A Zika Vaccine Generated Using the Chimeric Insect-Specific Binjari Virus Platform Protects against Fetal Brain Infection in Pregnant Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. ZIKVPRVABC59
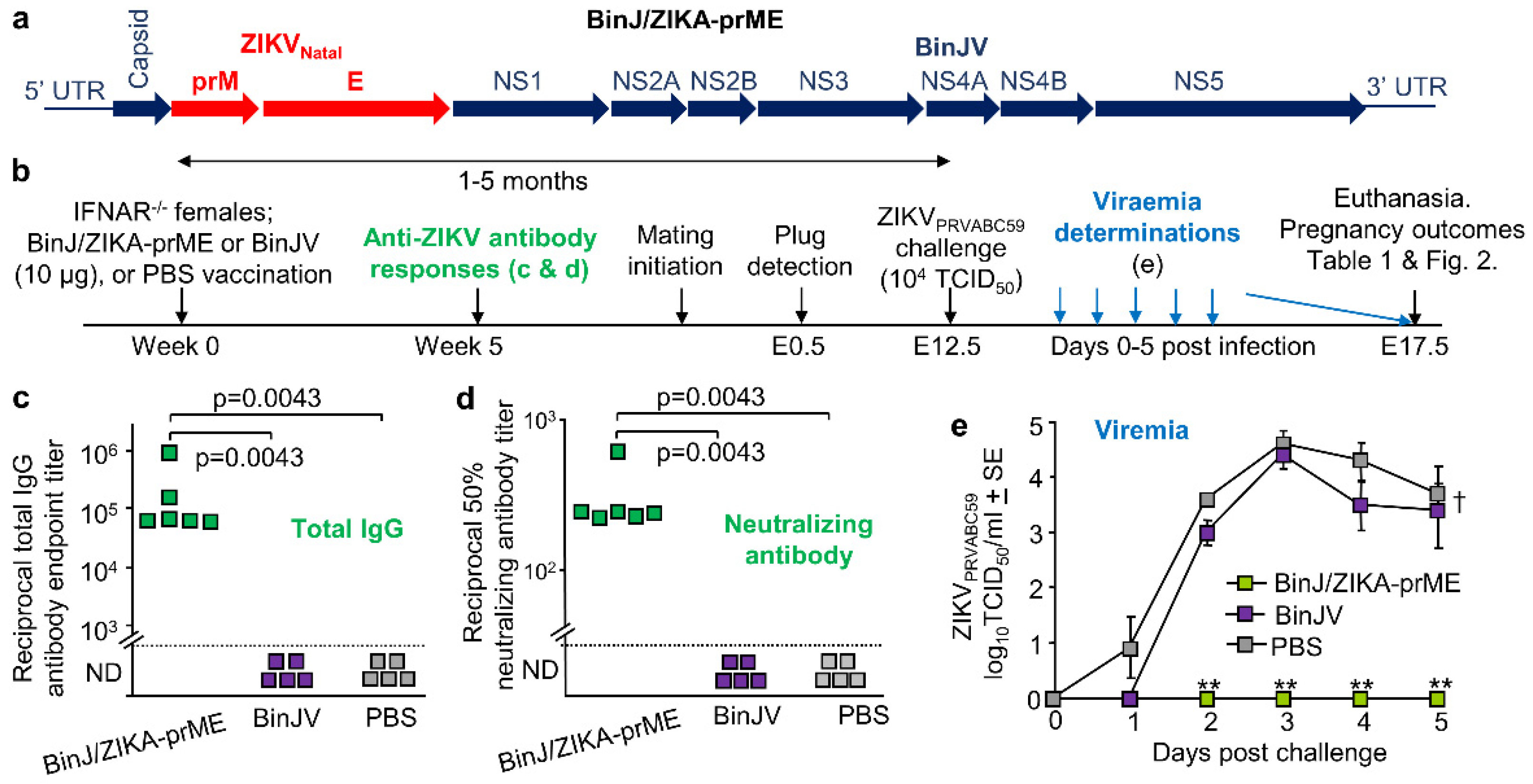
2.3. Vaccination and Antibody Responses
2.4. ZIKV Challenge of Pregnant IFNAR−/− Mice
2.5. Real Time Quantitative RT-PCR (qRT PCR)
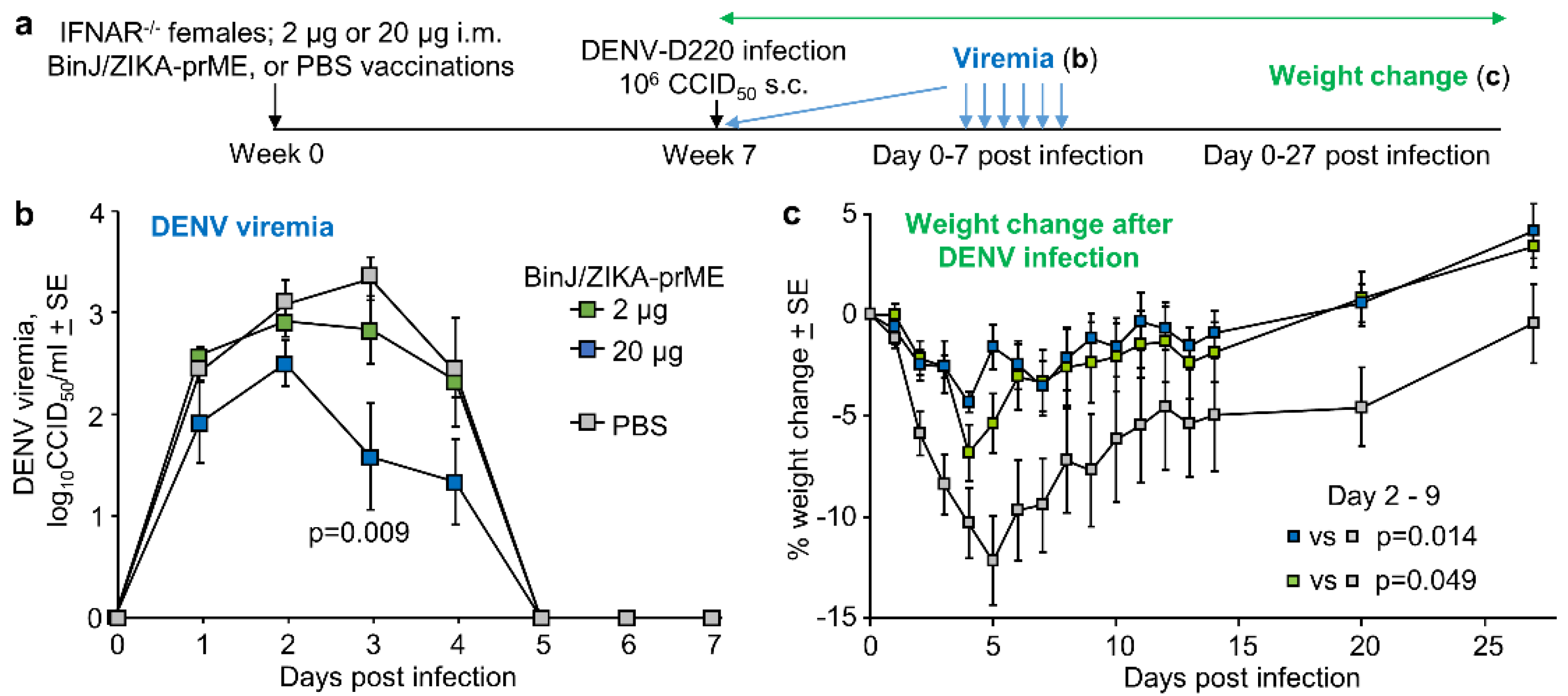
2.6. Model of DENV ADE
2.7. Statistical Analysis
3. Results
3.1. BinJ/ZIKA-prME Vaccination and Protection against ZIKV Challenge
3.2. Overt Pregnancy Outcomes after ZIKV Challenge
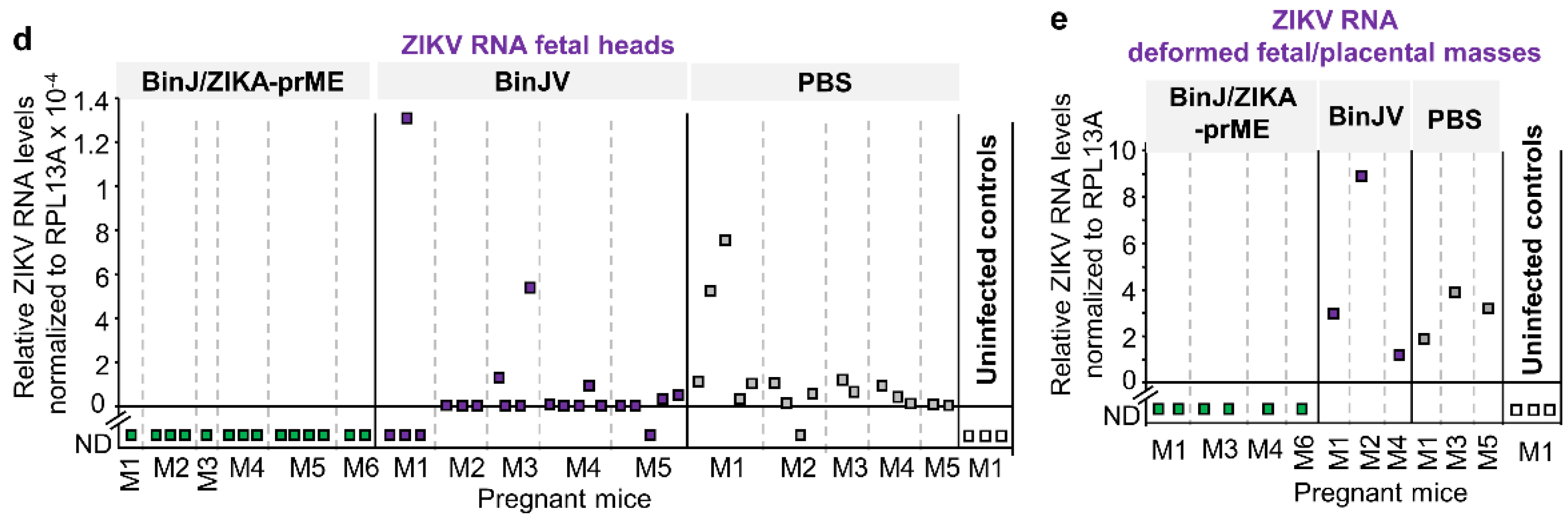
3.3. BinJ/ZIKA-prME Protected against Fetal Weight Loss and Infection of Placenta and Fetuses
3.4. Post-Challenge Viral RNA in Placenta and Fetal Heads
3.5. BinJ/ZIKA-prME Vaccination Did Not Induce ADE of DENV-2 Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martins, M.M.; Medronho, R.A.; Cunha, A. Zika virus in Brazil and worldwide: A narrative review. Paediatr. Int. Child Health 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Duttine, A.; Smythe, T.; Ribiero Calheiro de Sa, M.; Ferrite, S.; Zuurmond, M.; Moreira, M.E.; Collins, A.; Milner, K.; Kuper, H. Congenital Zika Syndrome-Assessing the Need for a Family Support Programme in Brazil. Int. J. Environ. Res. Public Health 2020, 17, 3559. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Barrett, A. Zika vaccine pre-clinical and clinical data review with perspectives on the future development. Hum. Vaccin. Immunother. 2020, 1–13. [Google Scholar] [CrossRef]
- Laris-Gonzalez, A.; Bernal-Serrano, D.; Jarde, A.; Kampmann, B. Safety of Administering Live Vaccines During Pregnancy: A Systematic Review and Meta-Analysis of Pregnancy Outcomes. Vaccines 2020, 8, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pattnaik, A.; Sahoo, B.R.; Pattnaik, A.K. Current Status of Zika Virus Vaccines: Successes and Challenges. Vaccines 2020, 8, 266. [Google Scholar] [CrossRef]
- Das Neves Almeida, R.; Racine, T.; Magalhaes, K.G.; Kobinger, G.P. Zika Virus Vaccines: Challenges and Perspectives. Vaccines 2018, 6, 62. [Google Scholar] [CrossRef] [Green Version]
- Luisi, K.; Morabito, K.M.; Burgomaster, K.E.; Sharma, M.; Kong, W.-P.; Foreman, B.M.; Patel, S.; Fisher, B.; Aleshnick, M.A.; Laliberte, J.; et al. Development of a potent Zika virus vaccine using self-amplifying messenger RNA. Sci. Adv. 2020, 6. [Google Scholar] [CrossRef]
- Li, X.-F.; Dong, H.-L.; Wang, H.-J.; Huang, X.-Y.; Qiu, Y.-F.; Ji, X.; Ye, Q.; Li, C.; Liu, Y.; Deng, Y.-Q.; et al. Development of a chimeric Zika vaccine using a licensed live-attenuated flavivirus vaccine as backbone. Nat. Commun. 2018, 9, 673. [Google Scholar] [CrossRef] [Green Version]
- Redoni, M.; Yacoub, S.; Rivino, L.; Giacobbe, D.R.; Luzzati, R.; Di Bella, S. Dengue: Status of current and under-development vaccines. Rev. Med. Virol. 2020, 30, e2101. [Google Scholar] [CrossRef]
- Hobson-Peters, J.; Harrison, J.J.; Watterson, D.; Hazlewood, J.E.; Vet, L.J.; Newton, N.D.; Warrilow, D.; Colmant, A.M.G.; Taylor, C.; Huang, B.; et al. A recombinant platform for flavivirus vaccines and diagnostics using chimeras of a new insect-specific virus. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef]
- Harrison, J.J.; Hobson-Peters, J.; Colmant, A.M.G.; Koh, J.; Newton, N.D.; Warrilow, D.; Bielefeldt-Ohmann, H.; Piyasena, T.B.H.; O’Brien, C.A.; Vet, L.J.; et al. Antigenic Characterization of New Lineage II Insect-Specific Flaviviruses in Australian Mosquitoes and Identification of Host Restriction Factors. mSphere 2020, 5, e00095-20. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Vet, L.J.; Tang, B.; Hobson-Peters, J.; Rawle, D.J.; Le, T.T.; Larcher, T.; Hall, R.A.; Suhrbier, A. A Yellow Fever Virus 17D Infection and Disease Mouse Model Used to Evaluate a Chimeric Binjari-Yellow Fever Virus Vaccine. Vaccines 2020, 8, 368. [Google Scholar] [CrossRef] [PubMed]
- Vet, L.J.; Setoh, Y.X.; Amarilla, A.A.; Habarugira, G.; Suen, W.W.; Newton, N.D.; Harrison, J.J.; Hobson-Peters, J.; Hall, R.A.; Bielefeldt-Ohmann, H. Protective Efficacy of a Chimeric Insect-Specific Flavivirus Vaccine against West Nile Virus. Vaccines 2020, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Prow, N.A.; Liu, L.; Nakayama, E.; Cooper, T.H.; Yan, K.; Eldi, P.; Hazlewood, J.E.; Tang, B.; Le, T.T.; Setoh, Y.X.; et al. A vaccinia-based single vector construct multi-pathogen vaccine protects against both Zika and chikungunya viruses. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbink, P.; Stephenson, K.E.; Barouch, D.H. Zika virus vaccines. Nat. Rev. Microbiol. 2018, 16, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Richner, J.M.; Diamond, M.S. Zika virus vaccines: Immune response, current status, and future challenges. Curr. Opin. Immunol. 2018, 53, 130–136. [Google Scholar] [CrossRef]
- Guo, Q.; Chan, J.F.; Poon, V.K.; Wu, S.; Chan, C.C.; Hou, L.; Yip, C.C.; Ren, C.; Cai, J.P.; Zhao, M.; et al. Immunization With a Novel Human Type 5 Adenovirus-Vectored Vaccine Expressing the Premembrane and Envelope Proteins of Zika Virus Provides Consistent and Sterilizing Protection in Multiple Immunocompetent and Immunocompromised Animal Models. J. Infect. Dis. 2018, 218, 365–377. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Ke, X.; Wang, T.; Tan, Z.; Luo, D.; Miao, Y.; Sun, J.; Zhang, Y.; Liu, Y.; Hu, Q.; et al. Zika Virus Attenuation by Codon Pair Deoptimization Induces Sterilizing Immunity in Mouse Models. J. Virol. 2018, 92, e00701-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prow, N.A.; Liu, L.; McCarthy, M.K.; Walters, K.; Kalkeri, R.; Geiger, J.; Koide, F.; Cooper, T.H.; Eldi, P.; Nakayama, E.; et al. The vaccinia virus based Sementis Copenhagen Vector vaccine against Zika and chikungunya is immunogenic in non-human primates. NPJ Vaccines 2020, 5, 44. [Google Scholar] [CrossRef]
- Forrester, J.V.; McMenamin, P.G.; Dando, S.J. CNS infection and immune privilege. Nat. Rev. Neurosci. 2018, 19, 655–671. [Google Scholar] [CrossRef]
- Adams Waldorf, K.M.; Nelson, B.R.; Stencel-Baerenwald, J.E.; Studholme, C.; Kapur, R.P.; Armistead, B.; Walker, C.L.; Merillat, S.; Vornhagen, J.; Tisoncik-Go, J.; et al. Congenital Zika virus infection as a silent pathology with loss of neurogenic output in the fetal brain. Nat. Med. 2018, 24, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Slon-Campos, J.L.; Dejnirattisai, W.; Jagger, B.W.; López-Camacho, C.; Wongwiwat, W.; Durnell, L.A.; Winkler, E.S.; Chen, R.E.; Reyes-Sandoval, A.; Rey, F.A.; et al. A protective Zika virus E-dimer-based subunit vaccine engineered to abrogate antibody-dependent enhancement of dengue infection. Nat. Immunol. 2019, 20, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Shanmugam, R.K.; Ramasamy, V.; Arora, U.; Batra, G.; Acklin, J.A.; Krammer, F.; Lim, J.K.; Swaminathan, S.; Khanna, N. Zika virus envelope nanoparticle antibodies protect mice without risk of disease enhancement. EBioMedicine 2020, 54. [Google Scholar] [CrossRef] [PubMed]
- Martin-Acebes, M.A.; Saiz, J.C.; Jimenez de Oya, N. Antibody-Dependent Enhancement and Zika: Real Threat or Phantom Menace? Front. Cell. Infect. Microbiol. 2018, 8, 44. [Google Scholar] [CrossRef] [Green Version]
- Katzelnick, L.C.; Narvaez, C.; Arguello, S.; Lopez Mercado, B.; Collado, D.; Ampie, O.; Elizondo, D.; Miranda, T.; Bustos Carillo, F.; Mercado, J.C.; et al. Zika virus infection enhances future risk of severe dengue disease. Science 2020, 369, 1123–1128. [Google Scholar] [CrossRef]
- Stettler, K.; Beltramello, M.; Espinosa, D.A.; Graham, V.; Cassotta, A.; Bianchi, S.; Vanzetta, F.; Minola, A.; Jaconi, S.; Mele, F.; et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 2016, 353, 823–826. [Google Scholar] [CrossRef] [Green Version]
- George, J.; Valiant, W.G.; Mattapallil, M.J.; Walker, M.; Huang, Y.-J.S.; Vanlandingham, D.L.; Misamore, J.; Greenhouse, J.; Weiss, D.E.; Verthelyi, D.; et al. Prior Exposure to Zika Virus Significantly Enhances Peak Dengue-2 Viremia in Rhesus Macaques. Sci. Rep. 2017, 7, 10498. [Google Scholar] [CrossRef]
- Terzian, A.C.B.; Schanoski, A.S.; Mota, M.T.O.; da Silva, R.A.; Estofolete, C.F.; Colombo, T.E.; Rahal, P.; Hanley, K.A.; Vasilakis, N.; Kalil, J.; et al. Viral Load and Cytokine Response Profile Does Not Support Antibody-Dependent Enhancement in Dengue-Primed Zika Virus-Infected Patients. Clin. Infect. Dis. 2017, 65, 1260–1265. [Google Scholar] [CrossRef] [Green Version]
- Vouga, M.; Chiu, Y.C.; Pomar, L.; de Meyer, S.V.; Masmejan, S.; Genton, B.; Musso, D.; Baud, D.; Stojanov, M. Dengue, Zika and chikungunya during pregnancy: Pre- and post-travel advice and clinical management. J. Travel Med. 2019, 26. [Google Scholar] [CrossRef]
- Kato, F.; Tajima, S.; Nakayama, E.; Kawai, Y.; Taniguchi, S.; Shibasaki, K.; Taira, M.; Maeki, T.; Lim, C.K.; Takasaki, T.; et al. Characterization of large and small-plaque variants in the Zika virus clinical isolate ZIKV/Hu/S36/Chiba/2016. Sci. Rep. 2017, 7, 16160. [Google Scholar] [CrossRef] [Green Version]
- Linn, M.L.; Bellett, A.J.D.; Parsons, P.G.; Suhrbier, A. Complete removal of mycoplasma from viral preparations using solvent extraction. J. Virol. Methods 1995, 52, 51–54. [Google Scholar] [CrossRef]
- Johnson, B.J.; Le, T.T.T.; Dobbin, C.A.; Banovic, T.; Howard, C.B.; de Maria Leon Flores, F.; Vanags, D.; Naylor, D.J.; Hill, G.R.; Suhrbier, A. Heat Shock Protein 10 Inhibits Lipopolysaccharide-induced Inflammatory Mediator Production. J. Biol. Chem. 2005, 280, 4037–4047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swann, J.B.; Hayakawa, Y.; Zerafa, N.; Sheehan, K.C.; Scott, B.; Schreiber, R.D.; Hertzog, P.; Smyth, M.J. Type I IFN contributes to NK cell homeostasis, activation, and antitumor function. J. Immunol. 2007, 178, 7540–7549. [Google Scholar] [CrossRef] [PubMed]
- Setoh, Y.X.; Prow, N.A.; Peng, N.; Hugo, L.E.; Devine, G.; Hazlewood, J.E.; Suhrbier, A.; Khromykh, A.A. De Novo Generation and Characterization of New Zika Virus Isolate Using Sequence Data from a Microcephaly Case. mSphere 2017, 2, e00190-17. [Google Scholar] [CrossRef] [Green Version]
- Barnard, T.R.; Rajah, M.M.; Sagan, S.M. Contemporary Zika Virus Isolates Induce More dsRNA and Produce More Negative-Strand Intermediate in Human Astrocytoma Cells. Viruses 2018, 10, 728. [Google Scholar] [CrossRef] [Green Version]
- Schroder, W.A.; Le, T.T.; Major, L.; Street, S.; Gardner, J.; Lambley, E.; Markey, K.; MacDonald, K.P.; Fish, R.J.; Thomas, R.; et al. A physiological function of inflammation-associated SerpinB2 is regulation of adaptive immunity. J. Immunol. 2010, 184, 2663–2670. [Google Scholar] [CrossRef] [Green Version]
- Miner, J.J.; Cao, B.; Govero, J.; Smith, A.M.; Fernandez, E.; Cabrera, O.H.; Garber, C.; Noll, M.; Klein, R.S.; Noguchi, K.K.; et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell 2016, 165, 1081–1091. [Google Scholar] [CrossRef] [Green Version]
- Woods, L.; Perez-Garcia, V.; Kieckbusch, J.; Wang, X.; DeMayo, F.; Colucci, F.; Hemberger, M. Decidualisation and placentation defects are a major cause of age-related reproductive decline. Nat. Commun. 2017, 8, 352. [Google Scholar] [CrossRef] [Green Version]
- Bonney, E.A. Demystifying animal models of adverse pregnancy outcomes: Touching bench and bedside. Am. J. Reprod. Immunol. 2013, 69, 567–584. [Google Scholar] [CrossRef] [Green Version]
- Meneses, J.D.A.; Ishigami, A.C.; de Mello, L.M.; de Albuquerque, L.L.; de Brito, C.A.A.; Cordeiro, M.T.; Pena, L.J. Lessons Learned at the Epicenter of Brazil’s Congenital Zika Epidemic: Evidence From 87 Confirmed Cases. Clin. Infect. Dis. 2017, 64, 1302–1308. [Google Scholar] [CrossRef]
- Montoya, M.; Collins, M.; Dejnirattisai, W.; Katzelnick, L.C.; Puerta-Guardo, H.; Jadi, R.; Schildhauer, S.; Supasa, P.; Vasanawathana, S.; Malasit, P.; et al. Longitudinal Analysis of Antibody Cross-neutralization Following Zika Virus and Dengue Virus Infection in Asia and the Americas. J. Infect. Dis. 2018, 218, 536–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, C.; Xie, X.; Luo, H.; Muruato, A.E.; Liu, Y.; Wakamiya, M.; La, J.H.; Chung, J.M.; Weaver, S.C.; Wang, T.; et al. Maternal vaccination and protective immunity against Zika virus vertical transmission. Nat. Commun. 2019, 10, 5677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walz, P.H.; Givens, M.D.; Rodning, S.P.; Riddell, K.P.; Brodersen, B.W.; Scruggs, D.; Short, T.; Grotelueschen, D. Evaluation of reproductive protection against bovine viral diarrhea virus and bovine herpesvirus-1 afforded by annual revaccination with modified-live viral or combination modified-live/killed viral vaccines after primary vaccination with modified-live viral vaccine. Vaccine 2017, 35, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Bielefeldt-Ohmann, H.; Tolnay, A.E.; Reisenhauer, C.E.; Hansen, T.R.; Smirnova, N.; Van Campen, H. Transplacental infection with non-cytopathic bovine viral diarrhoea virus types 1b and 2: Viral spread and molecular neuropathology. J. Comp. Pathol. 2008, 138, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, D.L.; Van Olphen, A.; Van Campen, H.; Hansen, T.R. The fetal brain in bovine viral diarrhea virus-infected calves: Lesions, distribution, and cellular heterogeneity of viral antigen at 190 days gestation. Vet. Pathol. 2008, 45, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Grant, G.B.; Desai, S.; Dumolard, L.; Kretsinger, K.; Reef, S.E. Progress Toward Rubella and Congenital Rubella Syndrome Control and Elimination—Worldwide, 2000-2018. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 855–859. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, S.K.; Goyal, A.; Dubey, M.; Kapur, A.; Ritwik, P. Congenital Rubella Syndrome: Dental manifestations and management in a 5 year old child. J. Clin. Pediatr. Dent. 2012, 37, 71–75. [Google Scholar] [CrossRef]
- Pardy, R.D.; Richer, M.J. Protective to a T: The Role of T Cells during Zika Virus Infection. Cells 2019, 8, 820. [Google Scholar] [CrossRef] [Green Version]
- Imanishi, T.; Saito, T. T Cell Co-stimulation and Functional Modulation by Innate Signals. Trends Immunol. 2020, 41, 200–212. [Google Scholar] [CrossRef]
- Kuka, M.; De Giovanni, M.; Iannacone, M. The role of type I interferons in CD4(+) T cell differentiation. Immunol. Lett. 2019, 215, 19–23. [Google Scholar] [CrossRef]
- Nix, C.D.; Salberg, J.; Coulter, F.J.; Kareko, B.W.; Lyski, Z.L.; Booty, B.L.; Messer, W.B. Potency and breadth of human primary ZIKV immune sera shows that Zika viruses cluster antigenically as a single serotype. PLoS Negl. Trop. Dis. 2020, 14, e0008006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halstead, S.B.; Mahalingam, S.; Marovich, M.A.; Ubol, S.; Mosser, D.M. Intrinsic antibody-dependent enhancement of microbial infection in macrophages: Disease regulation by immune complexes. Lancet Infect. Dis. 2010, 10, 712–722. [Google Scholar] [CrossRef] [Green Version]
- Suhrbier, A.; La Linn, M. Suppression of antiviral responses by antibody-dependent enhancement of macrophage infection. Trends Immunol. 2003, 24, 165–168. [Google Scholar] [CrossRef]
- Flores-Mendoza, L.K.; Estrada-Jimenez, T.; Sedeno-Monge, V.; Moreno, M.; Manjarrez, M.D.C.; Gonzalez-Ochoa, G.; Millan-Perez Pena, L.; Reyes-Leyva, J. IL-10 and socs3 Are Predictive Biomarkers of Dengue Hemorrhagic Fever. Mediat. Inflamm. 2017, 2017, 5197592. [Google Scholar] [CrossRef] [Green Version]
- Pinto, A.K.; Brien, J.D.; Lam, C.Y.; Johnson, S.; Chiang, C.; Hiscott, J.; Sarathy, V.V.; Barrett, A.D.; Shresta, S.; Diamond, M.S. Defining New Therapeutics Using a More Immunocompetent Mouse Model of Antibody-Enhanced Dengue Virus Infection. mBio 2015, 6, e01316-15. [Google Scholar] [CrossRef] [Green Version]
- Orozco, S.; Schmid, M.A.; Parameswaran, P.; Lachica, R.; Henn, M.R.; Beatty, R.; Harris, E. Characterization of a model of lethal dengue virus 2 infection in C57BL/6 mice deficient in the alpha/beta interferon receptor. J. Gen. Virol. 2012, 93, 2152–2157. [Google Scholar] [CrossRef]
- Zellweger, R.M.; Eddy, W.E.; Tang, W.W.; Miller, R.; Shresta, S. CD8+ T cells prevent antigen-induced antibody-dependent enhancement of dengue disease in mice. J. Immunol. 2014, 193, 4117–4124. [Google Scholar] [CrossRef] [Green Version]
- Shresta, S.; Sharar, K.L.; Prigozhin, D.M.; Beatty, P.R.; Harris, E. Murine model for dengue virus-induced lethal disease with increased vascular permeability. J. Virol. 2006, 80, 10208–10217. [Google Scholar] [CrossRef] [Green Version]

| Group | No. of Dams | Mean Number of Indicated Fetus Types + SE per Litter | Mean Dam Age | ||||
|---|---|---|---|---|---|---|---|
| Total | Normal | Reabsorbed | Deformed | IUGR | |||
| BinJ/ZIKA-prME | 6 | 6.3 ± 0.42 | 4.5 ± 0.85 | 0.2 ± 0.17 | 1.5 ± 0.56 | 0.2 ± 0.17 | 6.7 ± 0.56 |
| BinJV | 5 | 9 ± 0.55 | 7.4 ± 1.21 | 0 | 0.8 ± 0.37 | 0.8 ± 0.58 | 6.4 ± 0.93 |
| PBS | 5 | 7.6 ± 0.87 | 5.8 ± 0.49 | 0.2 ± 0.20 | 0.6 ± 0.24 | 1 ± 0.77 | 5 ± 0.84 |
| Unvaccinated & uninfected | 4 | 8.5 ± 0.50 | 7 ± 1.15 | 0 | 1.5 ± 0.96 | 0 | 3 ± 0.41 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazlewood, J.E.; Rawle, D.J.; Tang, B.; Yan, K.; Vet, L.J.; Nakayama, E.; Hobson-Peters, J.; Hall, R.A.; Suhrbier, A. A Zika Vaccine Generated Using the Chimeric Insect-Specific Binjari Virus Platform Protects against Fetal Brain Infection in Pregnant Mice. Vaccines 2020, 8, 496. https://doi.org/10.3390/vaccines8030496
Hazlewood JE, Rawle DJ, Tang B, Yan K, Vet LJ, Nakayama E, Hobson-Peters J, Hall RA, Suhrbier A. A Zika Vaccine Generated Using the Chimeric Insect-Specific Binjari Virus Platform Protects against Fetal Brain Infection in Pregnant Mice. Vaccines. 2020; 8(3):496. https://doi.org/10.3390/vaccines8030496
Chicago/Turabian StyleHazlewood, Jessamine E., Daniel J. Rawle, Bing Tang, Kexin Yan, Laura J. Vet, Eri Nakayama, Jody Hobson-Peters, Roy A. Hall, and Andreas Suhrbier. 2020. "A Zika Vaccine Generated Using the Chimeric Insect-Specific Binjari Virus Platform Protects against Fetal Brain Infection in Pregnant Mice" Vaccines 8, no. 3: 496. https://doi.org/10.3390/vaccines8030496
APA StyleHazlewood, J. E., Rawle, D. J., Tang, B., Yan, K., Vet, L. J., Nakayama, E., Hobson-Peters, J., Hall, R. A., & Suhrbier, A. (2020). A Zika Vaccine Generated Using the Chimeric Insect-Specific Binjari Virus Platform Protects against Fetal Brain Infection in Pregnant Mice. Vaccines, 8(3), 496. https://doi.org/10.3390/vaccines8030496






