Pathogenicity of West Nile Virus Lineage 1 to German Poultry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Domestic Poultry
2.3. Virus
2.4. Mosquito Strain
2.5. Subcutaneous Injections
2.6. Mosquito Bite Challenge
2.7. Vector Competence of Mosquitoes Used in the Pathogenicity Study
2.8. Sample Collection
2.9. Serology
2.10. Reverse Transcription Quantitative Real-Time PCR
2.11. Histopathology and Immunohistochemistry (IHC)
2.12. Data Analysis
3. Results
3.1. Clinical Signs during Infection
3.2. Infection Profile of Poultry Infected via Subcutaneous Injection (Viremia and Viral Shedding)
3.3. Infection Status of Mosquitoes after Intrathoracic Injection with WNV
3.4. Infection Profile of Geese Infected via Mosquito Bite (Viremia & Viral Shedding)
3.5. Serology
3.6. Tissue Tropism of WNV
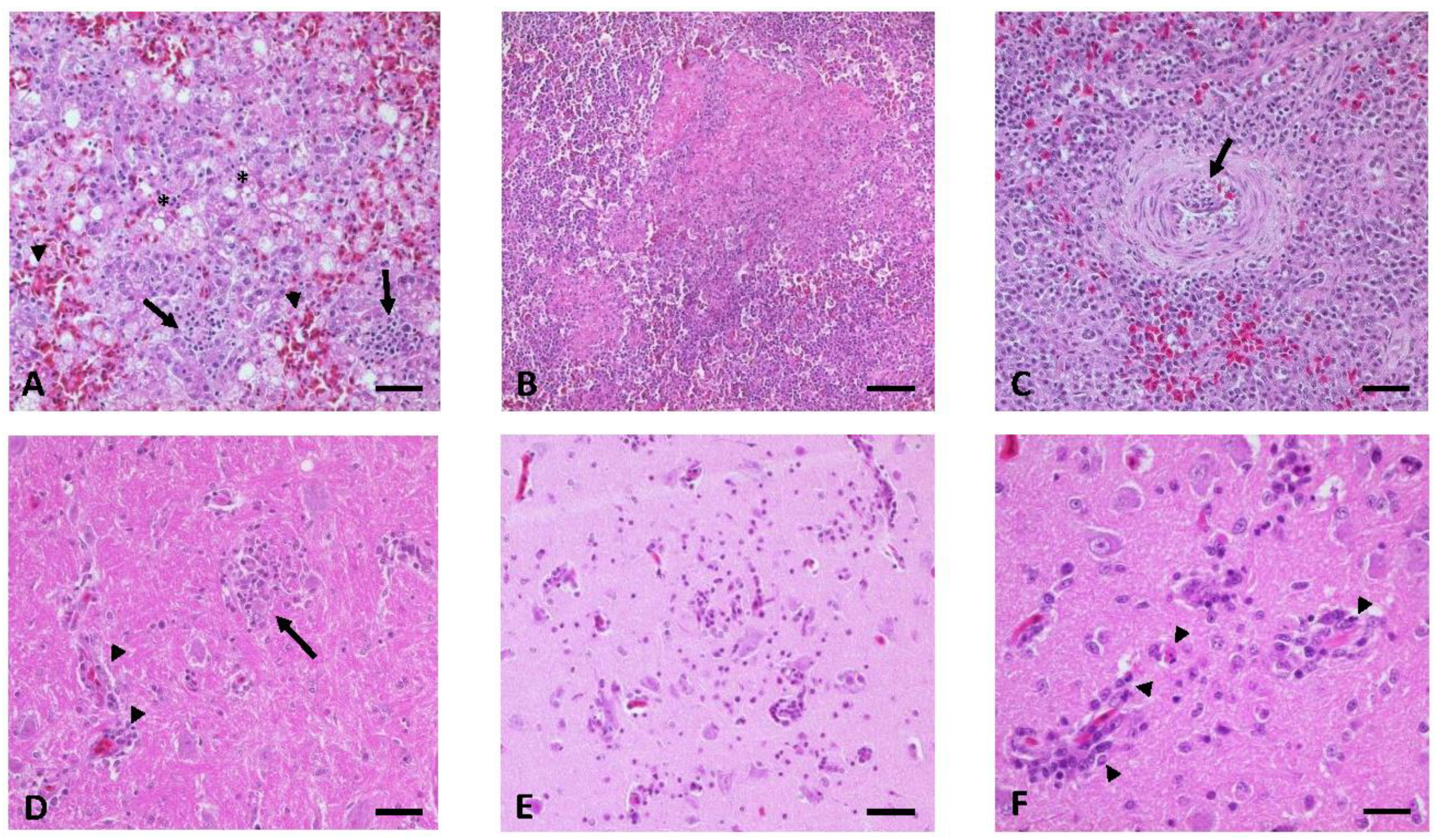
3.7. Gross Lesions, Histopathology, and Immunohistochemistry
3.8. Summary of Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kramer, L.D.; Li, J.; Shi, P.Y. West Nile virus. Lancet Neurol. 2007, 6, 171–181. [Google Scholar] [CrossRef]
- Chancey, C.; Grinev, A.; Volkova, E.; Rios, M. The global ecology and epidemiology of West Nile virus. BioMed Res. Int. 2015, 2015, 20. [Google Scholar] [CrossRef] [Green Version]
- Smithburn, K.C.; Hughes, T.P.; Burke, A.W.; Paul, J.H. A neurotropic virus isolated from the blood of a native of Uganda. Am. J. Trop. Med. Hyg. 1940, 471–492. [Google Scholar] [CrossRef]
- McLean, R.G.; Ubico, S.R.; Bourne, D.; Komar, N. West Nile virus in livestock and wildlife. Curr. Top. Microbiol. Immunol. 2002, 267, 271–308. [Google Scholar] [CrossRef] [PubMed]
- Van der Meulen, K.M.; Pensaert, M.B.; Nauwynck, H.J. West Nile virus in the vertebrate world. Arch. Virol. 2005, 150, 637–657. [Google Scholar] [CrossRef]
- Zeller, H.G.; Schuffenecker, I. West Nile virus: An overview of its spread in Europe and the Mediterranean basin in contrast to its spread in the Americas. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 147–156. [Google Scholar] [CrossRef]
- Bakonyi, T.; Ivanics, E.; Erdélyi, K.; Ursu, K.; Ferenczi, E.; Weissenböck, H.; Nowotny, N. Lineage 1 and 2 strains of encephalitic West Nile virus, central Europe. Emerg. Infect. Dis. 2006, 12, 618–623. [Google Scholar] [CrossRef]
- Tsai, T.F.; Popovici, F.; Cernescu, C.; Campbell, G.L.; Nedelcu, N.I. West Nile encephalitis epidemic in southeastern Romania. Lancet 1998, 352, 767–771. [Google Scholar] [CrossRef]
- Sirbu, A.; Ceianu, C.S.; Panculescu-Gatej, R.I.; Vazquez, A.; Tenorio, A.; Rebreanu, R.; Niedrig, M.; Nicolescu, G.; Pistol, A. Outbreak of West Nile virus infection in humans, Romania, July to October 2010. Euro. Surveill. 2011, 16, 19762. [Google Scholar] [CrossRef]
- Platonov, A.E.; Shipulin, G.A.; Shipulina, O.Y.; Tyutyunnik, E.N.; Frolochkina, T.I.; Lanciotti, R.S.; Yazyshina, S.; Platonova, O.V.; Obukhov, I.L.; Zhukov, A.N.; et al. Outbreak of West Nile virus infection, Volgograd Region, Russia, 1999. Emerg. Infect. Dis. 2001, 7, 128–132. [Google Scholar] [CrossRef]
- Murgue, B.; Murri, S.; Zientara, S.; Durand, B.; Durand, J.P.; Zeller, H. West Nile outbreak in horses in southern France, 2000: The return after 35 years. Emerg. Infect. Dis. 2001, 7, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Papa, A.; Danis, K.; Baka, A.; Bakas, A.; Dougas, G.; Lytras, T.; Theocharopoulos, G.; Chrysagis, D.; Vassiliadou, E.; Kamaria, F.; et al. Ongoing outbreak of West Nile virus infections in humans in Greece, July–August 2010. Euro. Surveill. 2010, 15, 19644. [Google Scholar] [CrossRef] [PubMed]
- Autorino, G.L.; Battisti, A.; Deubel, V.; Ferrari, G.; Forletta, R.; Giovannini, A.; Lelli, R.; Murri, S.; Scicluna, M.T. West Nile virus epidemic in horses, Tuscany region, Italy. Emerg. Infect. Dis. 2002, 8, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- Bagnarelli, P.; Marinelli, K.; Trotta, D.; Monachetti, A.; Tavio, M.; Del Gobbo, R.; Capobianchi, M.; Menzo, S.; Nicoletti, L.; Magurano, F.; et al. Human case of autochthonous West Nile virus lineage 2 infection in Italy, September 2011. Euro. Surveill. 2011, 16, 20002. [Google Scholar] [CrossRef]
- Magurano, F.; Remoli, M.E.; Baggieri, M.; Fortuna, C.; Marchi, A.; Fiorentini, C.; Bucci, P.; Benedetti, E.; Ciufolini, M.G.; Rizzo, C.; et al. Circulation of West Nile virus lineage 1 and 2 during an outbreak in Italy. Clin. Microbiol. Infect. 2012, 18, E545–547. [Google Scholar] [CrossRef] [Green Version]
- Popović, N.; Milošević, B.; Urošević, A.; Poluga, J.; Lavadinović, L.; Nedelijković, J.; Jevtović, D.; Dulović, O. Outbreak of West Nile virus infection among humans in Serbia, August to October 2012. Euro. Surveill. 2013, 18, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Gamino, V.; Höfle, U. Pathology and tissue tropism of natural West Nile virus infection in birds: A review. Vet. Res. 2013, 44, 39. [Google Scholar] [CrossRef] [Green Version]
- Lanciotti, R.S.; Roehrig, J.T.; Deubel, V.; Smith, J.; Parker, M.; Steele, K.; Crise, B.; Volpe, K.E.; Crabtree, M.B.; Scherret, J.H.; et al. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 1999, 286, 2333–2337. [Google Scholar] [CrossRef] [Green Version]
- Steele, K.E.; Linn, M.J.; Schoepp, R.J.; Komar, N.; Geisbert, T.W.; Manduca, R.M.; Calle, P.P.; Raphael, B.L.; Clippinger, T.L.; Larsen, T.; et al. Pathology of fatal West Nile virus infections in native and exotic birds during the 1999 outbreak in New York City, New York. Vet. Pathol. 2000, 37, 208–224. [Google Scholar] [CrossRef]
- Campbell, G.L.; Marfin, A.A.; Lanciotti, R.S.; Gubler, D.J. West Nile virus. Lancet Infect. Dis. 2002, 2, 519–529. [Google Scholar] [CrossRef]
- Deubel, V.; Fiette, L.; Gounon, P.; Drouet, M.T.; Khun, H.; Huerre, M.; Banet, C.; Malkinson, M.; Desprès, P. Variations in biological features of West Nile viruses. Ann. N. Y. Acad. Sci. 2001, 951, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.R.; Brault, A.C.; Nasci, R.S. West Nile virus: Review of the literature. JAMA 2013, 310, 308–315. [Google Scholar] [CrossRef] [PubMed]
- LaDeau, S.L.; Kilpatrick, A.M.; Marra, P.P. West Nile virus emergence and large-scale declines of North American bird populations. Nature 2007, 447, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Camp, J.V.; Nowotny, N. The knowns and unknowns of West Nile virus in Europe: What did we learn from the 2018 outbreak? Expert. Rev. Anti. Infect. Ther. 2020, 18, 145–154. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Epidemiological update: West Nile virus transmission season in Europe, 2018. 2018. Available online: https://www.ecdc.europa.eu/en/news-events/epidemiological-update-west-nile-virus-transmission-season-europe-2018 (accessed on 8 June 2020).
- European Centre for Disease Prevention and Control (ECDC). Epidemiological update: West Nile virus transmission season in Europe, 2019. Available online: https://www.ecdc.europa.eu/en/news-events/epidemiological-update-west-nile-virus-transmission-season-europe-2019 (accessed on 8 June 2020).
- Pérez-Ramírez, E.; Llorente, F.; Jiménez-Clavero, M.A. Experimental infections of wild birds with West Nile virus. Viruses 2014, 6, 752–781. [Google Scholar] [CrossRef] [Green Version]
- Bin, H.; Grossman, Z.; Pokamunski, S.; Malkinson, M.; Weiss, L.; Duvdevani, P.; Banet, C.; Weisman, Y.; Annis, E.; Gandaku, D.; et al. West Nile fever in Israel 1999–2000: From geese to humans. Ann. N. Y. Acad. Sci. 2001, 951, 127–142. [Google Scholar] [CrossRef]
- Austin, R.J.; Whiting, T.L.; Anderson, R.A.; Drebot, M.A. An outbreak of West Nile virus-associated disease in domestic geese (Anser anser domesticus) upon initial introduction to a geographic region, with evidence of bird to bird transmission. Can. Vet. J. 2004, 45, 117–123. [Google Scholar]
- Cox, S.L.; Campbell, G.D.; Nemeth, N.M. Outbreaks of West Nile virus in captive waterfowl in Ontario, Canada. Avian. Pathol. 2015, 44, 135–141. [Google Scholar] [CrossRef]
- Meece, J.K.; Kronenwetter-Koepel, T.A.; Vandermause, M.F.; Reed, K.D. West Nile virus infection in commercial waterfowl operation, Wisconsin. Emerg. Infect. Dis. 2006, 12, 1451–1453. [Google Scholar] [CrossRef] [Green Version]
- Himsworth, C.G.; Gurney, K.E.B.; Neimanis, A.S.; Wobeser, G.A.; Leighton, F.A. An outbreak of West Nile virus infection in captive lesser scaup (Aythya affinis) ducklings. Avian. Dis. 2009, 53, 129–134. [Google Scholar] [CrossRef]
- Komar, N.; Panella, N.A.; Burns, J.E.; Dusza, S.W.; Mascarenhas, T.M.; Talbot, T.O. Serologic evidence for West Nile virus infection in birds in the New York City vicinity during an outbreak in 1999. Emerg. Infect. Dis. 2001, 7, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Sa, E.S.M.; Ellis, A.; Karaca, K.; Minke, J.; Nordgren, R.; Wu, S.; Swayne, D.E. Domestic goose as a model for West Nile virus vaccine efficacy. Vaccine 2013, 31, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Banet-Noach, C.; Simanov, L.; Malkinson, M. Direct (non-vector) transmission of West Nile virus in geese. Avian. Pathol. 2003, 32, 489–494. [Google Scholar] [CrossRef]
- Langevin, S.A.; Bunning, M.; Davis, B.; Komar, N. Experimental infection of chickens as candidate sentinels for West Nile virus. Emerg. Infect. Dis. 2001, 7, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Komar, N. West Nile virus surveillance using sentinel birds. Ann. N. Y. Acad. Sci. 2001, 951, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Kwan, J.L.; Kluh, S.; Madon, M.B.; Nguyen, D.V.; Barker, C.M.; Reisen, W.K. Sentinel chicken seroconversions track tangential transmission of West Nile virus to humans in the greater Los Angeles area of California. Am. J. Trop. Med. Hyg. 2010, 83, 1137–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, C.D.; Baker, W.G.; Stark, L.; Burgess, J.; Lewis, A.L. Comparison of chickens and pheasants as sentinels for eastern equine encephalitis and St. Louis encephalitis viruses in Florida. J. Am. Mosq. Control Assoc. 1994, 10, 545–548. [Google Scholar]
- Rossini, G.; Carletti, F.; Bordi, L.; Cavrini, F.; Gaibani, P.; Landini, M.P.; Pierro, A.; Capobianchi, M.R.; Di Caro, A.; Sambri, V. Phylogenetic analysis of West Nile virus isolates, Italy, 2008-2009. Emerg. Infect. Dis. 2011, 17, 903–906. [Google Scholar] [CrossRef]
- Ziegler, U.; Lühken, R.; Keller, M.; Cadar, D.; van der Grinten, E.; Michel, F.; Albrecht, K.; Eiden, M.; Rinder, M.; Lachmann, L.; et al. West Nile virus epizootic in Germany, 2018. Antiviral Res. 2019, 162, 39–43. [Google Scholar] [CrossRef]
- Jansen, S.; Heitmann, A.; Lühken, R.; Leggewie, M.; Helms, M.; Badusche, M.; Rossini, G.; Schmidt-Chanasit, J.; Tannich, E. Culex torrentium: A potent vector for the transmission of West Nile virus in Central Europe. Viruses 2019, 11, 492. [Google Scholar] [CrossRef] [Green Version]
- Mayr, A.; Bachmann, P.A.; Bibrack, B.; Wittmann, G. Quantitative Bestimmung der Virusinfektiosität (Virustitration). In Virologische Arbeitsmethoden, Band I (Zellkulturen - Bebrütete Hühnereier - Versuchstiere), 1st ed.; Gustav Fischer Verlag: Stuttgart, Germany, 1974; Volume 39. [Google Scholar]
- Rudolf, M.; Czajka, C.; Börstler, J.; Melaun, C.; Jöst, H.; von Thien, H.; Badusche, M.; Becker, N.; Schmidt-Chanasit, J.; Krüger, A.; et al. First nationwide surveillance of Culex pipiens complex and Culex torrentium mosquitoes demonstrated the presence of Culex pipiens biotype pipiens/molestus hybrids in Germany. PLoS ONE 2013, 8, e71832. [Google Scholar] [CrossRef] [PubMed]
- Eiden, M.; Viña-Rodríguez, A.; Hoffmann, B.; Ziegler, U.; Groschup, M.H. Two new real-time quantitative reverse transcription polymerase chain reaction assays with unique target sites for the specific and sensitive detection of lineages 1 and 2 West Nile virus strains. J. Vet. Diagn. Invest. 2010, 22, 748–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Styer, L.M.; Kent, K.A.; Albright, R.G.; Bennett, C.J.; Kramer, L.D.; Bernard, K.A. Mosquitoes inoculate high doses of West Nile virus as they probe and feed on live hosts. PLoS Pathog. 2007, 3, 1262–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holicki, C.M.; Ziegler, U.; Răileanu, C.; Kampen, H.; Werner, D.; Silaghi, C.; Groschup, M.H.; Vasić, A. Intrathoracic injection of German Culex mosquitoes with West Nile virus to secure infection and virus transmission. manuscript in preparation.
- Heitmann, A.; Jansen, S.; Lühken, R.; Leggewie, M.; Schmidt-Chanasit, J.; Tannich, E. Forced salivation as a method to analyze vector competence of mosquitoes. J. Vis. Exp. 2018, 138, e57980. [Google Scholar] [CrossRef]
- Holicki, C.M.; Ziegler, U.; Răileanu, C.; Kampen, H.; Werner, D.; Schulz, J.; Silaghi, C.; Groschup, M.H.; Vasić, A. West Nile virus lineage 2 vector competence of indigenous Culex and Aedes mosquitoes from Germany at temperate climate conditions. Viruses 2020, 12, 561. [Google Scholar] [CrossRef]
- Seidowski, D.; Ziegler, U.; von Rönn, J.A.; Müller, K.; Hüppop, K.; Muller, T.; Freuling, C.; Mühle, R.U.; Nowotny, N.; Ulrich, R.G.; et al. West Nile virus monitoring of migratory and resident birds in Germany. Vector Borne Zoonotic Dis. 2010, 10, 639–647. [Google Scholar] [CrossRef]
- Mayr, A.; Bachmann, P.A.; Bibrack, B.; Wittmann, G. Neutraliationstest. In Virologische Arbeitsmethoden, Band II (Serologie), 1st ed.; Gustav Fischer Verlag: Stuttgart, Germany, 1977; Volume 39, pp. 457–534. [Google Scholar]
- Hoffmann, B.; Depner, K.; Schirrmeier, H.; Beer, M. A universal heterologous internal control system for duplex real-time RT-PCR assays used in a detection system for pestiviruses. J. Virol. Methods 2006, 136, 200–209. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; Global Biodiversity Information Facility: Vienna, Austria, 2019. [Google Scholar]
- Barrett, A.D.T. West Nile in Europe: An increasing public health problem. J. Travel. Med. 2018, 25. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, U.; Santos, P.D.; Groschup, M.H.; Hattendorf, C.; Eiden, M.; Höper, D.; Eisermann, P.; Keller, M.; Michel, F.; Klopfleisch, R.; et al. West Nile virus epidemic in Germany triggered by epizootic emergence, 2019. Viruses 2020, 12, 448. [Google Scholar] [CrossRef] [Green Version]
- Kampen, H.; Holicki, C.M.; Ziegler, U.; Groschup, M.H.; Tews, B.A.; Werner, D. West Nile virus mosquito vectors (Diptera: Culicidae) in Germany. Viruses 2020, 12, 493. [Google Scholar] [CrossRef]
- Sotelo, E.; Gutierrez-Guzmán, A.V.; del Amo, J.; Llorente, F.; El-Harrak, M.; Pérez-Ramírez, E.; Blanco, J.M.; Höfle, U.; Jiménez-Clavero, M.A. Pathogenicity of two recent Western Mediterranean West Nile virus isolates in a wild bird species indigenous to Southern Europe: The red-legged partridge. Vet. Res. 2011, 42, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamino, V.; Escribano-Romero, E.; Blázquez, A.B.; Gutiérrez-Guzmán, A.V.; Martín-Acebes, M.A.; Saiz, J.C.; Höfle, U. Experimental North American West Nile virus infection in the red-legged partridge (Alectoris rufa). Vet. Pathol. 2016, 53, 585–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spedicato, M.; Carmine, I.; Bellacicco, A.L.; Marruchella, G.; Marini, V.; Pisciella, M.; Di Francesco, G.; Lorusso, A.; Monaco, F.; Savini, G. Experimental infection of rock pigeons (Columba livia) with three West Nile virus lineage 1 strains isolated in Italy between 2009 and 2012. Epidemiol. Infect. 2016, 144, 1301–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dridi, M.; Rauw, F.; Muylkens, B.; Lecollinet, S.; van den Berg, T.; Lambrecht, B. Setting up a SPF chicken model for the pathotyping of West Nile virus (WNV) strains. Transbound. Emerg. Dis. 2013, 60 (Suppl. 2), 51–62. [Google Scholar] [CrossRef]
- Dridi, M.; Van Den Berg, T.; Lecollinet, S.; Lambrecht, B. Evaluation of the pathogenicity of West Nile virus (WNV) lineage 2 strains in a SPF chicken model of infection: NS3-249Pro mutation is neither sufficient nor necessary for conferring virulence. Vet. Res. 2015, 46, 130. [Google Scholar] [CrossRef] [Green Version]
- Association of Poultry Processors and Poultry Trade in the EU countries (AVEC). Annual Report 2019. Available online: https://www.avec-poultry.eu/resources/annual-reports/ (accessed on 8 June 2020).
- Samina, I.; Khinich, Y.; Simanov, M.; Malkinson, M. An inactivated West Nile virus vaccine for domestic geese-efficacy study and a summary of 4 years of field application. Vaccine 2005, 23, 4955–4958. [Google Scholar] [CrossRef]
- Glávits, R.; Ferenczi, E.; Ivanics, E.; Bakonyi, T.; Mató, T.; Zarka, P.; Palya, V. Co-occurrence of West Nile fever and circovirus infection in a goose flock in Hungary. Avian. Pathol. 2005, 34, 408–414. [Google Scholar] [CrossRef]
- Malkinson, M.; Banet, C.; Weisman, Y.; Pokamunski, S.; King, R.; Drouet, M.T.; Deubel, V. Introduction of West Nile virus in the Middle East by migrating white storks. Emerg. Infect. Dis. 2002, 8, 392–397. [Google Scholar] [CrossRef]
- Swayne, D.E.; Beck, J.R.; Smith, C.S.; Shieh, W.J.; Zaki, S.R. Fatal encephalitis and myocarditis in young domestic geese (Anser anser domesticus) caused by West Nile virus. Emerg. Infect. Dis. 2001, 7, 751–753. [Google Scholar] [CrossRef]
- Samina, I.; Havenga, M.; Koudstaal, W.; Khinich, Y.; Koldijk, M.; Malkinson, M.; Simanov, M.; Perl, S.; Gijsbers, L.; Weverling, G.J.; et al. Safety and efficacy in geese of a PER.C6-based inactivated West Nile virus vaccine. Vaccine 2007, 25, 8338–8345. [Google Scholar] [CrossRef]
- Lim, S.M.; Brault, A.C.; van Amerongen, G.; Sewbalaksing, V.D.; Osterhaus, A.D.; Martina, B.E.; Koraka, P. Susceptibility of European jackdaws (Corvus monedula) to experimental infection with lineage 1 and 2 West Nile viruses. J. Gen. Virol. 2014, 95, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; Brault, A.C.; van Amerongen, G.; Bosco-Lauth, A.M.; Romo, H.; Sewbalaksing, V.D.; Bowen, R.A.; Osterhaus, A.D.; Koraka, P.; Martina, B.E. Susceptibility of carrion crows to experimental infection with lineage 1 and 2 West Nile viruses. Emerg. Infect. Dis. 2015, 21, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Del Amo, J.; Llorente, F.; Pérez-Ramírez, E.; Soriguer, R.C.; Figuerola, J.; Nowotny, N.; Jiménez-Clavero, M.A. Experimental infection of house sparrows (Passer domesticus) with West Nile virus strains of lineages 1 and 2. Vet. Microbiol. 2014, 172, 542–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemeth, N.M.; Thomsen, B.V.; Spraker, T.R.; Benson, J.M.; Bosco-Lauth, A.M.; Oesterle, P.T.; Bright, J.M.; Muth, J.P.; Campbell, T.W.; Gidlewski, T.L.; et al. Clinical and pathologic responses of American crows (Corvus brachyrhynchos) and fish crows (C. ossifragus) to experimental West Nile virus infection. Vet. Pathol. 2011, 48, 1061–1074. [Google Scholar] [CrossRef] [Green Version]
- Bai, F.; Thompson, E.A.; Vig, P.J.S.; Leis, A.A. Current understanding of West Nile virus clinical manifestations, immune responses, neuroinvasion, and immunotherapeutic implications. Pathogens 2019, 8, 193. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, P. Advances in avian immunology—Prospects for disease control: A review. Avian. Pathol. 2010, 39, 309–324. [Google Scholar] [CrossRef]
- Evseev, D.; Magor, K.E. Innate immune responses to avian influenza viruses in ducks and chickens. Vet. Sci. 2019, 6, 5. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, E.A.; Bowen, R.A.; Brault, A.C. An ex vivo avian leukocyte culture model for West Nile virus infection. J. Virol. Methods 2015, 218, 19–22. [Google Scholar] [CrossRef] [Green Version]
- Weingartl, H.M.; Neufeld, J.L.; Copps, J.; Marszal, P. Experimental West Nile virus infection in blue jays (Cyanocitta cristata) and crows (Corvus brachyrhynchos). Vet. Pathol. 2004, 41, 362–370. [Google Scholar] [CrossRef] [Green Version]
- Sun, E.; Zhao, J.; Liu, N.; Yang, T.; Xu, Q.; Qin, Y.; Bu, Z.; Yang, Y.; Lunt, R.A.; Wang, L.; et al. Comprehensive mapping of common immunodominant epitopes in the West Nile virus nonstructural protein 1 recognized by avian antibody responses. PLoS ONE 2012, 7, e31434. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.G.; Hunt, J.L. On the use of spleen mass as a measure of avian immune system strength. Oecologia 2004, 138, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; West, N.; Vider, J.; Zhang, P.; Griffiths, R.E.; Wolvetang, E.; Burtonclay, P.; Warrilow, D. Inflammatory responses to a pathogenic West Nile virus strain. BMC Infect. Dis. 2019, 19, 912. [Google Scholar] [CrossRef] [PubMed]
- Fair, J.M.; Nemeth, N.M.; Taylor-McCabe, K.J.; Shou, Y.; Marrone, B.L. Clinical and acquired immunologic responses to West Nile virus infection of domestic chickens (Gallus gallus domesticus). Poult. Sci. 2011, 90, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Hamer, G.L.; Walker, E.D.; Brawn, J.D.; Loss, S.R.; Ruiz, M.O.; Goldberg, T.L.; Schotthoefer, A.M.; Brown, W.M.; Wheeler, E.; Kitron, U.D. Rapid amplification of West Nile virus: The role of hatch-year birds. Vector Borne Zoonotic Dis. 2008, 8, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Komar, N.; Langevin, S.; Hinten, S.; Nemeth, N.; Edwards, E.; Hettler, D.; Davis, B.; Bowen, R.; Bunning, M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg. Infect. Dis. 2003, 9, 311–322. [Google Scholar] [CrossRef]
- Styer, L.M.; Bernard, K.A.; Kramer, L.D. Enhanced early West Nile virus infection in young chickens infected by mosquito bite: Effect of viral dose. Am. J. Trop. Med. Hyg. 2006, 75, 337–345. [Google Scholar] [CrossRef]
- Shirafuji, H.; Kanehira, K.; Kubo, M.; Shibahara, T.; Kamio, T. Experimental West Nile virus infection in aigamo ducks, a cross between wild ducks (Anas platyrhynchos) and domestic ducks (Anas platyrhynchos var. domesticus). Avian. Dis. 2009, 53, 239–244. [Google Scholar] [CrossRef]
- Nemeth, N.M.; Hahn, D.C.; Gould, D.H.; Bowen, R.A. Experimental West Nile virus infection in eastern screech owls (Megascops asio). Avian. Dis. 2006, 50, 252–258. [Google Scholar] [CrossRef]
- Byas, A.D.; Ebel, G.D. Comparative pathology of West Nile virus in humans and non-human animals. Pathogens 2020, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, U.; Angenvoort, J.; Fischer, D.; Fast, C.; Eiden, M.; Rodríguez, A.V.; Revilla-Fernández, S.; Nowotny, N.; de la Fuente, J.G.; Lierz, M.; et al. Pathogenesis of West Nile virus lineage 1 and 2 in experimentally infected large falcons. Vet. Microbiol. 2013, 161, 263–273. [Google Scholar] [CrossRef]
- Feyer, S.; Bartenschlager, F.; Bertram, C.A.; Ziegler, U.; Fast, C.; Klopfleisch, R.; Müller, K. Clinical, pathological and virological aspects of fatal West Nile virus infections in 10 free-ranging goshawks (Accipiter gentilis) in Germany. Transbound. Emerg. Dis. 2020, 00, 1–13. [Google Scholar] [CrossRef]
- Senne, D.A.; Pedersen, J.C.; Hutto, D.L.; Taylor, W.D.; Schmitt, B.J.; Panigrahy, B. Pathogenicity of West Nile virus in chickens. Avian. Dis. 2000, 44, 642–649. [Google Scholar] [CrossRef]
- Michel, F.; Ziegler, U.; Fast, C.; Eiden, M.; Klaus, C.; Dobler, G.; Stiasny, K.; Groschup, M. Role of ducks in the transmission cycle of tick-borne encephalitis virus? Transbound. Emerg. Dis. 2020, 00, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Angenvoort, J.; Fischer, D.; Fast, C.; Ziegler, U.; Eiden, M.; de la Fuente, J.G.; Lierz, M.; Groschup, M.H. Limited efficacy of West Nile virus vaccines in large falcons (Falco spp.). Vet. Res. 2014, 45, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofmeister, E.; Porter, R.E.; Franson, J.C. Experimental susceptibility of wood ducks (Aix sponsa) for West Nile virus. J. Wildl. Dis. 2015, 51, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Kuno, G. Persistence of arboviruses and antiviral antibodies in vertebrate hosts: Its occurrence and impacts. Rev. Medical. Virol. 2001, 11, 165–190. [Google Scholar] [CrossRef] [PubMed]
- Appler, K.K.; Brown, A.N.; Stewart, B.S.; Behr, M.J.; Demarest, V.L.; Wong, S.J.; Bernard, K.A. Persistence of West Nile virus in the central nervous system and periphery of mice. PLoS ONE 2010, 5, e10649. [Google Scholar] [CrossRef]
- Wheeler, S.S.; Langevin, S.A.; Brault, A.C.; Woods, L.; Carroll, B.D.; Reisen, W.K. Detection of persistent West Nile virus RNA in experimentally and naturally infected avian hosts. Am. J. Trop. Med. Hyg. 2012, 87, 559–564. [Google Scholar] [CrossRef] [Green Version]
- Nemeth, N.; Young, G.; Ndaluka, C.; Bielefeldt-Ohmann, H.; Komar, N.; Bowen, R. Persistent West Nile virus infection in the house sparrow (Passer domesticus). Arch. Virol. 2009, 154, 783–789. [Google Scholar] [CrossRef]
- Duggal, N.K.; Langwig, K.E.; Ebel, G.D.; Brault, A.C. On the Fly: Interactions between birds, mosquitoes, and environment that have molded West Nile virus genomic structure over two decades. J. Med. Entomol. 2019, 56, 1467–1474. [Google Scholar] [CrossRef]
- Schneider, B.S.; Soong, L.; Girard, Y.A.; Campbell, G.; Mason, P.; Higgs, S. Potentiation of West Nile encephalitis by mosquito feeding. Viral. Immunol. 2006, 19, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Styer, L.M.; Lim, P.Y.; Louie, K.L.; Albright, R.G.; Kramer, L.D.; Bernard, K.A. Mosquito saliva causes enhancement of West Nile virus infection in mice. J. Virol. 2011, 85, 1517–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, B.S.; Higgs, S. The enhancement of arbovirus transmission and disease by mosquito saliva is associated with modulation of the host immune response. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 400–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demeure, C.E.; Brahimi, K.; Hacini, F.; Marchand, F.; Péronet, R.; Huerre, M.; St-Mezard, P.; Nicolas, J.F.; Brey, P.; Delespesse, G.; et al. Anopheles mosquito bites activate cutaneous mast cells leading to a local inflammatory response and lymph node hyperplasia. J. Immunol. 2005, 174, 3932–3940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swayne, D.E.; Beck, J.R.; Zaki, S. Pathogenicity of West Nile virus for turkeys. Avian. Dis. 2000, 44, 932–937. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, B.M.; Jupp, P.G. Infections in sentinel pigeons by Sindbis and West Nile viruses in South Africa, with observations on Culex (Culex) univittatus (Diptera: Culicidae) attracted to these birds. J. Med. Entomol. 1979, 16, 234–239. [Google Scholar] [CrossRef]
- McIntosh, B.M.; Jupp, P.G.; Dickinson, D.B.; McGillivray, G.M.; Sweetnam, J. Ecological studies on Sindbis and West Nile viruses in South Africa. I. Viral activity as revealed by infection of mosquitoes and sentinel fowls. S. Afr. J. Med. Sci. 1967, 32, 1–14. [Google Scholar]
- Doherty, R.L.; Carley, J.G.; Kay, B.H.; Filippich, C.; Marks, E.N. Murray Valley encephalitis virus infection in mosquitoes and domestic fowls in Queensland, 1974. Aust. J. Exp. Biol. Med. Sci. 1976, 54, 237–243. [Google Scholar] [CrossRef]
- Russell, R.C. Mosquito-borne arboviruses in Australia: The current scene and implications of climate change for human health. Int. J. Parasitol. 1998, 28, 955–969. [Google Scholar] [CrossRef]
- Cernescu, C.; Nedelcu, N.I.; Tardei, G.; Ruta, S.; Tsai, T.F. Continued transmission of West Nile virus to humans in southeastern Romania, 1997–1998. J. Infect. Dis. 2000, 181, 710–712. [Google Scholar] [CrossRef]
- Chaintoutis, S.C.; Gewehr, S.; Mourelatos, S.; Dovas, C.I. Serological monitoring of backyard chickens in Central Macedonia-Greece can detect low transmission of West Nile virus in the absence of human neuroinvasive disease cases. Acta. Trop. 2016, 163, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Marfin, A.A.; Petersen, L.R.; Eidson, M.; Miller, J.; Hadler, J.; Farello, C.; Werner, B.; Campbell, G.L.; Layton, M.; Smith, P.; et al. Widespread West Nile virus activity, eastern United States, 2000. Emerg. Infect. Dis. 2001, 7, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Shahhosseini, N.; Friedrich, J.; Moosa-Kazemi, S.H.; Sedaghat, M.M.; Kayedi, M.H.; Tannich, E.; Schmidt-Chanasit, J.; Lühken, R. Host-feeding patterns of Culex mosquitoes in Iran. Parasit. Vectors 2018, 11, 669. [Google Scholar] [CrossRef] [PubMed]




| Inoculation Method | Species | No. Coding | SPF Eggs | 1-2-Day-Old Hatchlings | Breeder |
|---|---|---|---|---|---|
| Subcutaneous injection | Chickens | C 01 – C 08; (C 09 – C 12 *) | × | commercial (SPF) | |
| Ducks | D 01 – D 08; (D 09 – D 12 *) | × | regional/conventional | ||
| Geese | G 01 – G 08; (G 09 – G 12 *) | × | regional/conventional | ||
| Mosquito bite | Geese | G 13 – G 16; (G 17 – G 20 †) | × |
| Infection | Dissemination | Transmission | ||
|---|---|---|---|---|
| WNV-Positive Heads | WNV-Positive Salivary Secretions | |||
| Efficiency (%) (95% CI) | 92/92 (100) (96.1–100) | 92/92 (100) (96.1–100) | 92/92 (100) (96.1–100) | 46/92 (50.0) (39.4–60.6) |
| Viral load (viral copies/µL of total RNA) | 9.1 × 105 | 2.9 × 104 | 1.8 × 105 | NA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holicki, C.M.; Michel, F.; Vasić, A.; Fast, C.; Eiden, M.; Răileanu, C.; Kampen, H.; Werner, D.; Groschup, M.H.; Ziegler, U. Pathogenicity of West Nile Virus Lineage 1 to German Poultry. Vaccines 2020, 8, 507. https://doi.org/10.3390/vaccines8030507
Holicki CM, Michel F, Vasić A, Fast C, Eiden M, Răileanu C, Kampen H, Werner D, Groschup MH, Ziegler U. Pathogenicity of West Nile Virus Lineage 1 to German Poultry. Vaccines. 2020; 8(3):507. https://doi.org/10.3390/vaccines8030507
Chicago/Turabian StyleHolicki, Cora M., Friederike Michel, Ana Vasić, Christine Fast, Martin Eiden, Cristian Răileanu, Helge Kampen, Doreen Werner, Martin H. Groschup, and Ute Ziegler. 2020. "Pathogenicity of West Nile Virus Lineage 1 to German Poultry" Vaccines 8, no. 3: 507. https://doi.org/10.3390/vaccines8030507
APA StyleHolicki, C. M., Michel, F., Vasić, A., Fast, C., Eiden, M., Răileanu, C., Kampen, H., Werner, D., Groschup, M. H., & Ziegler, U. (2020). Pathogenicity of West Nile Virus Lineage 1 to German Poultry. Vaccines, 8(3), 507. https://doi.org/10.3390/vaccines8030507





