Bacteriophage-Based Vaccines: A Potent Approach for Antigen Delivery
Abstract
1. Introduction
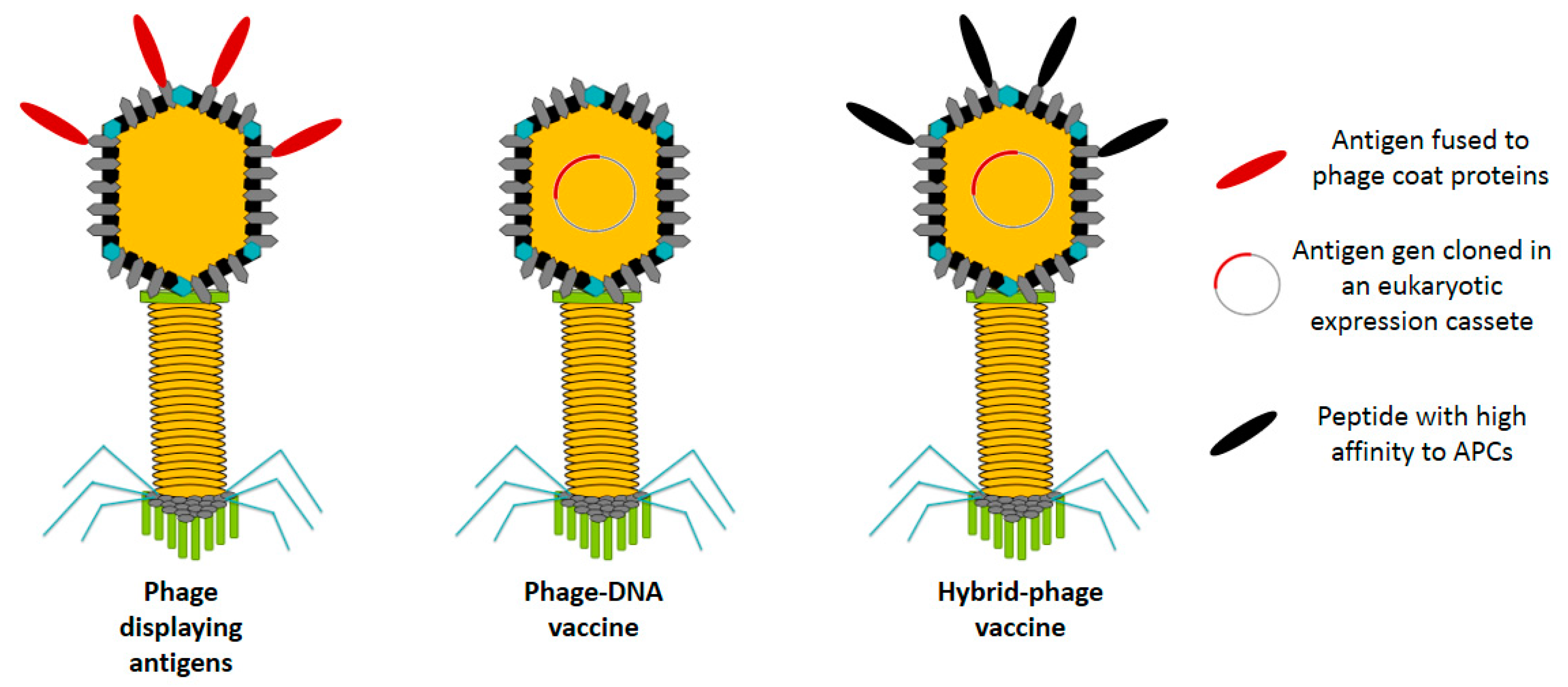
2. Phage-Based Vaccines
2.1. Phage Display Vaccines
2.1.1. General Overview of Phage Display Technology
Filamentous Phages
Lytic Phages
Temperate Phages
2.1.2. Phage Display in Vaccine Development
Phage Displaying Antigens
Antigen Identification
2.2. Bacteriophage DNA Vaccines
2.3. Hybrid Bacteriophage Vaccines
3. Pros and Cons of Bacteriophage-Based Vaccines
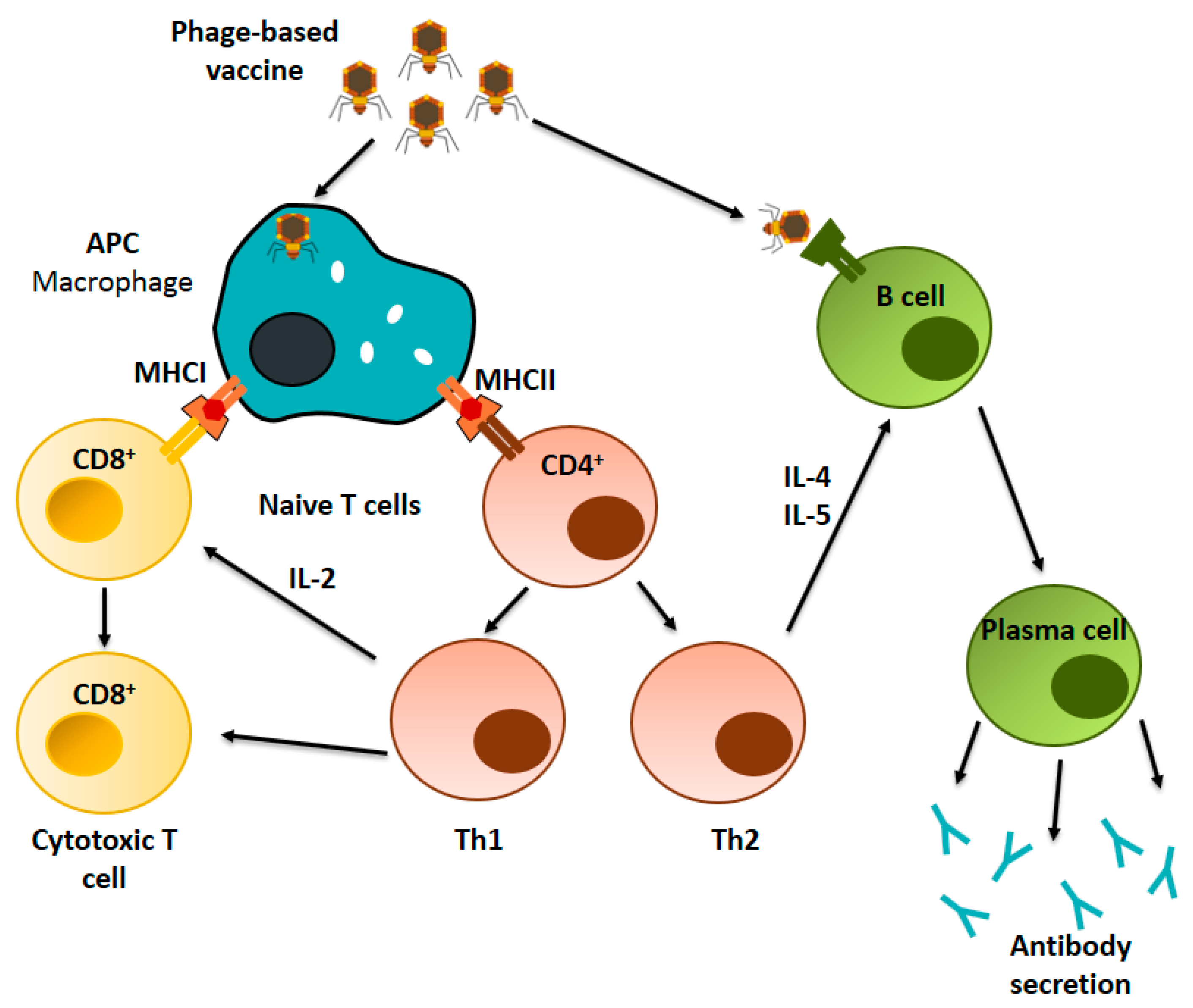
4. Immunological Basis of Phage-Based Vaccines
4.1. Immunogenic Properties of Phages
4.2. Immunological Mechanism of Phage-Based Vaccines
4.2.1. Cellular Immune Response
4.2.2. Humoral Immune Response
5. Administration Routes for Phage-Based Vaccines
6. Final Remarks and Challenges
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Greenwood, B. The contribution of vaccination to global health: Past, present and future. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130433. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.; Martínez, J.J.; Manoutcharian, K.; Hernández, M.; Fleury, A.; Gevorkian, G.; Acero, G.; Blancas, A.; Toledo, A.; Cervantes, J.; et al. Inexpensive anti-cysticercosis vaccine: S3Pvac expressed in heat inactivated M13 filamentous phage proves effective against naturally acquired Taenia solium porcine cysticercosis. Vaccine 2008, 26, 2899–2905. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.R.; Bartley, K.; Jepson, C.D.; Craik, V.; March, J.B. Comparison of a bacteriophage-delivered DNA vaccine and a commercially available recombinant protein vaccine against hepatitis B. FEMS Immunol. Med. Microbiol. 2011, 61, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Jorge, S.; Dellagostin, O.A. The development of veterinary vaccines: A review of traditional methods and modern biotechnology approaches. Biotechnol. Res. Innov. 2017, 1, 6–13. [Google Scholar] [CrossRef]
- Scheiblhofer, S.; Laimer, J.; Machado, Y.; Weiss, R.; Thalhamer, J. Influence of protein fold stability on immunogenicity and its implications for vaccine design. Expert Rev. Vaccines 2017, 16, 479–489. [Google Scholar] [CrossRef]
- Adhya, S.; Merril, C.R.; Biswas, B. Therapeutic and Prophylactic Applications of Bacteriophage Components in Modern Medicine. Cold Spring Harb. Perspect. Med. 2014, 4, a012518. [Google Scholar] [CrossRef]
- Toledo-Machado, C.M.; Bueno, L.L.; Menezes-Souza, D.; Machado-De-Ávila, R.A.; Nguyen, C.; Granier, C.; Bartholomeu, D.C.; Chávez-Olórtegui, C.; Fujiwara, R.T. Use of Phage Display technology in development of canine visceral leishmaniasis vaccine using synthetic peptide trapped in sphingomyelin/cholesterol liposomes. Parasites Vectors 2015, 8, 133. [Google Scholar] [CrossRef]
- Hobernik, D.; Bros, M. DNA Vaccines—How Far From Clinical Use? Int. J. Mol. Sci. 2018, 19, 3605. [Google Scholar] [CrossRef]
- Li, W.; Joshi, M.D.; Singhania, S.S.; Ramsey, K.H.; Murthy, A.K. Peptide Vaccine: Progress and Challenges. Vaccines 2014, 2, 515–536. [Google Scholar] [CrossRef]
- Nicastro, J.; Sheldon, K.; Slavcev, R. Bacteriophage lambda display systems: Developments and applications. Appl. Microbiol. Biotechnol. 2014, 98, 2853–2866. [Google Scholar] [CrossRef]
- Samoylova, T.I.; Norris, M.D.; Samoylov, A.M.; Cochran, A.M.; Wolfe, K.G.; Petrenko, V.A.; Cox, N.R. Infective and inactivated filamentous phage as carriers for immunogenic peptides. J. Virol. Methods 2012, 183, 63–68. [Google Scholar] [CrossRef] [PubMed]
- De La Cruz, V.F.; Lal, A.A.; McCutchan, T.F. Immunogenicity and epitope mapping of foreign sequences via genetically engineered filamentous phage. J. Biol. Chem. 1988, 263, 4318–4322. [Google Scholar]
- González-Mora, A.; Ruiz-Ruiz, F.; Benavides, J.; Willson, R.C.; Rito-Palomares, M. Recovery and primary purification of bacteriophage M13 using aqueous two-phase systems. J. Chem. Technol. Biotechnol. 2017, 92, 2808–2816. [Google Scholar] [CrossRef]
- Haq, I.U.; Chaudhry, W.; Akhtar, M.N.; Andleeb, S.; Qadri, I. Bacteriophages and their implications on future biotechnology: A review. Virol. J. 2012, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Carrera, M.R.A.; Kaufmann, G.F.; Mee, J.M.; Meijler, M.M.; Koob, G.F.; Janda, K.D. Treating cocaine addiction with viruses. Proc. Natl. Acad. Sci. USA 2004, 101, 10416–10421. [Google Scholar] [CrossRef]
- Frenkel, D.; Dewachter, I.; Van Leuven, F.; Solomon, B. Reduction of β-amyloid plaques in brain of transgenic mouse model of Alzheimer’s disease by EFRH-phage immunization. Vaccine 2003, 21, 1060–1065. [Google Scholar] [CrossRef]
- Asadi-Ghalehni, M.; Ghaemmaghami, M.; Klimka, A.; Javanmardi, M.; Navari, M.; Rasaee, M.J. Cancer immunotherapy by a recombinant phage vaccine displaying EGFR mimotope: Anin vivostudy. Immunopharmacol. Immunotoxicol. 2015, 37, 274–279. [Google Scholar] [CrossRef]
- Gu, Y.; Wei, J.; Yang, J.; Huang, J.; Yang, X.; Zhu, X. Protective Immunity against Trichinella spiralis Infection Induced by a Multi-Epitope Vaccine in a Murine Model. PLoS ONE 2013, 8, e77238. [Google Scholar] [CrossRef]
- Manoutcharian, K.; Díaz-Orea, A.; Gevorkian, G.; Fragoso, G.; Acero, G.; González, E.; De Aluja, A.; Villalobos, N.; Gómez-Conde, E.; Sciutto, E. Recombinant bacteriophage-based multiepitope vaccine against Taenia solium pig cysticercosis. Veter. Immunol. Immunopathol. 2004, 99, 11–24. [Google Scholar] [CrossRef]
- Rami, A.; Behdani, M.; Yardehnavi, N.; Habibi-Anbouhi, M.; Kazemi-Lomedasht, F. An overview on application of phage display technique in immunological studies. Asian Pac. J. Trop. Biomed. 2017, 7, 599–602. [Google Scholar] [CrossRef]
- Packer, M.S.; Rees, H.A.; Liu, D.R. Phage-assisted continuous evolution of proteases with altered substrate specificity. Nat. Commun. 2017, 8, 956. [Google Scholar] [CrossRef] [PubMed]
- Garet, E.; Cabado, A.; Vieites, J.; González-Fernández, Á. Rapid isolation of single-chain antibodies by phage display technology directed against one of the most potent marine toxins: Palytoxin. Toxicon 2010, 55, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Ben Rhaiem, R.; Houimel, M. Targeting Leishmania major parasite with peptides derived from a combinatorial phage display library. Acta Trop. 2016, 159, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-T.; Li, P.-C.; Liu, I.-J.; Liao, M.-Y.; Chiu, C.-Y.; Chao, D.-Y.; Wu, H.-C. An Epitope-Substituted DNA Vaccine Improves Safety and Immunogenicity against Dengue Virus Type 2. PLoS Negl. Trop. Dis. 2015, 9, e0003903. [Google Scholar] [CrossRef] [PubMed]
- Bazan, J.; Całkosiński, I.; Gamian, A. Phage display—A powerful technique for immunotherapy. Hum. Vaccines Immunother. 2012, 8, 1817–1828. [Google Scholar] [CrossRef]
- Jacinto, M.J.; Patinha, D.J.; Marrucho, I.M.; Gonçalves, J.; Willson, R.C.; Azevedo, A.M.; Aires-Barros, M.R. M13 bacteriophage purification using poly(ionic liquids) as alternative separation matrices. J. Chromatogr. A 2018, 1532, 246–250. [Google Scholar] [CrossRef]
- Rakonjac, J.; Bennett, N.J.; Spagnuolo, J.; Gagic, D.; Russel, M. Filamentous bacteriophage: Biology, phage display and nanotechnology applications. Curr. Issues Mol. Biol. 2011, 13, 51–76. [Google Scholar]
- Pande, J.; Szewczyk, M.M.; Grover, A.K. Phage display: Concept, innovations, applications and future. Biotechnol. Adv. 2010, 28, 849–858. [Google Scholar] [CrossRef]
- Hayes, S.; Gamage, L.N.; Hayes, C. Dual expression system for assembling phage lambda display particle (LDP) vaccine to porcine Circovirus 2 (PCV2). Vaccine 2010, 28, 6789–6799. [Google Scholar] [CrossRef]
- Prisco, A.; De Berardinis, P. Filamentous Bacteriophage Fd as an Antigen Delivery System in Vaccination. Int. J. Mol. Sci. 2012, 13, 5179–5194. [Google Scholar] [CrossRef]
- Van Houten, N.E.; Henry, K.A.; Smith, G.P.; Scott, J.K. Engineering filamentous phage carriers to improve focusing of antibody responses against peptides. Vaccine 2010, 28, 2174–2185. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Moreira, L.A.; Jacobs-Lorena, M. Plasmodium-mosquito interactions, phage display libraries and transgenic mosquitoes impaired for malaria transmission. Insect Biochem. Mol. Biol. 2002, 32, 1325–1331. [Google Scholar] [CrossRef]
- Javanmardi, M.; Rasaee, M.J.; Modjtahedi, H.; Asadi-Ghalehni, M.; Maghami, M.G. Triple Tandem Mimotope Peptide of Epidermal Growth Factor ReceptorDisplaying on the Surface of M13 Phage Induces Anti-Tumor Response in MiceTumor Model. Iran. J. Biotechnol. 2014, 12, 9–17. [Google Scholar] [CrossRef]
- Grabowska, A.M.; Jennings, R.; Laing, P.; Darsley, M.; Jameson, C.; Swift, L.; Irving, W. Immunisation with Phage Displaying Peptides Representing Single Epitopes of the Glycoprotein G Can Give Rise to Partial Protective Immunity to HSV-2. J. Virol. 2000, 269, 47–53. [Google Scholar] [CrossRef]
- Thomas, B.S.; Nishikawa, S.; Ito, K.; Chopra, P.; Sharma, N.; Evans, D.H.; Tyrrell, D.L.J.; Bathe, O.F.; Rancourt, D.E. Peptide vaccination is superior to genetic vaccination using a recombineered bacteriophage λ subunit vaccine. Vaccine 2012, 30, 998–1008. [Google Scholar] [CrossRef]
- Hamzeh-Mivehroud, M.; Alizadeh, A.A.; Morris, M.B.; Church, W.B.; Dastmalchi, S. Phage display as a technology delivering on the promise of peptide drug discovery. Drug Discov. Today 2013, 18, 1144–1157. [Google Scholar] [CrossRef]
- Hashemi, H.; Bamdad, T.; Jamali, A.; Pouyanfard, S.; Mohammadi, M.G. Evaluation of humoral and cellular immune responses against HSV-1 using genetic immunization by filamentous phage particles: A comparative approach to conventional DNA vaccine. J. Virol. Methods 2010, 163, 440–444. [Google Scholar] [CrossRef]
- Hess, G.T.; Cragnolini, J.J.; Popp, M.W.; Allen, M.A.; Dougan, S.K.; Spooner, E.; Ploegh, H.L.; Belcher, A.M.; Guimaraes, C.P. M13 Bacteriophage Display Framework That Allows Sortase-Mediated Modification of Surface-Accessible Phage Proteins. Bioconjug. Chem. 2012, 23, 1478–1487. [Google Scholar] [CrossRef]
- Bruttin, A.; Brüssow, H. Human Volunteers Receiving Escherichia coli Phage T4 Orally: A Safety Test of Phage Therapy. Antimicrob. Agents Chemother. 2005, 49, 2874–2878. [Google Scholar] [CrossRef]
- Ren, S.; Yu, F.; Zuo, S.; Zhao, M.; Wang, X.; Wang, X.; Chen, Y.; Wu, Z.; Ren, Z. Inhibition of tumor angiogenesis in lung cancer by T4 phage surface displaying mVEGFR2 vaccine. Vaccine 2011, 29, 5802–5811. [Google Scholar] [CrossRef]
- Dabrowska, K.; Miernikiewicz, P.; Piotrowicz, A.; Hodyra, K.; Owczarek, B.; Lecion, R.; Kaźmierczak, Z.; Letarov, A.; Górski, A. Immunogenicity Studies of Proteins Forming the T4 Phage Head Surface. J. Virol. 2014, 88, 12551–12557. [Google Scholar] [CrossRef] [PubMed]
- Tao, P.; Mahalingam, M.; Zhu, J.; Moayeri, M.; Sha, J.; Lawrence, W.S.; Leppla, S.H.; Chopra, A.K.; Rao, V.B.; Catalano, C.; et al. A Bacteriophage T4 Nanoparticle-Based Dual Vaccine against Anthrax and Plague. mBio 2018, 9, e01926-18. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Pouyanfard, S.; Bandehpour, M.; Noroozbabaei, Z.; Kazemi, B.; Saelens, X.; Mokhtari-Azad, T. Immunization with M2e-Displaying T7 Bacteriophage Nanoparticles Protects against Influenza A Virus Challenge. PLoS ONE 2012, 7, e45765. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Wang, L.; You, X.; Dai, P.; Zeng, Y. Advances in the T7 phage display system (Review). Mol. Med. Rep. 2017, 17, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Bao, X.; Lu, Y.; Liu, Y.; Deng, B.; Wang, Y.; Xu, Y.; Hou, J. Immunogenicity of T7 bacteriophage nanoparticles displaying G-H loop of foot-and-mouth disease virus (FMDV). Veter. Microbiol. 2017, 205, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, A.; Soleimanjahi, H.; Gill, P.; Hassan, Z.; Jahromi, S.R.; Roohvand, F. Recombinant λ-phage nanobioparticles for tumor therapy in mice models. Genet. Vaccines Ther. 2010, 8, 3. [Google Scholar] [CrossRef]
- Iwagami, Y.; Casulli, S.; Nagaoka, K.; Kim, M.; Carlson, R.I.; Ogawa, K.; Lebowitz, M.S.; Fuller, S.; Biswas, B.; Stewart, S.; et al. Lambda phage-based vaccine induces antitumor immunity in hepatocellular carcinoma. Heliyon 2017, 3, e00407. [Google Scholar] [CrossRef]
- Shivachandra, S.B.; Rao, M.; Janosi, L.; Sathaliyawala, T.; Matyas, G.R.; Alving, C.R.; Leppla, S.H.; Rao, V.B. In vitro binding of anthrax protective antigen on bacteriophage T4 capsid surface through Hoc–capsid interactions: A strategy for efficient display of large full-length proteins. J. Virol. 2006, 345, 190–198. [Google Scholar] [CrossRef]
- Wu, J.; Tu, C.; Yu, X.; Zhang, M.; Zhang, N.; Zhao, M.; Nie, W.; Ren, Z. Bacteriophage T4 nanoparticle capsid surface SOC and HOC bipartite display with enhanced classical swine fever virus immunogenicity: A powerful immunological approach. J. Virol. Methods 2007, 139, 50–60. [Google Scholar] [CrossRef]
- Pavoni, E.; Vaccaro, P.; D’Alessio, V.; De Santis, R.; Minenkova, O. Simultaneous display of two large proteins on the head and tail of bacteriophage lambda. BMC Biotechnol. 2013, 13, 79. [Google Scholar] [CrossRef]
- Hess, K.L.; Jewell, C.M. Phage display as a tool for vaccine and immunotherapy development. Bioeng. Transl. Med. 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Aghebati-Maleki, L.; Bakhshinejad, B.; Baradaran, B.; Motallebnezhad, M.; Aghebati-Maleki, A.; Nickho, H.; Yousefi, M.; Majidi, J. Phage display as a promising approach for vaccine development. J. Biomed. Sci. 2016, 23, 66. [Google Scholar] [CrossRef] [PubMed]
- Goracci, M.; Pignochino, Y.; Marchio’, S. Phage Display-Based Nanotechnology Applications in Cancer Immunotherapy. Molecular 2020, 25, 843. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Tang, L.; Zhou, C.; Tan, L. Immunotherapy of EGFR-positive tumor based on recombinant EGFR phage vaccine. Chinese-German J. Clin. Oncol. 2006, 5, 189–193. [Google Scholar] [CrossRef]
- Ren, S.-X.; Ren, Z.-J.; Zhao, M.-Y.; Wang, X.-B.; Zuo, S.-G.; Yu, F. Antitumor activity of endogenous mFlt4 displayed on a T4 phage nanoparticle surface. Acta Pharmacol. Sin. 2009, 30, 637–645. [Google Scholar] [CrossRef]
- Dabrowska, K.; Opolski, A.; Wietrzyk, J.; Switala-Jelen, K.; Godlewska, J.; Boratynski, J.; Syper, D.; Weber-Dabrowska, B.; Gorski, A. Anticancer activity of bacteriophage T4 and its mutant HAP1 in mouse experimental tumour models. Anticancer. Res. 2005, 24, 3991–3995. [Google Scholar]
- Eriksson, F.; Tsagozis, P.; Lundberg, K.; Parsa, R.; Mangsbo, S.M.; Persson, M.A.A.; Harris, R.A.; Pisa, P. Tumor-Specific Bacteriophages Induce Tumor Destruction through Activation of Tumor-Associated Macrophages. J. Immunol. 2009, 182, 3105–3111. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Y.; Bao, X.; Deng, B.; Li, P.; Lu, Y. Displaying of GnRH Peptides on Bacteriophage T7 and Its Immunogenicity in Mice Model. World Acad. Sci. Eng. Technol. Int. J. Bioeng. Life Sci. 2017, 11, 455–458. [Google Scholar]
- Bastien, N.; Trudel, M.; Simard, C. Protective Immune Responses Induced by the Immunization of Mice with a Recombinant Bacteriophage Displaying an Epitope of the Human Respiratory Syncytial Virus. J. Virol. 1997, 234, 118–122. [Google Scholar] [CrossRef]
- Fang, J.; Wang, G.; Yang, Q.; Song, J.; Wang, Y.; Wang, L. The potential of phage display virions expressing malignant tumor specific antigen MAGE-A1 epitope in murine model. Vaccine 2005, 23, 4860–4866. [Google Scholar] [CrossRef]
- Wan, Y.; Wu, Y.; Bian, J.; Wang, X.; Zhou, W.; Jia, Z.; Tan, Y.; Zhou, L. Induction of hepatitis B virus-specific cytotoxic T lymphocytes response in vivo by filamentous phage display vaccine. Vaccine 2001, 19, 2918–2923. [Google Scholar] [CrossRef]
- Sartorius, R.; Pisu, P.; D’Apice, L.; Pizzella, L.; Romano, C.; Cortese, G.; Giorgini, A.; Santoni, A.; Velotti, F.; De Berardinis, P. The use of filamentous bacteriophage fd to deliver MAGE-A10 or MAGE-A3 HLA-A2-restricted peptides and to induce strong antitumor CTL responses. J. Immunol. 2008, 180, 3719–3728. [Google Scholar] [CrossRef] [PubMed]
- Tao, P.; Mahalingam, M.; Kirtley, M.L.; Van Lier, C.J.; Sha, J.; Yeager, L.A.; Chopra, A.K.; Rao, V.B. Mutated and Bacteriophage T4 Nanoparticle Arrayed F1-V Immunogens from Yersinia pestis as Next Generation Plague Vaccines. PLoS Pathog. 2013, 9, e1003495. [Google Scholar] [CrossRef] [PubMed]
- Hell, R.; Amim, P.; De Andrade, H.; De Avila, R.; Felicori, L.; Oliveira, A.G.; Oliveira, C.; Nascimento, E.; Tavares, C.; Granier, C.; et al. Immunodiagnosis of human neurocysticercosis using a synthetic peptide selected by phage-display. Clin. Immunol. 2009, 131, 129–138. [Google Scholar] [CrossRef]
- Becker, M.; Felsberger, A.; Frenzel, A.; Shattuck, W.M.C.; Dyer, M.; Kügler, J.; Zantow, J.; Mather, T.N.; Hust, M. Application of M13 phage display for identifying immunogenic proteins from tick (Ixodes scapularis) saliva. BMC Biotechnol. 2015, 15, 43. [Google Scholar] [CrossRef]
- Ferdosian, M.; Khatami, M.R.; Malekshahi, Z.; Mohammadi, A.; Kashani, H.H.; Shooshtari, M. Identification of Immunotopes against Mycobacterium leprae as Immune Targets Using PhDTm- 12mer Phage Display Peptide Library. Trop. J. Pharm. Res. 2015, 14, 1153. [Google Scholar] [CrossRef]
- Meyer, T.; Schirrmann, T.; Frenzel, A.; Miethe, S.; Stratmann-Selke, J.; Gerlach, G.F.; Strutzberg-Minder, K.; Dübel, S.; Hust, M. Identification of immunogenic proteins and generation of antibodies against Salmonella Typhimurium using phage display. BMC Biotechnol. 2012, 12, 29. [Google Scholar] [CrossRef]
- Biradhar, N.; Nimmagadda, S.V.; Aavula, S.M.; Parthasarathy, S.; Sula, S.; Maithal, K. Identification and Characterization of Novel Binding Epitope of Tetanus Toxoid by Phage Display Peptide Library. Curr. Trends Biotechnol. Pharm. 2015, 9, 49–58. [Google Scholar]
- Jahdasani, R.; Jamnani, F.R.; Behdani, M.; Habibi-Anbouhi, M.; Yardehnavi, N.; Shahbazzadeh, D.; Kazemi-Lomedasht, F. Identification of the immunogenic epitopes of the whole venom component of the Hemiscorpius lepturus scorpion using the phage display peptide library. Toxicon 2016, 124, 83–93. [Google Scholar] [CrossRef]
- Knittelfelder, R.; Riemer, A.B.; Jensen-Jarolim, E. Mimotope vaccination—From allergy to cancer. Expert Opin. Biol. Ther. 2009, 9, 493–506. [Google Scholar] [CrossRef]
- Riemer, A.B.; Jensen-Jarolim, E. Mimotope vaccines: Epitope mimics induce anti-cancer antibodies. Immunol. Lett. 2007, 113, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mullaney, B.P.; Pallavicini, M.G.; Marks, J.D. Epitope Mapping of Neutralizing Botulinum Neurotoxin A Antibodies by Phage Display. Infect. Immun. 2001, 69, 6511–6514. [Google Scholar] [CrossRef] [PubMed]
- Gazarian, T.G.; Selisko, B.; Gurrola, G.B.; Hernández, R.; Possani, L.D.; Gazarian, K.G. Potential of peptides selected from random phage-displayed libraries to mimic conformational epitopes: A study on scorpion toxin Cn2 and the neutralizing monoclonal antibody BCF2. Comb. Chem. High. Throughput Screen. 2003, 6, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Galfré, G.; Monaci, P.; Nicosia, A.; Luzzago, A.; Felici, F.; Cortese, R. [6] Immunization with phage-displayed mimotopes. In Methods in Enzymology; Elsevier BV: Amsterdam, The Netherlands, 1996; Volume 267, pp. 109–115. [Google Scholar]
- Gnanasekar, M.; Rao, K.V.N.; He, Y.-X.; Mishra, P.K.; Nutman, T.B.; Kaliraj, P.; Ramaswamy, K. Novel Phage Display-Based Subtractive Screening To Identify Vaccine Candidates of Brugia malayi. Infect. Immun. 2004, 72, 4707–4715. [Google Scholar] [CrossRef]
- Liu, Y.; Brindley, P.J.; Zeng, Q.; Li, Y.; Zhou, J.; Chen, Y.; Yang, S.; Zhang, Z.; Liu, B.; Cai, L.; et al. Identification of phage display peptides with affinity for the tegument of Schistosoma japonicum schistosomula. Mol. Biochem. Parasitol. 2011, 180, 86–98. [Google Scholar] [CrossRef]
- Gazarian, K.; Solís, C.F.; Gazarian, T.G.; Rowley, M.; Laclette, J.P. Synthetic peptide-targeted selection of phage display mimotopes highlights immunogenic features of α-helical vs non-helical epitopes of Taenia solium paramyosin: Implications for parasite- and host-protective roles of the protein. Peptides 2012, 34, 232–241. [Google Scholar] [CrossRef]
- Khan, K.H. DNA vaccines: Roles against diseases. Germs 2013, 3, 26–35. [Google Scholar] [CrossRef]
- March, J.B.; Clark, J.R.; Jepson, C.D. Genetic immunisation against hepatitis B using whole bacteriophage λ particles. Vaccine 2004, 22, 1666–1671. [Google Scholar] [CrossRef]
- Coban, C.; Koyama, S.; Takeshita, F.; Akira, S.; Ishii, K.J. Molecular and cellular mechanisms of DNA vaccines. Hum. Vaccines 2008, 4, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Saade, F.; Petrovsky, N. The future of human DNA vaccines. J. Biotechnol. 2012, 162, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Manoutcharian, K.; Gevorkian, G.; Cano, J.A.; Almagro, J. Phage displayed biomolecules as preventive and therapeutic agents. Curr. Pharm. Biotechnol. 2001, 2, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Munira, S.L.; Hendriks, J.T.; Atmosukarto, I.I.; Friede, M.H.; Carter, L.M.; Butler, J.R.; Clements, A.C. A cost analysis of producing vaccines in developing countries. Vaccine 2019, 37, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Torres-Acosta, M.; González-Mora, A.; Ruiz-Ruiz, F.; Rito-Palomares, M.; Benavides, J.; Torres-Acosta, M. Economic evaluation of M13 bacteriophage production at large-Scale for therapeutic applications using aqueous Two-Phase systems. J. Chem. Technol. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Brigati, J.R.; Petrenko, V.A. Thermostability of landscape phage probes. Anal. Bioanal. Chem. 2005, 382, 1346–1350. [Google Scholar] [CrossRef]
- Huh, H.; Wong, S.; Jean, J.S.; Slavcev, R. Bacteriophage interactions with mammalian tissue: Therapeutic applications. Adv. Drug Deliv. Rev. 2019, 145, 4–17. [Google Scholar] [CrossRef]
- Clokie, M.R.; Millard, A.D.; Letarov, A.V.; Heaphy, S. Phages in nature. Bacteriophage 2011, 1, 31–45. [Google Scholar] [CrossRef]
- Krag, D.N.; Shukla, G.S.; Shen, G.-P.; Pero, S.; Ashikaga, T.; Fuller, S.; Weaver, D.L.; Burdette-Radoux, S.; Thomas, C. Selection of Tumor-binding Ligands in Cancer Patients with Phage Display Libraries. Cancer Res. 2006, 66, 7724–7733. [Google Scholar] [CrossRef]
- Jończyk, E.; Kłak, M.; Międzybrodzki, R.; Górski, A. The influence of external factors on bacteriophages—Review. Folia Microbiol. 2011, 56, 191–200. [Google Scholar] [CrossRef]
- Bakhshinejad, B.; Sadeghizadeh, M. Bacteriophages as vehicles for gene delivery into mammalian cells: Prospects and problems. Expert Opin. Drug Deliv. 2014, 11, 1561–1574. [Google Scholar] [CrossRef]
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Van Houten, N.; Zwick, M.; Menendez, A.; Scott, J.K. Filamentous phage as an immunogenic carrier to elicit focused antibody responses against a synthetic peptide. Vaccine 2006, 24, 4188–4200. [Google Scholar] [CrossRef]
- Van Belleghem, J.D.; Dąbrowska, K.; Vaneechoutte, M.; Barr, J.J.; Bollyky, P.L. Interactions between Bacteriophage, Bacteria, and the Mammalian Immune System. Viruses 2018, 11, 10. [Google Scholar] [CrossRef]
- Hashiguchi, S.; Yamaguchi, Y.; Takeuchi, O.; Akira, S.; Sugimura, K. Immunological basis of M13 phage vaccine: Regulation under MyD88 and TLR9 signaling. Biochem. Biophys. Res. Commun. 2010, 402, 19–22. [Google Scholar] [CrossRef]
- Mori, K.; Kubo, T.; Kibayashi, Y.; Ohkuma, T.; Kaji, A. Anti-vaccinia virus effect of M13 bacteriophage DNA. Antivir. Res. 1996, 31, 79–86. [Google Scholar] [CrossRef]
- Malanchère-Brès, E.; Payette, P.J.; Mancini, M.; Tiollais, P.; Davis, H.L.; Michel, M.-L. CpG Oligodeoxynucleotides with Hepatitis B Surface Antigen (HBsAg) for Vaccination in HBsAg-Transgenic Mice. J. Virol. 2001, 75, 6482–6491. [Google Scholar] [CrossRef] [PubMed]
- Golkar, Z.; Jamil, N. CpG Motif in Phage Genome DNA Enhanced the Efficacy of Phage Therapy by Immunostimulation. Cureus 2012, 4, 4. [Google Scholar] [CrossRef][Green Version]
- Liang, H.; Nishioka, Y.; Reich, C.F.; Pisetsky, D.S.; E Lipsky, P. Activation of human B cells by phosphorothioate oligodeoxynucleotides. J. Clin. Investig. 1996, 98, 1119–1129. [Google Scholar] [CrossRef]
- A Mason, K.; Ariga, H.; Neal, R.; Valdecanas, D.; Hunter, N.; Krieg, A.M.; Whisnant, J.K.; Milas, L. Targeting toll-like receptor 9 with CpG oligodeoxynucleotides enhances tumor response to fractionated radiotherapy. Clin. Cancer Res. 2005, 11, 361–369. [Google Scholar]
- Kim, A.; Shin, T.-H.; Shin, S.-M.; Pham, C.D.; Choi, D.-K.; Kwon, M.-H.; Kim, Y.-S. Cellular Internalization Mechanism and Intracellular Trafficking of Filamentous M13 Phages Displaying a Cell-Penetrating Transbody and TAT Peptide. PLoS ONE 2012, 7, e51813. [Google Scholar] [CrossRef]
- Jończyk-Matysiak, E.; Weber-Dąbrowska, B.; Owczarek, B.; Międzybrodzki, R.; Łusiak-Szelachowska, M.; Łodej, N.; Górski, A. Phage-Phagocyte Interactions and Their Implications for Phage Application as Therapeutics. Viruses 2017, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Maji, M.; Mazumder, S.; Bhattacharya, S.; Choudhury, S.T.; Sabur, A.; Shadab, M.; Bhattacharya, P.; Ali, N. A Lipid Based Antigen Delivery System Efficiently Facilitates MHC Class-I Antigen Presentation in Dendritic Cells to Stimulate CD8+ T Cells. Sci. Rep. 2016, 6, 27206. [Google Scholar] [CrossRef] [PubMed]
- Sartorius, R.; Bettua, C.; D’Apice, L.; Caivano, A.; Trovato, M.; Russo, D.; Zanoni, I.; Granucci, F.; Mascolo, D.; Barba, P.; et al. Vaccination with filamentous bacteriophages targeting DEC-205 induces DC maturation and potent anti-tumor T-cell responses in the absence of adjuvants. Eur. J. Immunol. 2011, 41, 2573–2584. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, Á.M.; Suárez, E.; Larsen, M.; Jensen, K.B.; Sanz, L.; Compte, M.; Kristensen, P.; Álvarez-Vallina, L. Enhancement of DNA vaccine potency through linkage of antigen to filamentous bacteriophage coat protein III domain I. Immunology 2006, 117, 502–506. [Google Scholar] [CrossRef]
- Mancera, A.E.V.; Quiroz-Romero, H.; Correa, D.; Ibarra, F.; Reyes-Pérez, M.; Reyes-Vivas, H.; López-Velázquez, G.; Gazarian, K.; Alonso, R.A.; Gazarian, T. Induction of immunity in sheep toFasciola hepaticawith mimotopes of cathepsin L selected from a phage display library. Parasitology 2008, 135, 1437–1445. [Google Scholar] [CrossRef]
- Hamzeh-Mivehroud, M.; Mahmoudpour, A.; Rezazadeh, H.; Dastmalchi, S. Non-specific translocation of peptide-displaying bacteriophage particles across the gastrointestinal barrier. Eur. J. Pharm. Biopharm. 2008, 70, 577–581. [Google Scholar] [CrossRef]
- Duerr, D.M.; White, S.J.; Schluesener, H.J. Identification of peptide sequences that induce the transport of phage across the gastrointestinal mucosal barrier. J. Virol. Methods 2004, 116, 177–180. [Google Scholar] [CrossRef]
- Sartorius, R.; D’Apice, L.; Prisco, A.; De Berardinis, P. Arming Filamentous Bacteriophage, a Nature-Made Nanoparticle, for New Vaccine and Immunotherapeutic Strategies. Pharmaceutics 2019, 11, 437. [Google Scholar] [CrossRef]
- Delmastro, P. Immunogenicity of filamentous phage displaying peptide mimotopes after oral administration. Vaccine 1997, 15, 1276–1285. [Google Scholar] [CrossRef]
- Ren, Z.; Tian, C.; Zhu, Q.; Zhao, M.; Xin, A.; Nie, W.; Ling, S.; Zhu, M.; Wu, J.; Lan, H.; et al. Orally delivered foot-and-mouth disease virus capsid protomer vaccine displayed on T4 bacteriophage surface: 100% protection from potency challenge in mice. Vaccine 2008, 26, 1471–1481. [Google Scholar] [CrossRef]

| Schematic Representation of Phage Display Vectors | Proteins Used for Fusion Display | Copy Number | Size of Displayed Antigen | References |
|---|---|---|---|---|
 Filamentous (M13, fd and f1) | pVIII (major coat protein 5 kDa)  | 2700 | 6–8 aa | [36,48] |
pIII (minor coat protein 45 kDa)  | 4–5 | Large proteins | ||
 T4 | Soc (small outer capsid 9 kDa)  | 810–960 | Up to 840 aa | [49] |
Hoc (highly antigenic outer capsid 40 kDa)  | 155–160 | Up to 183 aa | ||
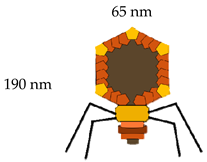 T7 | gp 10 A  | 415 | 40–50 aa | [6,43] |
| gp 10 B | 1 | Up to 1200 aa | ||
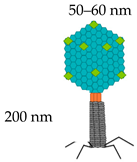 Lambda | gpD (outer capsid protein)  | 420 | Up to 1024 aa | [29,50] |
gpV (tail protein)  | 192 |
| Vaccine Type/Antigen Displayed | Model of Study | Main Effects of Phage-Based Vaccine Administration and Comments | Dose | Phage Used | Phagemid Vector | Protein Used for Phage Display | E. Coli Strain Used for Phage Assembly/Production | Reference |
|---|---|---|---|---|---|---|---|---|
| Anti-cancer Melanoma Epitope MAGE-A1161–169 | Mice and cell lines YAC-1 and B16.F10 | Protection from tumor growth Specific cytotoxic T cell (CTL) responses, increased NK activity, CD 8+ response and Delayed-type hypersensitivity | 50 ug phage particles i.p | fd | pfd8wf | pVIII | TG1 | [60] |
| Anti-viral Hepatitis B virus epitope S28–39 | Female BALB/c mice | MHC I restricted HBs specific CTL response | 10 ug i.d i.p | M13 | pC89 | pVIII | XL1-blue | [61] |
| Anti-viral HRSV G glycoprotein-epitope 173–187 | BALB/c mice | Induction/stimulation/promotion of strong immune response of animals against RSV infection | 1 mg i.p | fd | Bacteriophage vector Fuse 5 | pIII | K91 Kan | [59] |
| Anti-parasite Peptides GK1, KETc12, KETc1 and KETc7 Taenia solium cysticercosis | Pig | Reduction of 70% of tongue Cysticercosis, 54% of muscle-cysticercosis and 87% of total cysticerci Similar efficacy to the synthetic vaccine | 2 mL, 1012 phage particles of each s.c | M13 | M13KE | pVIII | TG-1 | [2] |
| Anti-liver cancer ASPH peptides | Murine | Reduced Hepatocellular carcinoma growth and progression. Increase in CD4+ and CD8+ response Specific Th1 and Th2 cytokine secretion. | 1010 pfu s.c | λ | pVCDcDL1A | gpD | n.r | [47] |
| Anti-viral Epitopes of the glycoprotein G of HSV-2 (gG2) | Balb/c mice | Promotion of protective immunity in a live challenge model of HSV-2 infection | n.r. | Fd-tet | n.r. | pVIII | K91Kan | [34] |
| Anti-cancer EGFR gene of extracellular domain of chicken xenogeneic EGFR | Male Kunming mice | Induction of specific anti-EGFR antibodies (Humoral immune response) Reduction of tumor progression immunity against EGFR-positive tumor. | 2.5 × 1013 pfu | T7 | n.r | 10 B | n.r | [54] |
| Anti-cancer EGFR ICR-62 binding peptide mimotope | BALB/c female mice | Humoral immunity induced in mice Reduced tumor growth in ectopic Lewis lung carcinoma animal model In vivo: no response | 1012 pfu s.c | M13 | pAK8-GVO | pVIII | TG-1 | [17] |
| Contraceptive 3 Gonadotrophin Releasing Hormone (GnRH) fragment 43 kDa | Male BALB/c mice | Specific anti-GnRH antibody faster than conventional vaccine Spermatogenesis suppression | 1010 pfu s.c | T7 | n.r | 10 B | BL21 | [58] |
| Anti-viral Ectodomain of influenza A virus channel protein M2 (M2e) | Female BALB/c mice | M2e-specific serum antibody responses T cell response Reduced viral load. Protection against Influenza A virus | 109 pfu s.c | T7 | n.r. | 10 B capsid protein | BL21 | [43] |
| Anti-cancer Mouse Fms-like tyrosine kinase 4 (Flt4) | Mice | Induction of anti-Flt4 antibody Induction of antitumor immunity Inhibition of metastasis in Lewis lung carcinoma | 1011 pfu s.c | T4 | T4-Z | Soc | BL21 | [55] |
| Hybrid Anti-cancer HLA-DR-restricted Th cell peptide epitope p23 and MAGE-A10254–262 or p23 and MAGE-A3271–279 from the HIV-1-RT | Human cell system in vitro humanized murine model in vivo | Induction of specific and potent CTL response Hampered tumor growth | 140 µg phage particles s.c | fd | pTfd8p-66 for p23 peptide | pVIII | TG1 rec O | [62] |
| Anti-cancer Vascular endothelial growth factor receptor 2 (VEGFR2) | Mouse tumor model Male C57BL/6J | Induced anti-tumor immunity Induction of anti-angiogenesis activity Induction of CD4+ T cell–mediated effector mechanisms | 5 × 1011 pfu s.c | T4-S-GPDS | pD-mVEGFR2 | Soc C-terminus fusion | BL21 or HB101 | [40] |
| Dual display of swine fever virus (CSFV) major antigenic determinant cluster mE2 and CSFV primary antigen E2 | Female BALB/c mice | Enhanced immune response High antibody titers | 1010 pfu s.c | T4-Zh− | pcDSW | Soc C-terminus fusion and Hoc N-terminus | BL21 | [49] |
| Anti-bacteria in vitro display Protective antigen (PA) from B. anthracis | Female CBA/J mice | Production of immunogenic particles High antibody titers Neutralization of anthrax lethal toxin | 1010 pfu i.m | T4 Hoc-soc- | pET-15b | Hoc N-terminus | P301 (sup-) | [48] |
| Yersinia pestis (Plague) capsular F1 and calcium response V antigen | Female BALB/c mice | Complete protection against pneumonic plague TH1 and TH2 responses | 10 ug phage particles i.m | T4 Hoc-soc- | pET-28b | Soc | BL21 | [63] |
| Antigen Source | Antigen Identified | Phage/Phagemid Vector | Screening Antibodies | Prophylactic and Therapeutic Effects | Reference |
|---|---|---|---|---|---|
| Ixodes scapularis ticks salivary gland | Metalloprotease (MP1) | pHORF3 M13 | 3 biopanning rounds with human serum antibodies against salivary gland homogenate (SGH) | No evaluated | [65] |
| EGFR gene | EGFR mimotope Triple tandem repeat | PAK-8 M13 pVIII | Anti EGFR monoclonal antibody ICR62 | Lewis lung carcinoma tumor model | [33] |
| Reduced tumor growth | |||||
| High level of cytokines Raised | |||||
| humoral response | |||||
| Anti-cancer activity | |||||
| Leishmania infantum (syn. L. chagasi) | Peptide 5 | M13 | Polyclonal IgGs from L. infantum infected dogs | High immunogenicity | [7] |
| Protective effect vs. L. infantum in mice model | |||||
| Mycobacterium leprae | Anti M.leprae epitopes | M13 (pIII) | Human antiserum | High immunogenicity in mice | [66] |
| High antibody titer | |||||
| Salmonella Typhimurium | Novel immunogenic antigens | pHORF3 M13 | Serum from infected pigs | High immunogenicity | [67] |
| Tetanus toxoid (TT) | Novel binding peptide anti TT | pGEX-4T1 M13 (pIII) | F13 Fab from human | No evaluated | [68] |
| Trichinella spirallis | Peptide 8F7 | M13 (pIII) | mAb 8F12 | Larval reduction in vaccinated mice | [18] |
| High levels of IgG1 |
| Vaccine | Model of Study | Main Effects | Doses | Phage Used | DNA Cloning Vector | Promoter | Reference |
|---|---|---|---|---|---|---|---|
| Anti-viral Hepatitis B Surface antigen (HB) | Mice and rabbits | Anti HB response | Mice: 5 × 109 pfu (250 ng of DNA) | λ | λ-gt11 | CMV | [79] |
| Rabbits: 4 × 1010 HBsAg pfu (2 ug of DNA) | |||||||
| Anti-viral Small surface antigen (HBsAg) of hepatitis B | Rabbits | Strong antibody response (IgG, IgM) | 4 × 1010 pfu | λ | pRcCMVHBs(S) λ-gt11 | n.r. | [3] |
| Anti-viral Herpes simplex virus 1 (HSV-1) glycoprotein D | Mice | Anti-HSV-1 neutralizing antibodies Dose-response effect CTL response | 1.4 × 1013 pfu (50 ug DNA) | M13 | pcDNA3-gD plasmid | Human cytomegalovirus immediate-early | [37] |
| Anti-viral Human papillomavirus (HPV)-16 E7 | C57BL/6 mice | Reduction of tumor volume | 2 × 1012 particles | λ ZAP | n.r. | CMV | [46] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Mora, A.; Hernández-Pérez, J.; Iqbal, H.M.N.; Rito-Palomares, M.; Benavides, J. Bacteriophage-Based Vaccines: A Potent Approach for Antigen Delivery. Vaccines 2020, 8, 504. https://doi.org/10.3390/vaccines8030504
González-Mora A, Hernández-Pérez J, Iqbal HMN, Rito-Palomares M, Benavides J. Bacteriophage-Based Vaccines: A Potent Approach for Antigen Delivery. Vaccines. 2020; 8(3):504. https://doi.org/10.3390/vaccines8030504
Chicago/Turabian StyleGonzález-Mora, Alejandro, Jesús Hernández-Pérez, Hafiz M. N. Iqbal, Marco Rito-Palomares, and Jorge Benavides. 2020. "Bacteriophage-Based Vaccines: A Potent Approach for Antigen Delivery" Vaccines 8, no. 3: 504. https://doi.org/10.3390/vaccines8030504
APA StyleGonzález-Mora, A., Hernández-Pérez, J., Iqbal, H. M. N., Rito-Palomares, M., & Benavides, J. (2020). Bacteriophage-Based Vaccines: A Potent Approach for Antigen Delivery. Vaccines, 8(3), 504. https://doi.org/10.3390/vaccines8030504







