Anti-Influenza Protective Efficacy of a H6 Virus-Like Particle in Chickens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell and Virus Cultures
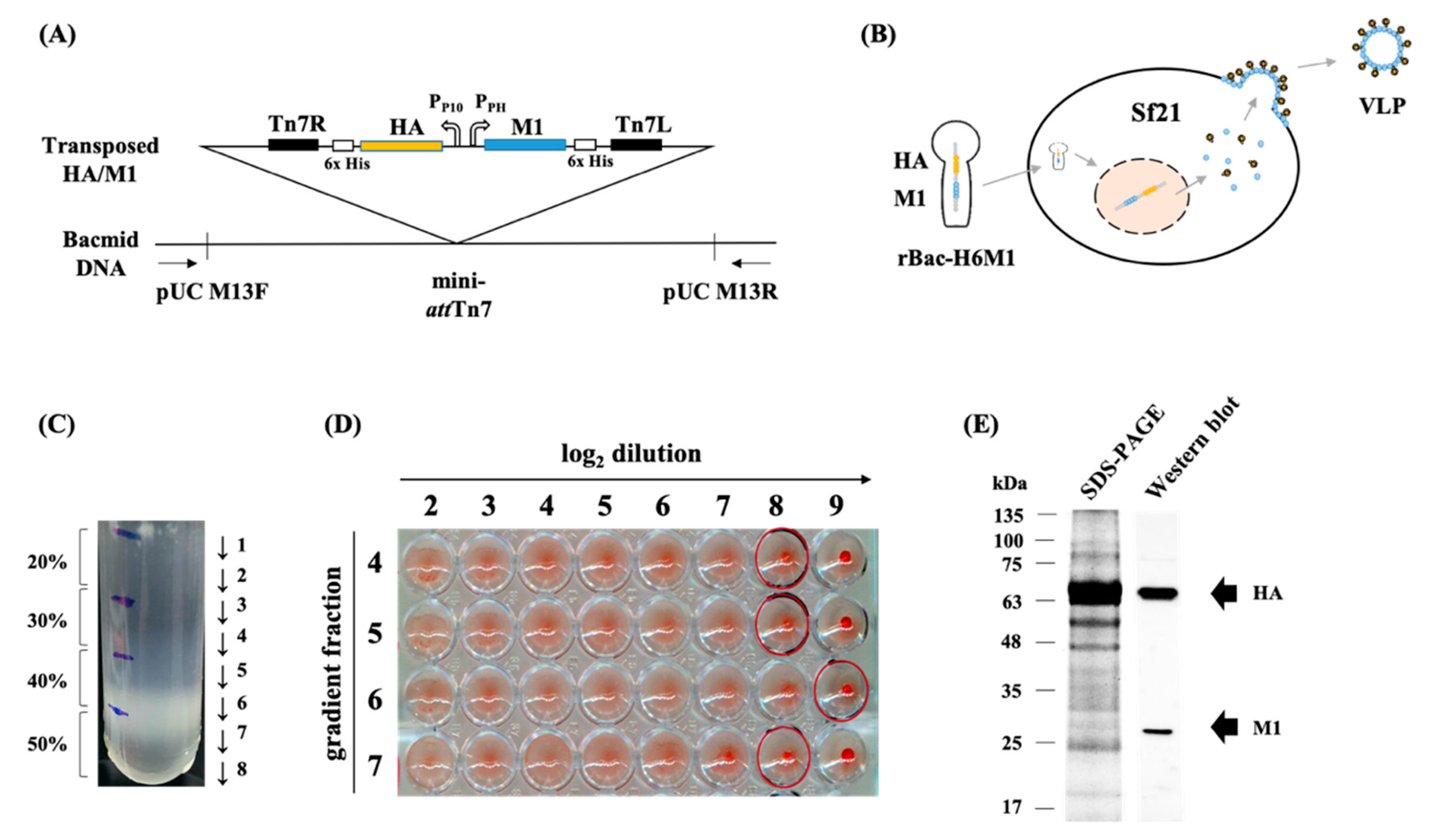
2.2. Influenza Genes and the Expression Construct
2.3. Production and Purification of VLPs
2.4. Sodium Dodecyl Sulfate (SDS)—Polyacrylamide Gel Electrophoresis (PAGE) and Western Blot
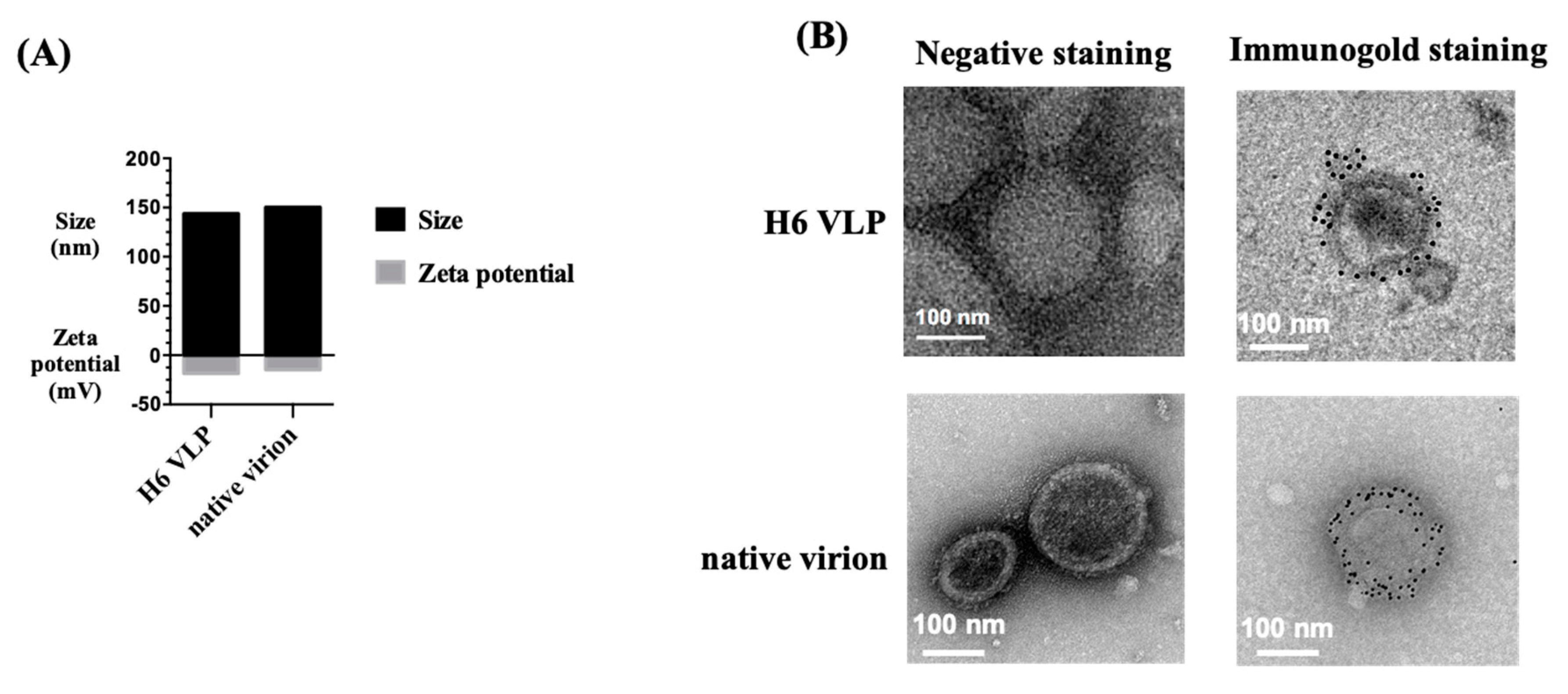
2.5. Transmission Electronic Microscopy (TEM) and Immunogold Staining
2.6. Hemagglutination Test and Hemagglutination-Inhibition Test
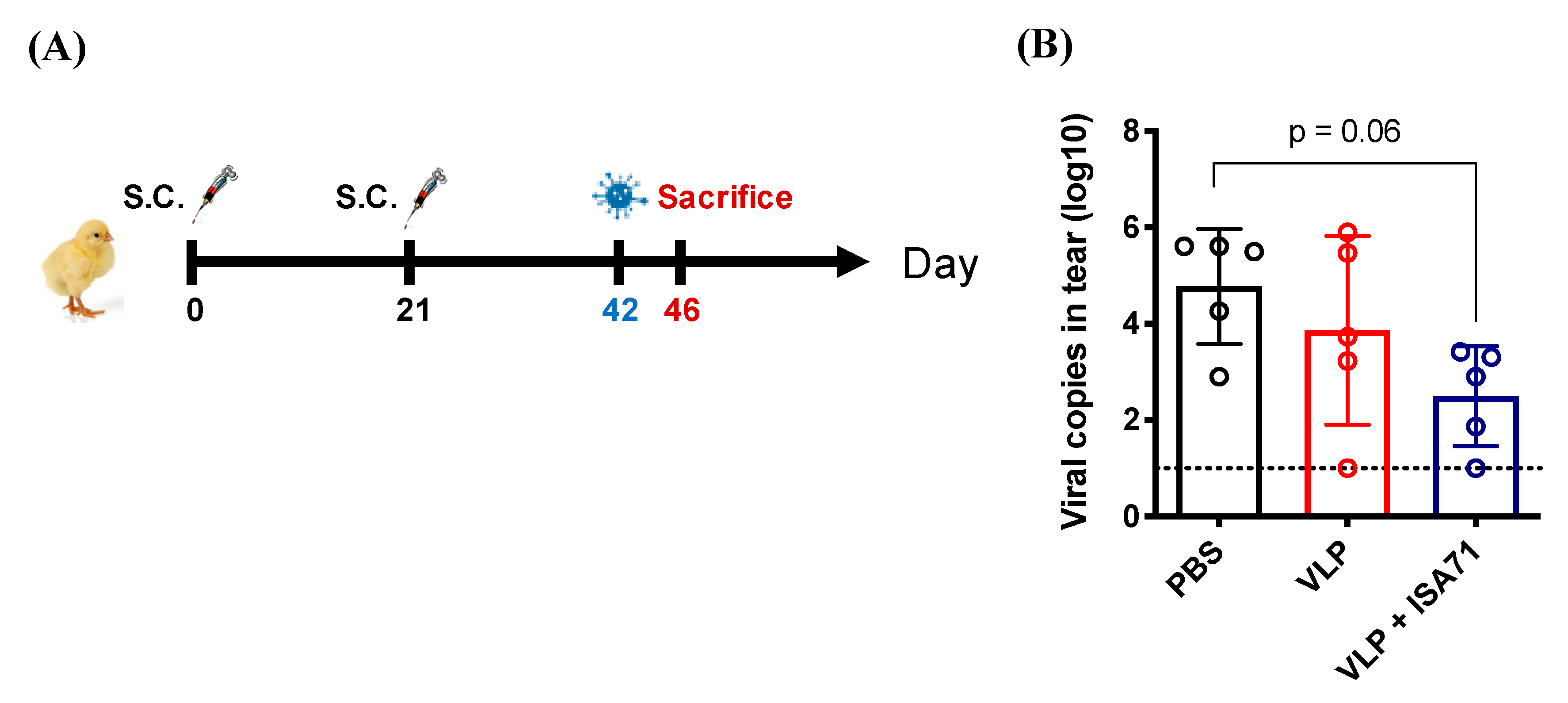
2.7. Chicken Immunization and Viral Challenge Studies
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Viral RNA Extraction, Reverse Transcription, and Quantitative PCR (qPCR)
2.10. Virus Re-Isolation in Embryo Eggs
2.11. Statistical Analyses
3. Results
3.1. Production and Purification of H6 VLPs
3.2. Characterization of H6 VLPs
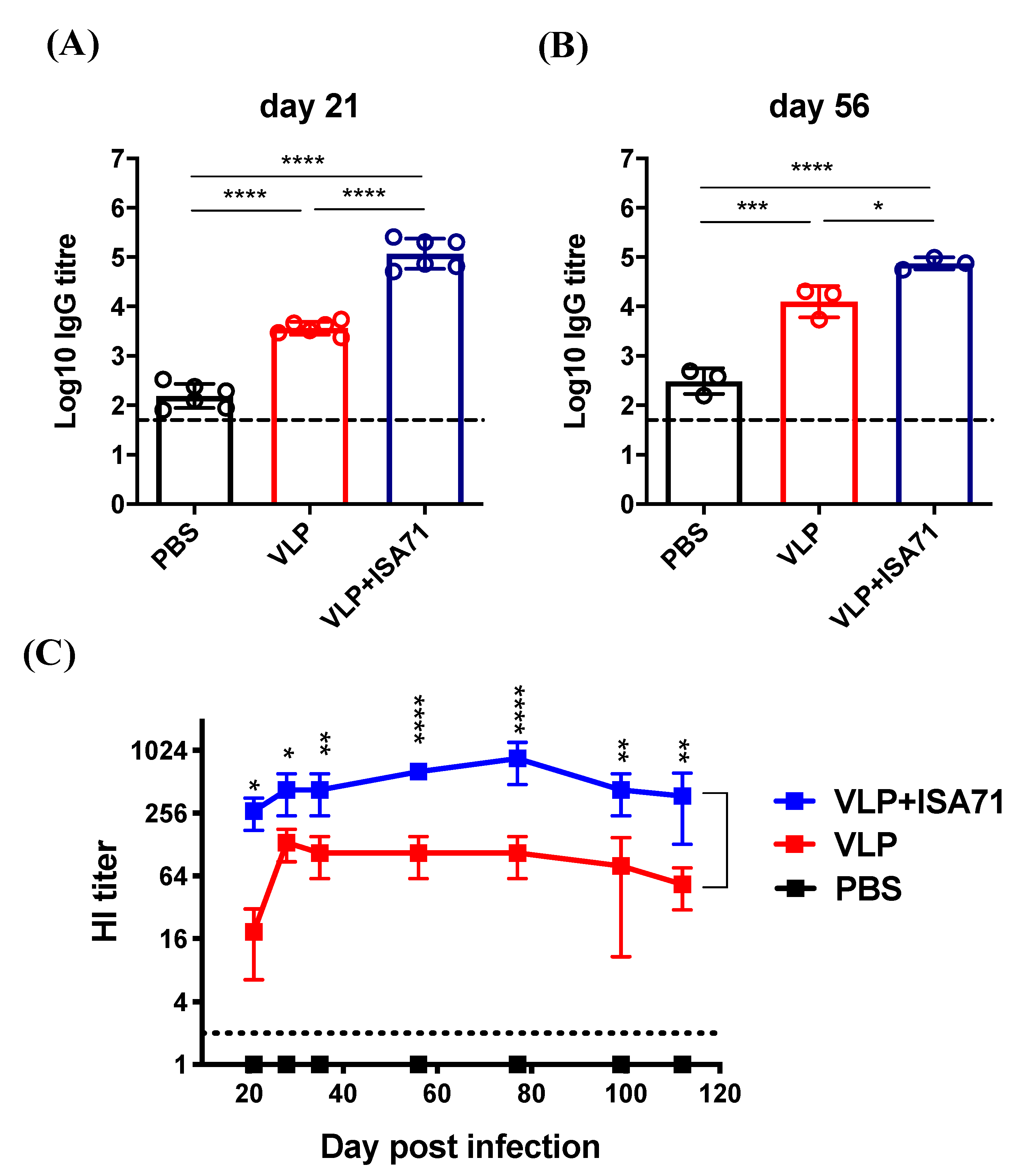
3.3. Immunogenicity of H6 VLPs with ISA 71 VG
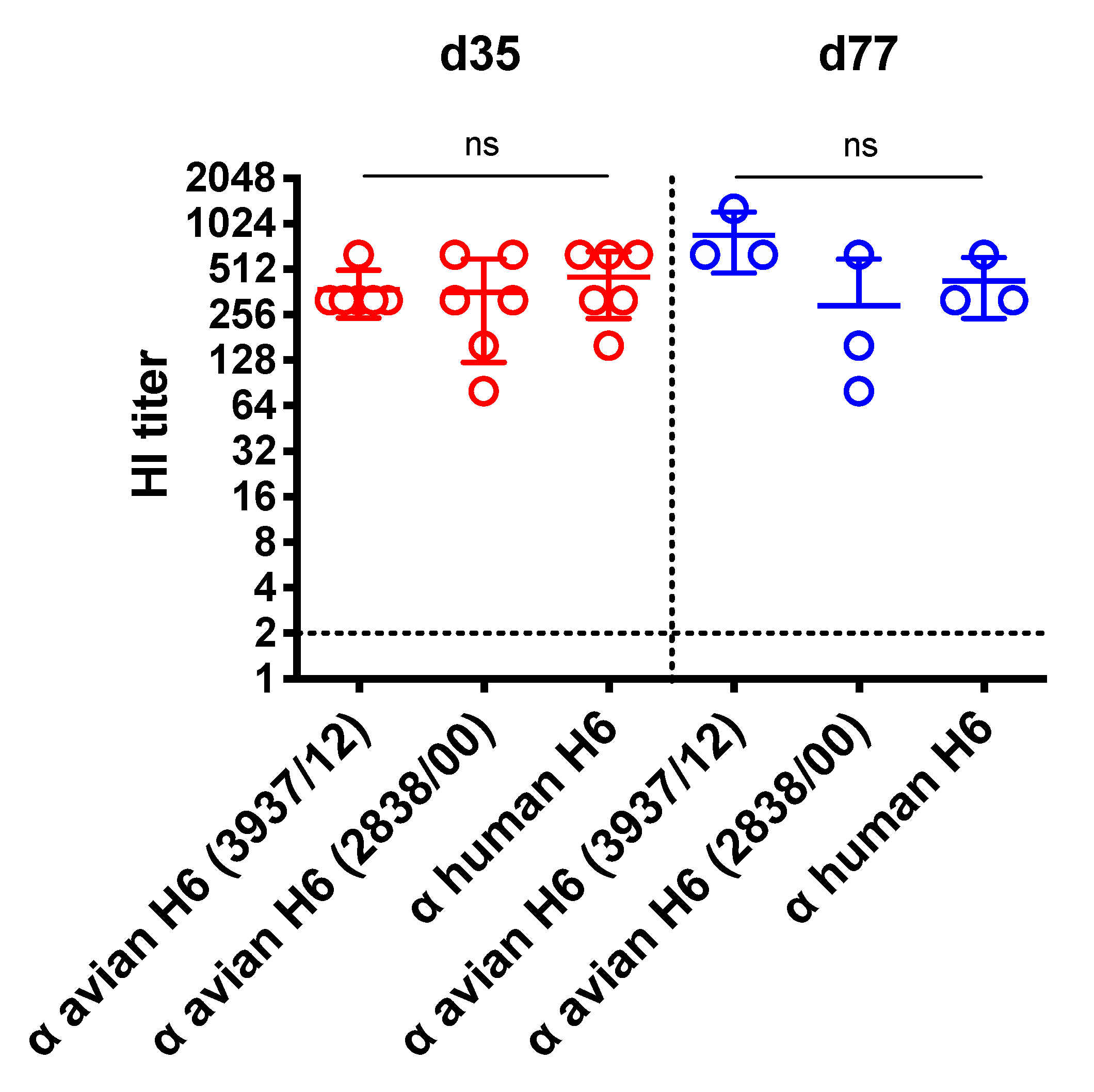
3.4. Protective Efficacy of H6 VLP Vaccine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bouvier, N.M.; Palese, P. The biology of influenza viruses. Vaccine 2008, 26, D49–D53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, S.; Zhu, X.; Li, Y.; Shi, M.; Zhang, J.; Bourgeois, M.; Yang, H.; Chen, X.; Recuenco, S.; Gomez, J.; et al. New world bats harbor diverse influenza A viruses. PLoS Pathog. 2013, 9, e1003657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munster, V.J.; Baas, C.; Lexmond, P.; Waldenström, J.; Wallensten, A.; Fransson, T.; Rimmelzwaan, G.F.; Beyer, W.E.; Schutten, M.; Olsen, B.; et al. Spatial, temporal, and species variation in prevalence of influenza A viruses in wild migratory birds. PLoS Pathog. 2007, 3, e61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Deng, G.; Shi, J.; Luo, W.; Zhang, G.; Zhang, Q.; Liu, L.; Jiang, Y.; Li, C.; Sriwilaijaroen, N.; et al. H6 influenza viruses pose a potential threat to human health. J. Virol. 2014, 88, 3953–3964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, Y.; Chai, H.; Fan, Z.; Wang, X.; Yao, Q.; Ma, J.; Chen, S.; Hua, Y.; Deng, G.; Chen, H. New H6 influenza virus reassortment strains isolated from Anser fabalis in Anhui Province, China. Virol. J. 2017, 14, 36. [Google Scholar] [CrossRef] [Green Version]
- Gillim-Ross, L.; Santos, C.; Chen, Z.; Aspelund, A.; Yang, C.F.; Ye, D.; Jin, H.; Kemble, G.; Subbarao, K. Avian influenza h6 viruses productively infect and cause illness in mice and ferrets. J. Virol. 2008, 82, 10854–10863. [Google Scholar] [CrossRef] [Green Version]
- Cheng, K.; Yu, Z.; Gao, Y.; Xia, X.; He, H.; Hua, Y.; Chai, H. Experimental infection of dogs with H6N1 avian influenza A virus. Arch. Virol. 2014, 159, 2275–2282. [Google Scholar] [CrossRef]
- Lee, M.-S.; Chang, P.-C.; Shien, J.-H.; Cheng, M.-C.; Chen, C.-L.; Shieh, H.K. Genetic and pathogenic characterization of H6N1 avian influenza viruses isolated in Taiwan between 1972 and 2005. Avian Dis. 2006, 50, 561–571. [Google Scholar] [CrossRef]
- Lin, H.-T.; Wang, C.-H.; Chueh, L.-L.; Su, B.-L.; Wang, L.-C. Influenza A (H6N1) virus in dogs, Taiwan. Emerg. Infect. Dis. 2015, 21, 2154–2157. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.H.; Yang, J.R.; Wu, H.S.; Chang, M.C.; Lin, J.S.; Lin, C.Y.; Liu, Y.L.; Lo, Y.C.; Yang, C.H.; Chuang, J.H.; et al. Human infection with avian influenza A H6N1 virus: An epidemiological analysis. Lancet Respir. Med. 2013, 1, 771–778. [Google Scholar] [CrossRef]
- Huang, S.; Yang, J.; Lin, Y.; Yang, C.; Cheng, M.; Liu, M.; Wu, H.; Chang, F. Serological comparison of antibodies to avian influenza viruses, subtypes H5N2, H6N1, H7N3 and H7N9 between poultry workers and non-poultry workers in Taiwan in 2012. Epidemiol. Infect. 2015, 143, 2965–2974. [Google Scholar] [CrossRef] [PubMed]
- Durous, L.; Rosa-Calatrava, M.; Petiot, E. Advances in influenza virus-like particles bioprocesses. Expert Rev. Vaccines 2019, 18, 1285–1300. [Google Scholar] [CrossRef] [PubMed]
- Frietze, K.M.; Peabody, D.S.; Chackerian, B. Engineering virus-like particles as vaccine platforms. Curr. Opin. Virol. 2016, 18, 44–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeltins, A. Construction and characterization of virus-like particles: A review. Mol. Biotechnol. 2013, 53, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.; Gomes, A.; Vogel, M.; Bachmann, M. Interaction of viral capsid-derived virus-like particles (VLPs) with the innate immune system. Vaccines 2018, 6, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roldão, A.; Mellado, M.C.M.; Castilho, L.R.; Carrondo, M.J.; Alves, P.M. Virus-like particles in vaccine development. Expert Rev. Vaccines 2010, 9, 1149–1176. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Zha, L.; Cabral-Miranda, G.; Bachmann, M.F. Major findings and recent advances in virus-like particle (VLP)-based vaccines. Semin. Immunol. 2017, 34, 123–132. [Google Scholar] [CrossRef]
- Wang, C.W.; Wang, C.H. Experimental selection of virus derivatives with variations in virulence from a single low-pathogenicity H6N1 avian influenza virus field isolate. Avian Dis. 2003, 47, 1416–1422. [Google Scholar] [CrossRef]
- Chen, H.W.; Liu, P.F.; Liu, Y.T.; Kuo, S.; Zhang, X.Q.; Schooley, R.T.; Rohde, H.; Gallo, R.L.; Huang, C.M. Nasal commensal Staphylococcus epidermidis counteracts influenza virus. Sci. Rep. 2016, 6, 27870. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.J.; Chien, C.Y.; Liu, M.T.; Fang, Z.S.; Chang, S.Y.; Juang, R.H.; Chang, S.C.; Chen, H.W. Multi-antigen avian influenza a (H7N9) virus-like particles: Particulate characterizations and immunogenicity evaluation in murine and avian models. BMC Biotechnol. 2017, 17, 2. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-T.; Juang, R.-H.; He, J.-L.; Chu, W.-Y.; Wang, C.-H. Detection of H6 influenza antibody by blocking enzyme-linked immunosorbent assay. Vet. Microbiol. 2010, 142, 205–210. [Google Scholar] [CrossRef] [PubMed]
- OIE. Chapter 2.3.4. Avian Influenza (Infection with Avian Influenza Viruses). Available online: http://www.oie.int/fileadmin/Home/fr/Health_standards/tahm/2.03.04_AI.pdf (accessed on 20 June 2020).
- Ward, C.; Dempsey, M.; Ring, C.; Kempson, R.; Zhang, L.; Gor, D.; Snowden, B.; Tisdale, M. Design and performance testing of quantitative real time PCR assays for influenza A and B viral load measurement. J. Clin. Virol. 2004, 29, 179–188. [Google Scholar] [CrossRef]
- Chen, H.W.; Fang, Z.S.; Chen, Y.T.; Chen, Y.I.; Yao, B.Y.; Cheng, J.Y.; Chien, C.Y.; Chang, Y.C.; Hu, C.J. Targeting and Enrichment of Viral Pathogen by Cell Membrane Cloaked Magnetic Nanoparticles for Enhanced Detection. ACS Appl. Mater. Interfaces 2017, 9, 39953–39961. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.J.; Chen, Y.T.; Fang, Z.S.; Chang, W.S.; Chen, H.W. Antiviral efficacy of nanoparticulate vacuolar ATPase inhibitors against influenza virus infection. Int. J. Nanomed. 2018, 13, 8579–8593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, J.C.; Chan, W.W.; Kien, F.; Nicholls, J.M.; Peiris, J.S.; Garcia, J.M. Formation of virus-like particles from human cell lines exclusively expressing influenza neuraminidase. J. Gen. Virol. 2010, 91, 2322–2330. [Google Scholar] [CrossRef]
- Kapczynski, D.R.; Tumpey, T.M.; Hidajat, R.; Zsak, A.; Chrzastek, K.; Tretyakova, I.; Pushko, P. Vaccination with virus-like particles containing H5 antigens from three H5N1 clades protects chickens from H5N1 and H5N8 influenza viruses. Vaccine 2016, 34, 1575–1581. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.; O’Kennedy, M.M.; Wandrag, D.B.R.; Adeyemi, M.; Abolnik, C. Efficacy of a plant-produced virus-like particle vaccine in chickens challenged with Influenza A H6N2 virus. Plant Biotechnol. J. 2020, 18, 502–512. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Jarvis, D.L. Protein N-glycosylation in the baculovirus-insect cell system. Curr. Drug Targets 2007, 8, 1116–1125. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.R.; Chen, C.Y.; Kuo, C.Y.; Cheng, C.Y.; Lee, M.S.; Cheng, M.C.; Yang, Y.C.; Wu, C.Y.; Wu, H.S.; Liu, M.T.; et al. A novel H6N1 virus-like particle vaccine induces long-lasting cross-clade antibody immunity against human and avian H6N1 viruses. Antivir. Res. 2016, 126, 8–17. [Google Scholar] [CrossRef]
- Dou, D.; Revol, R.; Östbye, H.; Wang, H.; Daniels, R. Influenza A virus cell entry, replication, virion assembly and movement. Front. Immunol. 2018, 9, 1581. [Google Scholar] [CrossRef]
- Sha, B.; Luo, M. Structure of a bifunctional membrane-RNA binding protein, influenza virus matrix protein M1. Nat. Struct. Biol. 1997, 4, 239. [Google Scholar] [CrossRef] [PubMed]
- Safo, M.K.; Musayev, F.N.; Mosier, P.D.; Zhou, Q.; Xie, H.; Desai, U.R. Crystal structures of influenza A virus matrix protein M1: Variations on a theme. PloS ONE 2014, 9, e109510. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, L.; Ascarateil, S.; Aucouturier, J.; Ganne, V. SEPPIC vaccine adjuvants for poultry. Ann. N.Y. Acad. Sci. 2006, 1081, 202–205. [Google Scholar] [CrossRef]
- Tan, P.T.; Khan, A.M.; August, J.T. Highly conserved influenza A sequences as T cell epitopes-based vaccine targets to address the viral variability. Hum. Vaccines 2011, 7, 402–409. [Google Scholar] [CrossRef]
- Pushko, P.; Tretyakova, I.; Hidajat, R.; Zsak, A.; Chrzastek, K.; Tumpey, T.M.; Kapczynski, D.R. Virus-like particles displaying H5, H7, H9 hemagglutinins and N1 neuraminidase elicit protective immunity to heterologous avian influenza viruses in chickens. Virology 2017, 501, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Sunwoo, S.-Y.; Schotsaert, M.; Morozov, I.; Davis, A.; Li, Y.; Lee, J.; McDowell, C.; Meade, P.; Nachbagauer, R.; García-Sastre, A.; et al. A universal influenza virus vaccine candidate tested in a pig vaccination-infection model in the presence of maternal antibodies. Vaccines 2018, 6, 64. [Google Scholar] [CrossRef] [Green Version]
- Steel, J.; Lowen, A.C.; Wang, T.T.; Yondola, M.; Gao, Q.; Haye, K.; García-Sastre, A.; Palese, P. Influenza virus vaccine based on the conserved hemagglutinin stalk domain. MBio 2010, 1, e00018-10. [Google Scholar] [CrossRef] [Green Version]
- Rajão, D.S.; Pérez, D.R. Universal vaccines and vaccine platforms to protect against influenza viruses in humans and agriculture. Front. Microbiol. 2018, 9, 123. [Google Scholar] [CrossRef]
- Kang, S.-M.; Kim, M.-C.; Compans, R.W. Virus-like particles as universal influenza vaccines. Expert Rev. Vaccines 2012, 11, 995–1007. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.H.; Park, J.K.; Lee, Y.N.; Song, J.M.; Kang, S.M.; Lee, J.B.; Park, S.Y.; Choi, I.S.; Song, C.S. H9N2 avian influenza virus-like particle vaccine provides protective immunity and a strategy for the differentiation of infected from vaccinated animals. Vaccine 2011, 29, 4003–4007. [Google Scholar] [CrossRef] [Green Version]
| Vaccination Group | 106 EID50 Challenge | 109 EID50 Challenge | ||
|---|---|---|---|---|
| Cloacal Swab | Lung | Kidney | Lung | |
| PBS | 4/5 | 5/5 | 4/5 | 3/3 |
| VLP | 1/5 | 2/5 | 0/5 | n/a 1 |
| VLP + ISA 71 VG | 0/5 | 0/5 | 0/5 | 0/3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.-Z.; Wen, Y.-C.; Lin, S.-Y.; Chen, T.-C.; Chen, H.-W. Anti-Influenza Protective Efficacy of a H6 Virus-Like Particle in Chickens. Vaccines 2020, 8, 465. https://doi.org/10.3390/vaccines8030465
Zhu W-Z, Wen Y-C, Lin S-Y, Chen T-C, Chen H-W. Anti-Influenza Protective Efficacy of a H6 Virus-Like Particle in Chickens. Vaccines. 2020; 8(3):465. https://doi.org/10.3390/vaccines8030465
Chicago/Turabian StyleZhu, Wan-Zhen, Yi-Chi Wen, Shu-Yi Lin, Ting-Chih Chen, and Hui-Wen Chen. 2020. "Anti-Influenza Protective Efficacy of a H6 Virus-Like Particle in Chickens" Vaccines 8, no. 3: 465. https://doi.org/10.3390/vaccines8030465
APA StyleZhu, W. -Z., Wen, Y. -C., Lin, S. -Y., Chen, T. -C., & Chen, H. -W. (2020). Anti-Influenza Protective Efficacy of a H6 Virus-Like Particle in Chickens. Vaccines, 8(3), 465. https://doi.org/10.3390/vaccines8030465





