Determinant
6.2. S-Gene Mutants
7. The Requirement for Booster Vaccination
8. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. World Health Organization Factsheets for Chronic Hepatitis B (Last Updated 27 July 2020). Available online: https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-b (accessed on 1 August 2020).
- Lai, C.L.; Wong, D.; Ip, P.; Kopaniszen, M.; Seto, W.K.; Fung, J.; Huang, F.Y.; Lee, B.; Cullaro, G.; Chong, C.K.; et al. Reduction of covalently closed circular DNA with long-term nucleos (t) ide analogue treatment in chronic hepatitis B. J. Hepatol. 2017, 66, 275–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, C.L.; Yuen, M.F. The natural history and treatment of chronic hepatitis B: A critical evaluation of standard treatment criteria and end points. Ann. Intern. Med. 2007, 147, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Kao, J.H.; Chen, D.S. Global control of hepatitis B virus infection. Lancet Infect. Dis. 2002, 2, 395–403. [Google Scholar] [CrossRef]
- Chen, D.S. Hepatitis B vaccination: The key towards elimination and eradication of hepatitis B. J. Hepatol. 2009, 50, 805–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szmuness, W.; Stevens, C.E.; Harley, E.J.; Zang, E.A.; Oleszko, W.R.; William, D.C.; Sadovsky, R.; Morrison, J.M.; Kellner, A. Hepatitis B vaccine: Demonstration of efficacy in a controlled clinical trial in a high-risk population in the United States. N. Engl. J. Med. 1980, 303, 833–841. [Google Scholar] [CrossRef]
- Casey, R.M.; Dumolard, L.; Danovaro-Holliday, M.C.; Gacic-Dobo, M.; Diallo, M.S.; Hampton, L.M.; Wallace, A.S. Global Routine Vaccination Coverage, 2015. Morb. Mortal. Wkly. Rep. 2016, 65, 1270–1273. [Google Scholar] [CrossRef] [Green Version]
- Ni, Y.H.; Huang, L.M.; Chang, M.H.; Yen, C.J.; Lu, C.Y.; You, S.L.; Kao, J.H.; Lin, Y.C.; Chen, H.L.; Hsu, H.Y.; et al. Two decades of universal hepatitis B vaccination in taiwan: Impact and implication for future strategies. Gastroenterology 2007, 132, 1287–1293. [Google Scholar] [CrossRef]
- Kuo, A.; Gish, R. Chronic hepatitis B infection. Clin. Liver Dis. 2012, 16, 347–369. [Google Scholar] [CrossRef]
- Kurbanov, F.; Tanaka, Y.; Mizokami, M. Geographical and genetic diversity of the human hepatitis B virus. Hepatol. Res. 2010, 40, 14–30. [Google Scholar] [CrossRef]
- Okamoto, H.; Tsuda, F.; Sakugawa, H.; Sastrosoewignjo, R.I.; Imai, M.; Miyakawa, Y.; Mayumi, M. Typing hepatitis B virus by homology in nucleotide sequence: Comparison of surface antigen subtypes. J. Gen. Virol. 1988, 69, 2575–2583. [Google Scholar] [CrossRef]
- Orito, E.; Mizokami, M.; Ina, Y.; Moriyama, E.N.; Kameshima, N.; Yamamoto, M.; Gojobori, T. Host-independent evolution and a genetic classification of the hepadnavirus family based on nucleotide sequences. Proc. Natl. Acad. Sci. USA 1989, 86, 7059–7062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norder, H.; Hammas, B.; Löfdahl, S.; Couroucé, A.M.; Magnius, L.O. Comparison of the amino acid sequences of nine different serotypes of hepatitis B surface antigen and genomic classification of the corresponding hepatitis B virus strains. J. Gen. Virol. 1992, 73, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Naumann, H.; Schaefer, S.; Yoshida, C.F.; Gaspar, A.M.; Repp, R.; Gerlich, W.H. Identification of a new hepatitis B virus (HBV) genotype from Brazil that expresses HBV surface antigen subtype adw4. J. Gen. Virol. 1993, 74, 1627–1632. [Google Scholar] [CrossRef] [PubMed]
- Stuyver, L.; De Gendt, S.; Van Geyt, C.; Zoulim, F.; Fried, M.; Schinazi, R.F.; Rossau, R. A new genotype of hepatitis B virus: Complete genome and phylogenetic relatedness. J. Gen. Virol. 2000, 81, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Arauz-Ruiz, P.; Norder, H.; Robertson, B.H.; Magnius, L.O. Genotype H: A new Amerindian genotype of hepatitis B virus revealed in Central America. J. Gen. Virol. 2002, 83, 2059–2073. [Google Scholar] [CrossRef]
- Kramvis, A. Genotypes and genetic variability of hepatitis B virus. Intervirology 2014, 57, 141–150. [Google Scholar] [CrossRef]
- Tatematsu, K.; Tanaka, Y.; Kurbanov, F.; Sugauchi, F.; Mano, S.; Maeshiro, T.; Nakayoshi, T.; Wakuta, M.; Miyakawa, Y.; Mizokami, M. A genetic variant of hepatitis B virus divergent from known human and ape genotypes isolated from a Japanese patient and provisionally assigned to new genotype J. J. Virol. 2009, 83, 10538–10547. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Zhong, G.; Xu, G.; He, W.; Jing, Z.; Gao, Z.; Huang, Y.; Qi, Y.; Peng, B.; Wang, H.; et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife 2012, 1, e00049. [Google Scholar] [CrossRef]
- Shokrgozar, M.A.; Shokri, F. Subtype specificity of anti-HBs antibodies produced by human B-cell lines isolated from normal individuals vaccinated with recombinant hepatitis B vaccine. Vaccine 2002, 20, 2215–2220. [Google Scholar] [CrossRef]
- Inoue, T.; Tanaka, Y. Hepatitis B Virus and Its Sexually Transmitted Infection—An Update. Microb. Cell 2016, 3, 420–437. [Google Scholar] [CrossRef] [Green Version]
- Thedja, M.D.; Muljono, D.H.; Ie, S.I.; Sidarta, E.; Turyadi; Verhoef, J.; Marzuki, S. Genogeography and Immune Epitope Characteristics of Hepatitis B Virus Genotype C Reveals Two Distinct Types: Asian and Papua-Pacific. PLoS ONE 2015, 10, e0132533. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadeq, D.W.; Taleb, S.A. Hepatitis B Virus Molecular Epidemiology, Host-Virus Interaction, Coinfection, and Laboratory Diagnosis in the MENA Region: An Update. Pathogens 2019, 8, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, Y.; Hasegawa, I.; Kato, T.; Orito, E.; Hirashima, N.; Acharya, S.K.; Gish, R.G.; Kramvis, A.; Kew, M.C.; Yoshihara, N.; et al. A case-control study for differences among hepatitis B virus infections of genotypes A (subtypes Aa and Ae) and D. Hepatology 2004, 40, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Kramvis, A. Molecular characteristics and clinical relevance of African genotypes and subgenotypes of hepatitis B virus. S. Afr. Med. J. 2018, 108, 17–21. [Google Scholar] [PubMed]
- Panduro, A.; Maldonado-Gonzalez, M.; Fierro, N.A.; Roman, S. Distribution of HBV genotypes F and H in Mexico and Central America. Antivir. Ther. 2013, 18, 475–484. [Google Scholar] [CrossRef] [Green Version]
- Te, H.S.; Jensen, D.M. Epidemiology of hepatitis B and C viruses: A global overview. Clin. Liver Dis. 2010, 14, 1–21. [Google Scholar] [CrossRef]
- Fujisaki, S.; Yokomaku, Y.; Shiino, T.; Koibuchi, T.; Hattori, J.; Ibe, S.; Iwatani, Y.; Iwamoto, A.; Shirasaka, T.; Hamaguchi, M.; et al. Outbreak of infections by hepatitis B virus genotype A and transmission of genetic drug resistance in patients coinfected with HIV-1 in Japan. J. Clin. Microbiol. 2011, 49, 1017–1024. [Google Scholar] [CrossRef] [Green Version]
- Sagnelli, C.; Ciccozzi, M.; Pisaturo, M.; Lo Presti, A.; Cella, E.; Coppola, N.; Sagnelli, E. The impact of viral molecular diversity on the clinical presentation and outcome of acute hepatitis B in Italy. New Microbiol. 2015, 38, 137–147. [Google Scholar]
- Araujo, N.M.; Waizbort, R.; Kay, A. Hepatitis B virus infection from an evolutionary point of view: How viral, host, and environmental factors shape genotypes and subgenotypes. Infect. Genet. Evol. 2011, 11, 1199–1207. [Google Scholar] [CrossRef]
- Ito, K.; Yotsuyanagi, H.; Yatsuhashi, H.; Karino, Y.; Takikawa, Y.; Saito, T.; Arase, Y.; Imazeki, F.; Kurosaki, M.; Umemura, T.; et al. Risk factors for long-term persistence of serum hepatitis B surface antigen following acute hepatitis B virus infection in Japanese adults. Hepatology 2014, 59, 89–97. [Google Scholar] [CrossRef]
- Ito, K.; Yotsuyanagi, H.; Sugiyama, M.; Yatsuhashi, H.; Karino, Y.; Takikawa, Y.; Saito, T.; Arase, Y.; Imazeki, F.; Kurosaki, M.; et al. Geographic distribution and characteristics of genotype A hepatitis B virus infection in acute and chronic hepatitis B patients in Japan. J. Gastroenterol. Hepatol. 2016, 31, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Tanaka, Y.; Simonetti, J.; Osiowy, C.; Borresen, M.L.; Koch, A.; Kurbanov, F.; Sugiyama, M.; Minuk, G.Y.; McMahon, B.J.; et al. Classification of hepatitis B virus genotype B into 2 major types based on characterization of a novel subgenotype in Arctic indigenous populations. J. Infect. Dis. 2007, 196, 1487–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kao, J.H.; Chen, P.J.; Lai, M.Y.; Chen, D.S. Hepatitis B genotypes correlate with clinical outcomes in patients with chronic hepatitis B. Gastroenterology 2000, 118, 554–559. [Google Scholar] [CrossRef]
- Fung, S.K.; Lok, A.S. Hepatitis B virus genotypes: Do they play a role in the outcome of HBV infection? Hepatology 2004, 40, 790–792. [Google Scholar] [CrossRef]
- Croagh, C.M.; Desmond, P.V.; Bell, S.J. Genotypes and viral variants in chronic hepatitis B: A review of epidemiology and clinical relevance. World J. Hepatol. 2015, 7, 289–303. [Google Scholar] [CrossRef]
- Marciano, S.; Galdame, O.A.; Gadano, A.C. HBV genotype F: Natural history and treatment. Antivir. Ther. 2013, 18, 485–488. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.; Liang, X.; Hamed, K.; Tanaka, Y.; Omagari, K.; Fan, R.; Xie, Q.; Tan, D.; Zhou, B.; Jia, J.D.; et al. Effect of hepatitis B virus subgenotype on antiviral response in nucleoside-treated hepatitis B envelope antigen-positive patients. Hepatol Res. 2018, 48, 134–143. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection; World Health Organization: Geneva, Switzerland, 2015; Available online: https://www.who.int/hiv/pub/hepatitis/hepatitis-b-guidelines/en/ (accessed on 1 July 2020).
- Tanaka, Y.; Mukaide, M.; Orito, E.; Yuen, M.F.; Ito, K.; Kurbanov, F.; Sugauchi, F.; Asahina, Y.; Izumi, N.; Kato, M.; et al. Specific mutations in enhancer II/core promoter of hepatitis B virus subgenotypes C1/C2 increase the risk of hepatocellular carcinoma. J. Hepatol. 2006, 45, 646–653. [Google Scholar] [CrossRef]
- Chu, C.M.; Liaw, Y.F. Genotype C hepatitis B virus infection is associated with a higher risk of reactivation of hepatitis B and progression to cirrhosis than genotype B: A longitudinal study of hepatitis B e antigen-positive patients with normal aminotransferase levels at baseline. J. Hepatol. 2005, 43, 411–417. [Google Scholar]
- Chan, H.L.; Wong, G.L.; Tse, C.H.; Chim, A.M.; Yiu, K.K.; Chan, H.Y.; Sung, J.J.; Wong, V.W. Hepatitis B virus genotype C is associated with more severe liver fibrosis than genotype B. Clin. Gastroenterol. Hepatol. 2009, 7, 1361–1366. [Google Scholar] [CrossRef]
- Lee, M.H.; Yang, H.I.; Liu, J.; Batrla-Utermann, R.; Jen, C.L.; Iloeje, U.H.; Lu, S.N.; You, S.L.; Wang, L.Y.; Chen, C.J. Prediction models of long-term cirrhosis and hepatocellular carcinoma risk in chronic hepatitis B patients: Risk scores integrating host and virus profiles. Hepatology 2013, 58, 546–554. [Google Scholar] [CrossRef]
- Inoue, J.; Akahane, T.; Nakayama, H.; Kimura, O.; Kobayashi, T.; Kisara, N.; Sato, T.; Morosawa, T.; Izuma, M.; Kakazu, E.; et al. Comparison of hepatitis B virus genotypes B and C among chronically hepatitis B virus-infected patients who received nucleos (t) ide analogs: A multicenter retrospective study. Hepatol. Res. 2019, 49, 1263–1274. [Google Scholar] [CrossRef]
- Fattovich, G.; Bortolotti, F.; Donato, F. Natural history of chronic hepatitis B: Special emphasis on disease progression and prognostic factors. J. Hepatol. 2008, 48, 335–352. [Google Scholar] [CrossRef]
- McMahon, B.J. Natural history of chronic hepatitis B. Clin. Liver Dis. 2010, 14, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Kramvis, A.; Kew, M.; Francois, G. Hepatitis B virus genotypes. Vaccine 2005, 23, 2409–2423. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Kao, J.H. Hepatitis B virus genotypes and variants. Cold Spring Harb. Perspect. Med. 2015, 5, a021436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orito, E.; Mizokami, M.; Sakugawa, H.; Michitaka, K.; Ishikawa, K.; Ichida, T.; Okanoue, T.; Yotsuyanagi, H.; Iino, S. A case-control study for clinical and molecular biological differences between hepatitis B viruses of genotypes B and C. Japan HBV Genotype Research Group. Hepatology 2001, 33, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Kramvis, A.; Arakawa, K.; Yu, M.C.; Nogueira, R.; Stram, D.O.; Kew, M.C. Relationship of serological subtype, basic core promoter and precore mutations to genotypes/subgenotypes of hepatitis B virus. J. Med. Virol. 2008, 80, 27–46. [Google Scholar] [CrossRef]
- Sugauchi, F.; Orito, E.; Ichida, T.; Kato, H.; Sakugawa, H.; Kakumu, S.; Ishida, T.; Chutaputti, A.; Lai, C.L.; Gish, R.G.; et al. Epidemiologic and virologic characteristics of hepatitis B virus genotype B having the recombination with genotype C. Gastroenterology 2003, 124, 925–932. [Google Scholar] [CrossRef]
- Sendi, H.; Mehrab-Mohseni, M.; Zali, M.R.; Norder, H.; Magnius, L.O. T1764G1766 core promoter double mutants are restricted to Hepatitis B virus strains with an A1757 and are common in genotype D. J. Gen. Virol. 2005, 86, 2451–2458. [Google Scholar] [CrossRef]
- Bell, T.G.; Yousif, M.; Kramvis, A. Bioinformatic curation and alignment of genotyped hepatitis B virus (HBV) sequence data from the GenBank public database. Springerplus 2016, 5, 1896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.S.; Tong, S.P.; Wen, Y.M.; Vitvitski, L.; Zhang, Q.; Trépo, C. Hepatitis B virus genotype A rarely circulates as an HBe-minus mutant: Possible contribution of a single nucleotide in the precore region. J. Virol. 1993, 67, 5402–5410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lok, A.S.; Akarca, U.; Greene, S. Mutations in the pre-core region of hepatitis B virus serve to enhance the stability of the secondary structure of the pre-genome encapsidation signal. Proc. Natl. Acad. Sci. USA 1994, 91, 4077–4081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revill, P.; Yuen, L.; Walsh, R.; Perrault, M.; Locarnini, S.; Kramvis, A. Bioinformatic analysis of the hepadnavirus e-antigen and its precursor identifies remarkable sequence conservation in all orthohepadnaviruses. J. Med. Virol. 2010, 82, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Kramvis, A.; Kostaki, E.G.; Hatzakis, A.; Paraskevis, D. Immunomodulatory Function of HBeAg Related to Short-Sighted Evolution, Transmissibility, and Clinical Manifestation of Hepatitis B Virus. Front. Microbiol. 2018, 9, 2521. [Google Scholar] [CrossRef] [PubMed]
- Kao, J.H.; Chen, P.J.; Lai, M.Y.; Chen, D.S. Hepatitis B virus genotypes and spontaneous hepatitis B e antigen seroconversion in Taiwanese hepatitis B carriers. J. Med. Virol. 2004, 72, 363–369. [Google Scholar] [CrossRef]
- Shimakawa, Y.; Lemoine, M.; Njai, H.F.; Bottomley, C.; Ndow, G.; Goldin, R.D.; Jatta, A.; Jeng-Barry, A.; Wegmuller, R.; Moore, S.E.; et al. Natural history of chronic HBV infection in West Africa: A longitudinal population-based study from The Gambia. Gut 2016, 65, 2007–2016. [Google Scholar] [CrossRef]
- Mahgoub, S.; Candotti, D.; El Ekiaby, M.; Allain, J.P. Hepatitis B virus (HBV) infection and recombination between HBV genotypes D and E in asymptomatic blood donors from Khartoum, Sudan. J. Clin. Microbiol. 2011, 49, 298–306. [Google Scholar] [CrossRef] [Green Version]
- Yousif, M.; Mudawi, H.; Bakhiet, S.; Glebe, D.; Kramvis, A. Molecular characterization of hepatitis B virus in liver disease patients and asymptomatic carriers of the virus in Sudan. BMC Infect. Dis. 2013, 13, 328. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Yuan, Q.; Ge, S.X.; Wang, H.Y.; Zhang, Y.L.; Chen, Q.R.; Zhang, J.; Chen, P.J.; Xia, N.S. Molecular and phylogenetic analyses suggest an additional hepatitis B virus genotype “I”. PLoS ONE 2010, 5, e9297. [Google Scholar] [CrossRef] [Green Version]
- Shepard, C.W.; Simard, E.P.; Finelli, L.; Fiore, A.E.; Bell, B.P. Hepatitis B virus infection: Epidemiology and vaccination. Epidemiol. Rev. 2006, 28, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Tufon, K.A.; Meriki, H.D.; Kwenti, T.E.; Tony, N.J.; Malika, E.; Bolimo, A.F.; Kouanou, Y.S.; Nkuo-Akenji, T.; Anong, D.N. HBV Transmission Risk Assessment in Healthcare Workers, Household and Sexual Contacts of HBV Infected Patients in the Southwest Region of Cameroon. Oman Med. J. 2019, 34, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Coppola, N.; De Pascalis, S.; Onorato, L.; Calò, F.; Sagnelli, C.; Sagnelli, E. Hepatitis B virus and hepatitis C virus infection in healthcare workers. World J. Hepatol. 2016, 8, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Liao, L.Y.; Liu, C.J.; Yu, M.W.; Chen, P.J.; Lai, M.Y.; Chen, D.S.; Kao, J.H. Hepatitis B viral factors in HBeAg-negative carriers with persistently normal serum alanine aminotransferase levels. Hepatology 2007, 45, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.B. Importance of perinatal versus horizontal transmission of hepatitis B virus infection in China. Gut 1996, 38 (Suppl. 2), S39–S42. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, P.; Cramm, M.; Van der Auwera, J.C.; Vranckx, R.; Meheus, A. Horizontal transmission of hepatitis B virus. Lancet 1995, 345, 27–29. [Google Scholar] [CrossRef]
- Alter, M.J.; Ahtone, J.; Weisfuse, I.; Starko, K.; Vacalis, T.D.; Maynard, J.E. Hepatitis B virus transmission between heterosexuals. J. Am. Med. Assoc. 1986, 256, 1307–1310. [Google Scholar] [CrossRef]
- Alter, M.J.; Coleman, P.J.; Alexander, W.J.; Kramer, E.; Miller, J.K.; Mandel, E.; Hadler, S.C.; Margolis, H.S. Importance of heterosexual activity in the transmission of hepatitis B and non-A, non-B hepatitis. J. Am. Med. Assoc. 1989, 262, 1201–1205. [Google Scholar] [CrossRef]
- Mast, E.E.; Margolis, H.S.; Fiore, A.E.; Brink, E.W.; Goldstein, S.T.; Wang, S.A.; Moyer, L.A.; Bell, B.P.; Alter, M.J. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: Immunization of infants, children, and adolescents. MMWR Recomm. Rep. 2005, 54, 1–31. [Google Scholar]
- Mast, E.E.; Weinbaum, C.M.; Fiore, A.E.; Alter, M.J.; Bell, B.P.; Finelli, L.; Rodewald, L.E.; Douglas, J.M., Jr.; Janssen, R.S.; Ward, J.W. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: Immunization of adults. MMWR Recomm. Rep. 2006, 55, 1–33. [Google Scholar]
- SAOL® Therapeutics; Hepagam B®. Dosing with High Levels of Anti-HBs. Available online: https://hepagamb.com/dosing.php (accessed on 1 August 2020).
- Miller, K.E.; Ruiz, D.E.; Graves, J.C. Update on the prevention and treatment of sexually transmitted diseases. Am. Fam. Physician 2003, 67, 1915–1922. [Google Scholar] [PubMed]
- Kane, M. Global programme for control of hepatitis B infection. Vaccine 1995, 13 (Suppl. 1), S47–S49. [Google Scholar] [CrossRef]
- Zanetti, A.R.; Van Damme, P.; Shouval, D. The global impact of vaccination against hepatitis B: A historical overview. Vaccine 2008, 26, 6266–6273. [Google Scholar] [CrossRef] [PubMed]
- Workowski, K.A.; Bolan, G.A. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm. Rep. 2015, 64, 1–137. [Google Scholar]
- Schillie, S.; Murphy, T.V.; Sawyer, M.; Ly, K.; Hughes, E.; Jiles, R.; de Perio, M.A.; Reilly, M.; Byrd, K.; Ward, J.W. CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering postexposure management. MMWR Recomm. Rep. 2013, 62, 1–19. [Google Scholar]
- Valenzuela, P.; Medina, A.; Rutter, W.J.; Ammerer, G.; Hall, B.D. Synthesis and assembly of hepatitis B virus surface antigen particles in yeast. Nature 1982, 298, 347–350. [Google Scholar] [CrossRef]
- Niu, M.T.; Salive, M.E.; Ellenberg, S.S. Neonatal deaths after hepatitis B vaccine: The vaccine adverse event reporting system, 1991–1998. Arch. Pediatr. Adolesc. Med. 1999, 153, 1279–1282. [Google Scholar] [CrossRef] [Green Version]
- Eriksen, E.M.; Perlman, J.A.; Miller, A.; Marcy, S.M.; Lee, H.; Vadheim, C.; Zangwill, K.M.; Chen, R.T.; DeStefano, F.; Lewis, E.; et al. Lack of association between hepatitis B birth immunization and neonatal death: A population-based study from the vaccine safety datalink project. Pediatr. Infect. Dis. J. 2004, 23, 656–662. [Google Scholar] [CrossRef]
- Arevalo, J.A.; Washington, A.E. Cost-effectiveness of prenatal screening and immunization for hepatitis B virus. J. Am. Med. Assoc. 1988, 259, 365–369. [Google Scholar] [CrossRef]
- Chen, D.S.; Hsu, N.H.; Sung, J.L.; Hsu, T.C.; Hsu, S.T.; Kuo, Y.T.; Lo, K.J.; Shih, Y.T. A mass vaccination program in Taiwan against hepatitis B virus infection in infants of hepatitis B surface antigen-carrier mothers. J. Am. Med. Assoc. 1987, 257, 2597–2603. [Google Scholar] [CrossRef]
- Locarnini, S.; Hatzakis, A.; Chen, D.S.; Lok, A. Strategies to control hepatitis B: Public policy, epidemiology, vaccine and drugs. J. Hepatol. 2015, 62, S76–S86. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Mossman, S.; Olivieri, A.; De Ridder, M.; Leroux-Roels, G. Hepatitis B vaccine effectiveness in the face of global HBV genotype diversity. Expert Rev. Vaccines 2011, 10, 1709–1715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graber-Stiehl, I. The silent epidemic killing more people than HIV, malaria or TB. Nature 2018, 564, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Men, P.; Xiao, Y.; Gao, P.; Lv, M.; Yuan, Q.; Chen, W.; Bai, S.; Wu, J. Hepatitis B infection in the general population of China: A systematic review and meta-analysis. BMC Infect. Dis. 2019, 19, 811. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Bi, S.; Yang, W.; Wang, L.; Cui, G.; Cui, F.; Zhang, Y.; Liu, J.; Gong, X.; Chen, Y.; et al. Epidemiological serosurvey of hepatitis B in China--declining HBV prevalence due to hepatitis B vaccination. Vaccine 2009, 27, 6550–6557. [Google Scholar] [CrossRef]
- Spradling, P.R.; Xing, J.; Williams, R.; Masunu-Faleafaga, Y.; Dulski, T.; Mahamud, A.; Drobeniuc, J.; Teshale, E.H. Immunity to hepatitis B virus (HBV) infection two decades after implementation of universal infant HBV vaccination: Association of detectable residual antibodies and response to a single HBV challenge dose. Clin. Vaccine Immunol. 2013, 20, 559–561. [Google Scholar] [CrossRef] [Green Version]
- Mendy, M.; Peterson, I.; Hossin, S.; Peto, T.; Jobarteh, M.L.; Jeng-Barry, A.; Sidibeh, M.; Jatta, A.; Moore, S.E.; Hall, A.J.; et al. Observational study of vaccine efficacy 24 years after the start of hepatitis B vaccination in two Gambian villages: No need for a booster dose. PLoS ONE 2013, 8, e58029. [Google Scholar] [CrossRef]
- Poovorawan, Y.; Chongsrisawat, V.; Theamboonlers, A.; Leroux-Roels, G.; Crasta, P.D.; Hardt, K. Persistence and immune memory to hepatitis B vaccine 20 years after primary vaccination of Thai infants, born to HBsAg and HBeAg positive mothers. Hum. Vaccines Immunother. 2012, 8, 896–904. [Google Scholar] [CrossRef] [Green Version]
- Coates, T.; Wilson, R.; Patrick, G.; André, F.; Watson, V. Hepatitis B vaccines: Assessment of the seroprotective efficacy of two recombinant DNA vaccines. Clin. Ther. 2001, 23, 392–403. [Google Scholar] [CrossRef]
- Li, Z.K.; Nie, J.J.; Li, J.; Zhuang, H. The effect of HLA on immunological response to hepatitis B vaccine in healthy people: A meta-analysis. Vaccine 2013, 31, 4355–4361. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, H. Hepatitis B virus: Where do we stand and what is the next step for eradication? World J. Gastroenterol. 2014, 20, 8998–9016. [Google Scholar]
- Leads from the MMWR. Suboptimal response to hepatitis B vaccine given by injection into the buttock. J. Am. Med. Assoc. 1985, 253, 1705–1707. [Google Scholar] [CrossRef]
- Clemens, R.; Sänger, R.; Kruppenbacher, J.; Höbel, W.; Stanbury, W.; Bock, H.L.; Jilg, W. Booster immunization of low- and non-responders after a standard three dose hepatitis B vaccine schedule--results of a post-marketing surveillance. Vaccine 1997, 15, 349–352. [Google Scholar] [CrossRef]
- Craven, D.E.; Awdeh, Z.L.; Kunches, L.M.; Yunis, E.J.; Dienstag, J.L.; Werner, B.G.; Polk, B.F.; Syndman, D.R.; Platt, R.; Crumpacker, C.S.; et al. Nonresponsiveness to hepatitis B vaccine in health care workers. Results of revaccination and genetic typings. Ann. Intern. Med. 1986, 105, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.; Xie, S.B.; Liu, J.; Zhao, Z.X.; Chong, Y.T.; Gao, Z.L. Effect of revaccination using different schemes among adults with low or undetectable anti-HBs titers after hepatitis B virus vaccination. Clin. Vaccine Immunol. 2010, 17, 1548–1551. [Google Scholar] [CrossRef] [Green Version]
- Miyakawa, Y.; Mizokami, M. Classifying hepatitis B virus genotypes. Intervirology 2003, 46, 329–338. [Google Scholar] [CrossRef]
- Avazova, D.; Kurbanov, F.; Tanaka, Y.; Sugiyama, M.; Radchenko, I.; Ruziev, D.; Musabaev, E.; Mizokami, M. Hepatitis B virus transmission pattern and vaccination efficiency in Uzbekistan. J. Med. Virol. 2008, 80, 217–224. [Google Scholar] [CrossRef]
- Matsuura, K.; Tanaka, Y.; Hige, S.; Yamada, G.; Murawaki, Y.; Komatsu, M.; Kuramitsu, T.; Kawata, S.; Tanaka, E.; Izumi, N.; et al. Distribution of hepatitis B virus genotypes among patients with chronic infection in Japan shifting toward an increase of genotype A. J. Clin. Microbiol. 2009, 47, 1476–1483. [Google Scholar] [CrossRef] [Green Version]
- Iwarson, S.; Tabor, E.; Thomas, H.C.; Goodall, A.; Waters, J.; Snoy, P.; Shih, J.W.; Gerety, R.J. Neutralization of hepatitis B virus infectivity by a murine monoclonal antibody: An experimental study in the chimpanzee. J. Med. Virol. 1985, 16, 89–96. [Google Scholar] [CrossRef]
- Jin, A.; Ozawa, T.; Tajiri, K.; Obata, T.; Kondo, S.; Kinoshita, K.; Kadowaki, S.; Takahashi, K.; Sugiyama, T.; Kishi, H.; et al. A rapid and efficient single-cell manipulation method for screening antigen-specific antibody-secreting cells from human peripheral blood. Nat. Med. 2009, 15, 1088–1092. [Google Scholar] [CrossRef]
- Tajiri, K.; Kishi, H.; Tokimitsu, Y.; Kondo, S.; Ozawa, T.; Kinoshita, K.; Jin, A.; Kadowaki, S.; Sugiyama, T.; Muraguchi, A. Cell-microarray analysis of antigen-specific B-cells: Single cell analysis of antigen receptor expression and specificity. Cytom. Part A J. Int. Soc. Anal. Cytol. 2007, 71, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Tokimitsu, Y.; Kishi, H.; Kondo, S.; Honda, R.; Tajiri, K.; Motoki, K.; Ozawa, T.; Kadowaki, S.; Obata, T.; Fujiki, S.; et al. Single lymphocyte analysis with a microwell array chip. Cytom. Part A J. Int. Soc. Anal. Cytol. 2007, 71, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, K.; Ozawa, T.; Jin, A.; Tokimitsu, Y.; Minemura, M.; Kishi, H.; Sugiyama, T.; Muraguchi, A. Analysis of the epitope and neutralizing capacity of human monoclonal antibodies induced by hepatitis B vaccine. Antivir. Res. 2010, 87, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Hamada-Tsutsumi, S.; Iio, E.; Watanabe, T.; Murakami, S.; Isogawa, M.; Iijima, S.; Inoue, T.; Matsunami, K.; Tajiri, K.; Ozawa, T.; et al. Validation of cross-genotype neutralization by hepatitis B virus-specific monoclonal antibodies by in vitro and in vivo infection. PLoS ONE 2015, 10, e0118062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stramer, S.L.; Wend, U.; Candotti, D.; Foster, G.A.; Hollinger, F.B.; Dodd, R.Y.; Allain, J.P.; Gerlich, W. Nucleic acid testing to detect HBV infection in blood donors. N. Engl. J. Med. 2011, 364, 236–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, M.; Hamada-Tsutsumi, S.; Okuse, C.; Sakai, A.; Matsumoto, N.; Sato, M.; Sato, T.; Arito, M.; Omoteyama, K.; Suematsu, N.; et al. Effects of vaccine-acquired polyclonal anti-HBs antibodies on the prevention of HBV infection of non-vaccine genotypes. J. Gastroenterol. 2017, 52, 1051–1063. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Chang, M.H.; Ni, Y.H.; Hsu, H.Y.; Lee, P.I.; Lee, C.Y.; Chen, D.S. Seroepidemiology of hepatitis B virus infection in children: Ten years of mass vaccination in Taiwan. J. Am. Med. Assoc. 1996, 276, 906–908. [Google Scholar] [CrossRef]
- Chongsrisawat, V.; Yoocharoen, P.; Theamboonlers, A.; Tharmaphornpilas, P.; Warinsathien, P.; Sinlaparatsamee, S.; Paupunwatana, S.; Chaiear, K.; Khwanjaipanich, S.; Poovorawan, Y. Hepatitis B seroprevalence in Thailand: 12 years after hepatitis B vaccine integration into the national expanded programme on immunization. Trop. Med. Int. Health 2006, 11, 1496–1502. [Google Scholar] [CrossRef]
- Wichajarn, K.; Kosalaraksa, P.; Wiangnon, S. Incidence of hepatocellular carcinoma in children in Khon Kaen before and after national hepatitis B vaccine program. Asian Pac. J. Cancer Prev. 2008, 9, 507–509. [Google Scholar]
- Romanò, L.; Paladini, S.; Galli, C.; Raimondo, G.; Pollicino, T.; Zanetti, A.R. Hepatitis B vaccination. Hum. Vaccines Immunother. 2015, 11, 53–57. [Google Scholar] [CrossRef] [Green Version]
- Qiu, X.; Schroeder, P.; Bridon, D. Identification and characterization of a C(K/R)TC motif as a common epitope present in all subtypes of hepatitis B surface antigen. J. Immunol. 1996, 156, 3350–3356. [Google Scholar] [PubMed]
- Carman, W.F.; Korula, J.; Wallace, L.; MacPhee, R.; Mimms, L.; Decker, R. Fulminant reactivation of hepatitis B due to envelope protein mutant that escaped detection by monoclonal HBsAg ELISA. Lancet 1995, 345, 1406–1407. [Google Scholar] [CrossRef]
- Wu, C.; Deng, W.; Deng, L.; Cao, L.; Qin, B.; Li, S.; Wang, Y.; Pei, R.; Yang, D.; Lu, M.; et al. Amino acid substitutions at positions 122 and 145 of hepatitis B virus surface antigen (HBsAg) determine the antigenicity and immunogenicity of HBsAg and influence in vivo HBsAg clearance. J. Virol. 2012, 86, 4658–4669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komatsu, H.; Inui, A.; Sogo, T.; Konishi, Y.; Tateno, A.; Fujisawa, T. Hepatitis B surface gene 145 mutant as a minor population in hepatitis B virus carriers. BMC Res. Notes 2012, 5, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, P.I.; Chang, L.Y.; Lee, C.Y.; Huang, L.M.; Chang, M.H. Detection of hepatitis B surface gene mutation in carrier children with or without immunoprophylaxis at birth. J. Infect. Dis. 1997, 176, 427–430. [Google Scholar] [CrossRef] [Green Version]
- Zanetti, A.R.; Tanzi, E.; Manzillo, G.; Maio, G.; Sbreglia, C.; Caporaso, N.; Thomas, H.; Zuckerman, A.J. Hepatitis B variant in Europe. Lancet 1988, 2, 1132–1133. [Google Scholar] [CrossRef]
- Carman, W.F.; Zanetti, A.R.; Karayiannis, P.; Waters, J.; Manzillo, G.; Tanzi, E.; Zuckerman, A.J.; Thomas, H.C. Vaccine-induced escape mutant of hepatitis B virus. Lancet 1990, 336, 325–329. [Google Scholar] [CrossRef]
- Ogata, N.; Zanetti, A.R.; Yu, M.; Miller, R.H.; Purcell, R.H. Infectivity and pathogenicity in chimpanzees of a surface gene mutant of hepatitis B virus that emerged in a vaccinated infant. J. Infect. Dis. 1997, 175, 511–523. [Google Scholar] [CrossRef] [Green Version]
- Hsu, H.Y.; Chang, M.H.; Liaw, S.H.; Ni, Y.H.; Chen, H.L. Changes of hepatitis B surface antigen variants in carrier children before and after universal vaccination in Taiwan. Hepatology 1999, 30, 1312–1317. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Chang, M.H.; Ni, Y.H.; Chen, H.L. Survey of hepatitis B surface variant infection in children 15 years after a nationwide vaccination programme in Taiwan. Gut 2004, 53, 1499–1503. [Google Scholar] [CrossRef] [Green Version]
- Hsu, H.Y.; Chang, M.H.; Ni, Y.H.; Chiang, C.L.; Chen, H.L.; Wu, J.F.; Chen, P.J. No increase in prevalence of hepatitis B surface antigen mutant in a population of children and adolescents who were fully covered by universal infant immunization. J. Infect. Dis. 2010, 201, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Chang, M.H.; Ni, Y.H.; Lin, H.H.; Wang, S.M.; Chen, D.S. Surface gene mutants of hepatitis B virus in infants who develop acute or chronic infections despite immunoprophylaxis. Hepatology 1997, 26, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Lin, L.H.; Hu, F.C.; Lee, J.T.; Lin, W.T.; Yang, Y.J.; Huang, F.C.; Wu, S.F.; Chen, S.C.; Wen, W.H.; et al. Effects of maternal screening and universal immunization to prevent mother-to-infant transmission of HBV. Gastroenterology 2012, 142, 773–781.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, W.H.; Chang, M.H.; Zhao, L.L.; Ni, Y.H.; Hsu, H.Y.; Wu, J.F.; Chen, P.J.; Chen, D.S.; Chen, H.L. Mother-to-infant transmission of hepatitis B virus infection: Significance of maternal viral load and strategies for intervention. J. Hepatol. 2013, 59, 24–30. [Google Scholar] [CrossRef]
- Fujii, H.; Moriyama, K.; Sakamoto, N.; Kondo, T.; Yasuda, K.; Hiraizumi, Y.; Yamazaki, M.; Sakaki, Y.; Okochi, K.; Nakajima, E. Gly145 to Arg substitution in HBs antigen of immune escape mutant of hepatitis B virus. Biochem. Biophys. Res. Commun. 1992, 184, 1152–1157. [Google Scholar] [CrossRef]
- Hino, K.; Okuda, M.; Hashimoto, O.; Ishiko, H.; Okazaki, M.; Fujii, K.; Hanada, H.; Okita, K. Glycine-to-arginine substitution at codon 145 of HBsAg in two infants born to hepatitis B e antigen-positive carrier. Dig. Dis. Sci. 1995, 40, 566–570. [Google Scholar] [CrossRef]
- Miyake, Y.; Oda, T.; Li, R.; Sugiyama, K. A comparison of amino acid sequences of hepatitis B virus S gene in 46 children presenting various clinical features for immunoprophylaxis. Tohoku J. Exp. Med. 1996, 180, 233–247. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, T.; Nakata, K.; Hamasaki, K.; Daikokoku, M.; Nakao, K.; Yamashita, Y.; Shirahama, S.; Kato, Y. Efficacy of immunization of high-risk infants against hepatitis B virus evaluated by polymerase chain reaction. J. Med. Virol. 1997, 53, 255–260. [Google Scholar] [CrossRef]
- Komatsu, H.; Inui, A.; Umetsu, S.; Tsunoda, T.; Sogo, T.; Konishi, Y.; Fujisawa, T. Evaluation of the G145R Mutant of the Hepatitis B Virus as a Minor Strain in Mother-to-Child Transmission. PLoS ONE 2016, 11, e0165674. [Google Scholar] [CrossRef] [Green Version]
- Oon, C.J.; Lim, G.K.; Ye, Z.; Goh, K.T.; Tan, K.L.; Yo, S.L.; Hopes, E.; Harrison, T.J.; Zuckerman, A.J. Molecular epidemiology of hepatitis B virus vaccine variants in Singapore. Vaccine 1995, 13, 699–702. [Google Scholar] [CrossRef]
- Ngui, S.L.; O’Connell, S.; Eglin, R.P.; Heptonstall, J.; Teo, C.G. Low detection rate and maternal provenance of hepatitis B virus S gene mutants in cases of failed postnatal immunoprophylaxis in England and Wales. J. Infect. Dis. 1997, 176, 1360–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.W.; Lu, Q.; Zhu, Q.R.; Duan, S.C.; Wen, Y.M. Mutations in the ‘a’ determinant of hepatitis B surface antigen among Chinese infants receiving active postexposure hepatitis B immunization. Vaccine 1998, 16, 170–173. [Google Scholar] [CrossRef]
- Yan, B.; Lv, J.; Feng, Y.; Liu, J.; Ji, F.; Xu, A.; Zhang, L. Temporal trend of hepatitis B surface mutations in the post-immunization period: 9 years of surveillance (2005–2013) in eastern China. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basuni, A.A.; Butterworth, L.; Cooksley, G.; Locarnini, S.; Carman, W.F. Prevalence of HBsAg mutants and impact of hepatitis B infant immunisation in four Pacific Island countries. Vaccine 2004, 22, 2791–2799. [Google Scholar] [CrossRef] [PubMed]
- Purwono, P.B.; Amin, M.; Bramanthi, R.; Resi, E.M.; Wahyuni, R.M.; Yano, Y.; Hotta, H.; Hayashi, Y.; Utsumi, T.; Lusida, M.I. Hepatitis B Virus Infection in Indonesia 15 Years After Adoption of a Universal Infant Vaccination Program: Possible Impacts of Low Birth Dose Coverage and a Vaccine-Escape Mutant. Am. J. Trop. Med. Hyg. 2016, 95, 674–679. [Google Scholar] [CrossRef] [Green Version]
- Nainan, O.V.; Khristova, M.L.; Byun, K.; Xia, G.; Taylor, P.E.; Stevens, C.E.; Margolis, H.S. Genetic variation of hepatitis B surface antigen coding region among infants with chronic hepatitis B virus infection. J. Med. Virol. 2002, 68, 319–327. [Google Scholar] [CrossRef]
- Bian, T.; Yan, H.; Shen, L.; Wang, F.; Zhang, S.; Cao, Y.; Zhang, S.; Zhang, Y.; Bi, S. Change in hepatitis B virus large surface antigen variant prevalence 13 years after implementation of a universal vaccination program in China. J. Virol. 2013, 87, 12196–12206. [Google Scholar] [CrossRef] [Green Version]
- Jilg, W.; Norder, H.; Kane, M.; Van Damme, P.; Vorsters, A. Reduced prevalence of HBsAg variants following a successful immunization program in China. J. Virol. 2014, 88, 4605–4606. [Google Scholar] [CrossRef] [Green Version]
- Locarnini, S.; Shouval, D. Commonly found variations/mutations in the HBsAg of hepatitis B virus in the context of effective immunization programs: Questionable clinical and public health significance. J. Virol. 2014, 88, 6532. [Google Scholar] [CrossRef] [Green Version]
- Kalinina, T.; Iwanski, A.; Will, H.; Sterneck, M. Deficiency in virion secretion and decreased stability of the hepatitis B virus immune escape mutant G145R. Hepatology 2003, 38, 1274–1281. [Google Scholar] [CrossRef]
- Mele, A.; Tancredi, F.; Romanò, L.; Giuseppone, A.; Colucci, M.; Sangiuolo, A.; Lecce, R.; Adamo, B.; Tosti, M.E.; Taliani, G.; et al. Effectiveness of hepatitis B vaccination in babies born to hepatitis B surface antigen-positive mothers in Italy. J. Infect. Dis. 2001, 184, 905–908. [Google Scholar] [CrossRef] [PubMed]
- West, D.J.; Calandra, G.B. Vaccine induced immunologic memory for hepatitis B surface antigen: Implications for policy on booster vaccination. Vaccine 1996, 14, 1019–1027. [Google Scholar] [CrossRef]
- Lu, C.Y.; Ni, Y.H.; Chiang, B.L.; Chen, P.J.; Chang, M.H.; Chang, L.Y.; Su, I.J.; Kuo, H.S.; Huang, L.M.; Chen, D.S.; et al. Humoral and cellular immune responses to a hepatitis B vaccine booster 15–18 years after neonatal immunization. J. Infect. Dis. 2008, 197, 1419–1426. [Google Scholar] [CrossRef]
- Wu, T.W.; Lin, H.H.; Wang, L.Y. Chronic hepatitis B infection in adolescents who received primary infantile vaccination. Hepatology 2013, 57, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S. Long-term protection of hepatitis B vaccine: Lessons from Alaskan experience after 15 years. Ann. Intern. Med. 2005, 142, 384–385. [Google Scholar] [CrossRef]
- Edmunds, W.J.; Medley, G.F.; Nokes, D.J.; Hall, A.J.; Whittle, H.C. The influence of age on the development of the hepatitis B carrier state. Proc. Biol. Sci. 1993, 253, 197–201. [Google Scholar] [PubMed]
- European Consensus Group on Hepatitis B Immunity. Are booster immunisations needed for lifelong hepatitis B immunity? Lancet 2000, 355, 561–565. [Google Scholar] [CrossRef]
- Chaves, S.S.; Fischer, G.; Groeger, J.; Patel, P.R.; Thompson, N.D.; Teshale, E.H.; Stevenson, K.; Yano, V.M.; Armstrong, G.L.; Samandari, T.; et al. Persistence of long-term immunity to hepatitis B among adolescents immunized at birth. Vaccine 2012, 30, 1644–1649. [Google Scholar] [CrossRef]
- Tajiri, K.; Shimizu, Y. Unsolved problems and future perspectives of hepatitis B virus vaccination. World J. Gastroenterol. 2015, 21, 7074–7083. [Google Scholar] [CrossRef]
- Hahné, S.; van Houdt, R.; Koedijk, F.; van Ballegooijen, M.; Cremer, J.; Bruisten, S.; Coutinho, R.; Boot, H. Selective hepatitis B virus vaccination has reduced hepatitis B virus transmission in the Netherlands. PLoS ONE 2013, 8, e67866. [Google Scholar] [CrossRef] [Green Version]
- Liao, X.; Liang, Z. Strategy vaccination against Hepatitis B in China. Hum. Vaccines Immunother. 2015, 11, 1534–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Z.; Li, X.; Ma, L.; Yang, Y. Hepatitis B immunoglobulin injection in pregnancy to interrupt hepatitis B virus mother-to-child transmission-a meta-analysis. Int. J. Infect. Dis. 2010, 14, e622–e634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Wei, Y.; Chen, T.; Lu, J.; Zhu, C.L.; Ni, Z.; Huang, F.; Du, J.; Sun, Z.; Qu, C. Occult HBV infection in anti-HBs-positive young adults after neonatal HB vaccination. Vaccine 2010, 28, 5986–5992. [Google Scholar] [CrossRef] [PubMed]
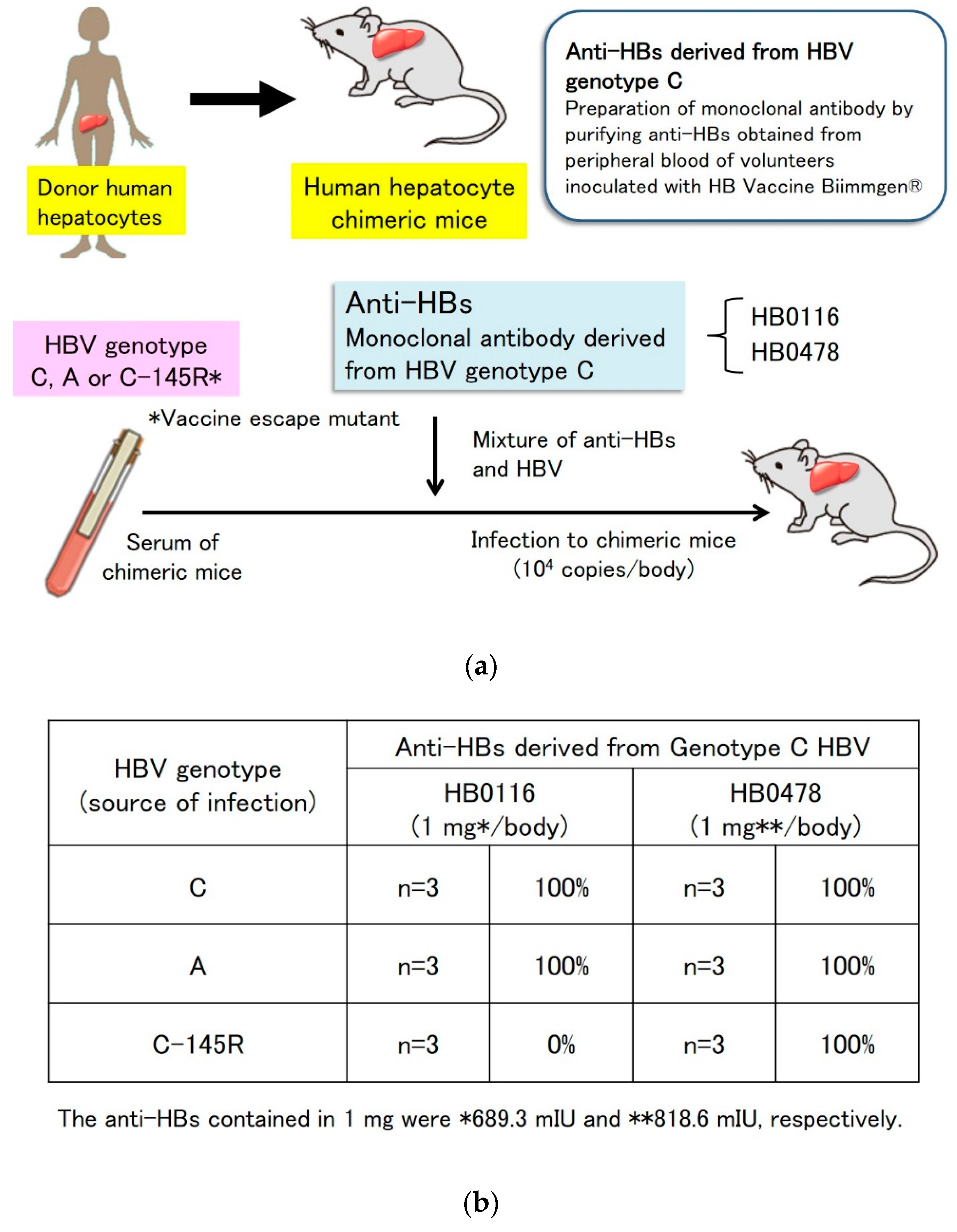
| Publication | Country/Region | Reference | VEM/HBV Infection (%) | Status of HBV Infection | Immunization | Site of Amino Acid Substitution |
|---|---|---|---|---|---|---|
| 1984 * | Taiwan | Hsu et al. [122] | 8/103 (8%) | Persistence | Before vaccine | T126A (1), M133L (1), F134L (1), C138S (1), T140R (1), T140I (1), T143M (1), D144A (1) |
| 1989 * | Taiwan | Hsu et al. [122] | 10/51 (20%) | Persistence | HBIG+vaccine | T126A (2), P127T (1), Q129H (1), S143W (2), G145R (3), W156L (1) |
| 1994 * | Taiwan | Hsu et al. [122] | 9/32 (28%) | Persistence | HBIG+vaccine | T125A (1), P120Q + P127T (1), T126A + T143M (1), T126S + D144H (1), D144H + G145R (1), T140P (1), N146S (1), T148I (1), C147R + C149R (1) |
| 1999 * | Taiwan | Hsu et al. [123] | 3/13 (23%) | Persistence | HBIG+vaccine | T131I (1), G145R (2) |
| 2004 * | Taiwan | Hsu et al. [124] | 7/31 (23%) | Persistence | HBIG+vaccine | T126A (2), M133T (1), F134L + T148A (1), G145R (1), G145A (1), W156C (1) |
| 1997 | Taiwan | Hsu et al. [125] | 1/7 (14%) | Acute or fulminant hepatitis | Vaccine | T126A + G145R (1) |
| 1997 | Taiwan | Hsu et al. [125] | 5/15 (33%) | Persistence | HBIG+vaccine | T126A (2), Q129R (1), G145R (2) |
| 2012 | Taiwan | Chen et al. [126] | 8/25 (32%) | Persistence | HBIG+vaccine | K122R (1), I126T (1), G145R (6) |
| 2013 | Taiwan | Wen WH et al. [127] | 3/10 (30%) | Persistence | HBIG+vaccine | Q129H + T140S (1), P142L + G145R (1), G145R (1) |
| 1992 | Japan | Fujii et al. [128] | 1/2 (50%) | Persistence | HBIG+vaccine | G145R (1) |
| 1995 | Japan | Hino et al. [129] | 2/2 (100%) | Persistence | HBIG+vaccine | G145R (2) |
| 1996 | Japan | Miyake et al. [130] | 8/46 (17%) | Persistence | HBIG+vaccine | I/T126S (3), T140S (3), G145R (1), G145K (1) |
| 1997 | Japan | Matsumoto et al. [131] | 1/2 (50%) | Acute hepatitis | HBIG+vaccine | P120Q + G145R (1) |
| 2016 | Japan | Komatsu et al. [132] | 5/25 (20%) | Persistence | HBIG+vaccine | I/T126S (1), G130N (1), G145R (2), G145K (1) |
| 1995 | Singapore | Oon et al. [133] | 16/41 (39%) | Persistence | HBIG+vaccine | I/T126A (1), Q129H (1), M133L (1), D144A (1), G145R (10), G145R + D144A (1), G145R + P142S (1) |
| 1997 | England, Wales | Ngui et al. [134] | 2/17 (12%) | Persistence | HBIG+vaccine | P120Q + Y134F + D144A (1), I126N (1) |
| 1998 | China | He et al. [135] | 4/24 (17%) | Persistence | HBIG+vaccine | I/T126S (1), Q129H (1), Q129L (1), G145R (1) |
| 2005 * | China | Yan et al. [136] | 8/131 (6%) | Persistence | HBIG+vaccine | I126S (1), I126S + T131N + M133T (1), T131P (1), M133T (1), G145A (1) |
| 2006 * | China | Yan et al. [136] | 10/101 (10%) | Persistence | HBIG+vaccine | I126S (2), P127T (2), T131P (3) |
| 2007 * | China | Yan et al. [136] | 11/113 (10%) | Persistence | HBIG+vaccine | I126S (2), I126S + T131N + M133T (1), G145A (1) |
| 2008 * | China | Yan et al. [136] | 9/136 (7%) | Persistence | HBIG+vaccine | I126S (1), D144E (1), G145A (3) |
| 2009 * | China | Yan et al. [136] | 19/206 (9%) | Persistence | HBIG+vaccine | I126S (3), I126N (1), P127T (2), Q129H (2), M133I + D144A (1), D144A (1), D144N + G145R (1), G145A (1) |
| 2010 * | China | Yan et al. [136] | 7/75 (9%) | Persistence | HBIG+vaccine | I126S (1), I126N + P127T(1), D144E (1), G145R (1) |
| 2011 * | China | Yan et al. [136] | 13/102 (13%) | Persistence | HBIG+vaccine | T126A (1), I126S (1), P127T (2), Q129H (1), D144A (1), G145A (1), |
| 2012 * | China | Yan et al. [136] | 8/78 (10%) | Persistence | HBIG+vaccine | T131N (1), F134L (1), G145R (1), G145A (1) |
| 2013 * | China | Yan et al. [136] | 12/135 (9%) | Persistence | HBIG+vaccine | I126S + G130E (1), G145A (6), |
| 2004 | Pacific islands | Basuni et al. [137] | 0/22 (0%) | Persistence | Vaccine | - |
| 2016 | Indonesia | Purwono et al. [138] | 6/61 (12%) | Persistence | Vaccine | P120S + A159V (1), M133L (1), M133T + C147S (1), T140I (2), S155F (1) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, T.; Tanaka, Y. Cross-Protection of Hepatitis B Vaccination among Different Genotypes. Vaccines 2020, 8, 456. https://doi.org/10.3390/vaccines8030456
Inoue T, Tanaka Y. Cross-Protection of Hepatitis B Vaccination among Different Genotypes. Vaccines. 2020; 8(3):456. https://doi.org/10.3390/vaccines8030456
Chicago/Turabian StyleInoue, Takako, and Yasuhito Tanaka. 2020. "Cross-Protection of Hepatitis B Vaccination among Different Genotypes" Vaccines 8, no. 3: 456. https://doi.org/10.3390/vaccines8030456
APA StyleInoue, T., & Tanaka, Y. (2020). Cross-Protection of Hepatitis B Vaccination among Different Genotypes. Vaccines, 8(3), 456. https://doi.org/10.3390/vaccines8030456




