Vaccination during the First Diagnosis of Multiple Myeloma: A Cohort Study of the French National Health Insurance Database
Abstract
:1. Introduction
2. Method
2.1. Data Sources
2.2. Selection of MM Incident Cases
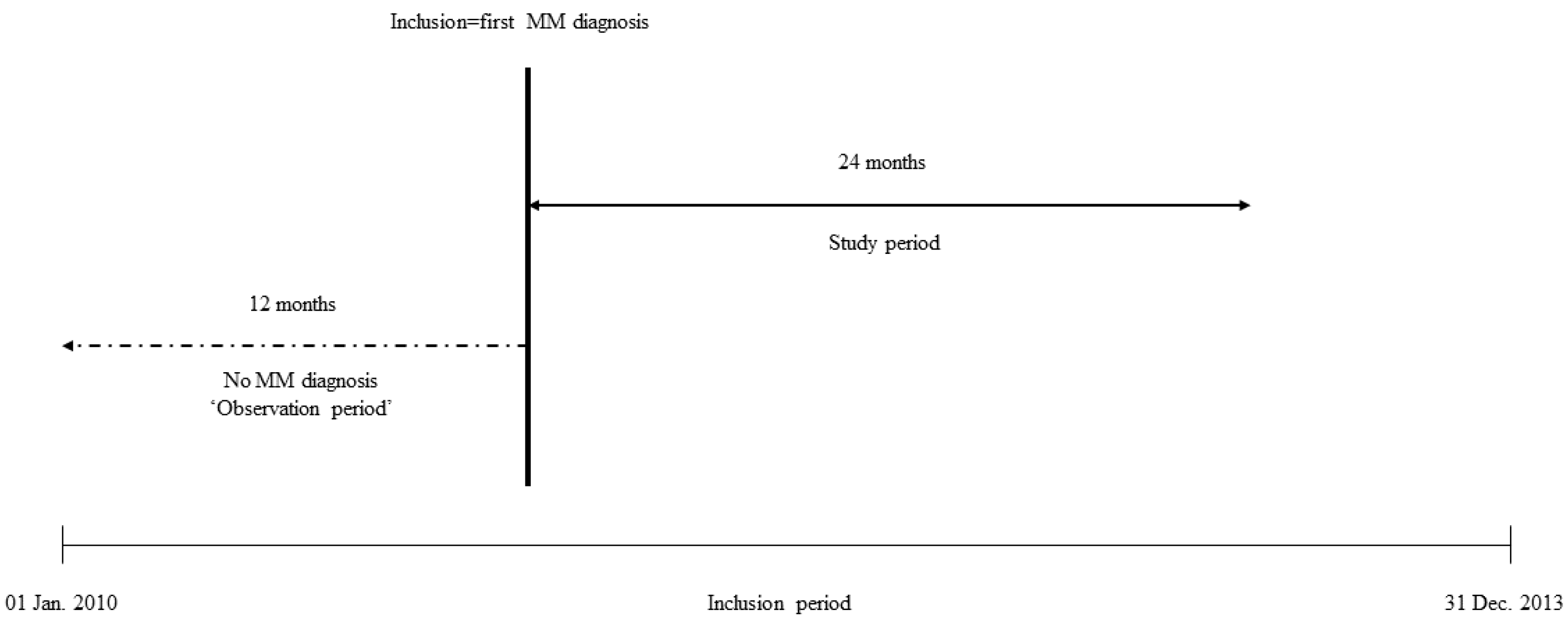
2.3. Observation and Study Periods
2.4. Definition of Outcomes
2.5. Covariates
- -
- At diagnosis: age, gender, and complementary universal health insurance (CMU-C). In France, this supplementary insurance is available free of charge for people with a low income who are entitled to universal healthcare coverage.
- -
- During the observation period: Comorbidities were assessed by calculating a SNDS database adaptation of the Charlson Comorbidity Index [22,23,24]. We used the Charlson items and French recommendations for SP vaccination [25] to identify patients with a dual recommendation for SP vaccination (MM and another disease). We also included the healthcare consumption profile: number of different drugs used (categorized as ATC classes), number of different drugs used excluding vaccines (categorized as ATC classes), reimbursed vaccines (none versus at least one), number of medical visits (as a continuous variable), and number of hospital stays (none versus at least one). Lastly, we included two socioeconomic variables calculated using the community (smallest administrative unit in France) code [26]: the patient geographic area (urban versus rural) and the Fdep09, a deprivation index [27], with patients in the fifth quintile being the most deprived.
- -
- During the study period: antiviral prophylaxis (Herpes simplex virus (HSV) and Varicella-zoster-virus (VZV)) with at least two valaciclovir (ATC code J05AB11) reimbursements and pneumocystis jirovecii prophylaxis with at least two cotrimoxazole (ATC code J01EE01) or two pentamidine (ATC code P01CX01) reimbursements.
2.6. Analyses
3. Results
3.1. Characteristics of MM Patients
3.2. Vaccine Use in MM Patients
3.3. Factors Associated with Vaccination
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, S.K.; Dispenzieri, A.; Lacy, M.Q.; Gertz, M.A.; Buadi, F.K.; Pandey, S.; Kapoor, P.; Dingli, D.; Hayman, S.R.; Leung, N.; et al. Continued improvement in survival in multiple myeloma: Changes in early mortality and outcomes in older patients. Leukemia 2014, 28, 1122–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristinsson, S.Y.; Anderson, W.F.; Landgren, O. Improved long-term survival in multiple myeloma up to the age of 80 years. Leukemia 2014, 28, 1346–1348. [Google Scholar] [CrossRef] [PubMed]
- Kristinsson, S.Y.; Landgren, O.; Dickman, P.W.; Derolf, A.R.; Björkholm, M. Patterns of survival in multiple myeloma: A population-based study of patients diagnosed in Sweden from 1973 to to 2003. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 1993–1999. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Mina, R. Management of older adults with multiple myeloma. Blood Rev. 2013, 27, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Kyle, R.A.; Rajkumar, S.V. Multiple myeloma. N. Engl. J. Med. 2004, 351, 1860–1873. [Google Scholar] [CrossRef]
- Nucci, M.; Anaissie, E. Infections in patients with multiple myeloma in the era of high-dose therapy and novel agents. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2009, 49, 1211–1225. [Google Scholar] [CrossRef] [Green Version]
- Blimark, C.; Holmberg, E.; Mellqvist, U.-H.; Landgren, O.; Björkholm, M.; Hultcrantz, M.; Kjellander, C.; Turesson, I.; Kristinsson, S.Y. Multiple myeloma and infections: A population-based study on 9253 multiple myeloma patients. Haematologica 2015, 100, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Pratt, G.; Goodyear, O.; Moss, P. Immunodeficiency and immunotherapy in multiple myeloma. Br. J. Haematol. 2007, 138, 563–579. [Google Scholar] [CrossRef]
- Gregersen, H.; Madsen, K.M.; Sørensen, H.T.; Schønheyder, H.C.; Ibsen, J.S.; Dahlerup, J.F. The risk of bacteremia in patients with monoclonal gammopathy of undetermined significance. Eur. J. Haematol. 1998, 61, 140–144. [Google Scholar] [CrossRef]
- Kristinsson, S.Y.; Tang, M.; Pfeiffer, R.M.; Björkholm, M.; Goldin, L.R.; Blimark, C.; Mellqvist, U.-H.; Wahlin, A.; Turesson, I.; Landgren, O. Monoclonal gammopathy of undetermined significance and risk of infections: A population-based study. Haematologica 2012, 97, 854–858. [Google Scholar] [CrossRef] [Green Version]
- Hargreaves, R.M.; Lea, J.R.; Griffiths, H.; Faux, J.A.; Holt, J.M.; Reid, C.; Bunch, C.; Lee, M.; Chapel, H.M. Immunological factors and risk of infection in plateau phase myeloma. J. Clin. Pathol. 1995, 48, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.; Andréasson, B.; Kondori, N.; Erman, E.; Riesbeck, K.; Hogevik, H.; Wennerås, C. Comparative study of immune status to infectious agents in elderly patients with multiple myeloma, Waldenstrom’s macroglobulinemia, and monoclonal gammopathy of undetermined significance. Clin. Vaccine Immunol. CVI 2011, 18, 969–977. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, H.; Miguel, J.S.; Dimopoulos, M.A.; Palumbo, A.; Garcia Sanz, R.; Powles, R.; Lentzsch, S.; Chen, W.M.; Hou, J.; Jurczyszyn, A.; et al. International Myeloma Working Group recommendations for global myeloma care. Leukemia 2014, 28, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.D.; Nagesh, K.; Jowitt, S.N.; Dougal, M.; Anderson, H.; Mutton, K.; Zambon, M.; Scarffe, J.H. Immunogenicity of vaccination against influenza, Streptococcus pneumoniae and Haemophilus influenzae type B in patients with multiple myeloma. Br. J. Cancer 2000, 82, 1261–1265. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Gertz, M.A.; Kyle, R.A.; Greipp, P.R.; Myeloma, M.C. Current therapy for multiple myeloma. Mayo Clin. Proc. 2002, 77, 813–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, H.; Delforge, M.; Facon, T.; Einsele, H.; Gay, F.; Moreau, P.; Avet-Loiseau, H.; Boccadoro, M.; Hajek, R.; Mohty, M.; et al. Prevention and management of adverse events of Novel agents in multiple myeloma: A consensus of the european myeloma network. Leukemia 2018, 32, 1542–1560. [Google Scholar] [CrossRef] [PubMed]
- Moulis, G.; Lapeyre-Mestre, M.; Palmaro, A.; Pugnet, G.; Montastruc, J.-L.; Sailler, L. French health insurance databases: What interest for medical research? Rev. Med. Interne. 2015, 36, 411–417. [Google Scholar] [CrossRef] [Green Version]
- Conte, C.; Rueter, M.; Laurent, G.; Bourrel, R.; Lapeyre-Mestre, M.; Despas, F. Psychotropic drug initiation during the first diagnosis and the active treatment phase of B cell non-Hodgkin’s lymphoma: A cohort study of the French national health insurance database. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2016, 24, 4791–4799. [Google Scholar] [CrossRef]
- Palmaro, A.; Gauthier, M.; Conte, C.; Grosclaude, P.; Despas, F.; Lapeyre-Mestre, M. Identifying multiple myeloma patients using data from the French health insurance databases: Validation using a cancer registry. Medicine 2017, 96, e6189. [Google Scholar] [CrossRef]
- HCSP. Actualisation de la Liste des Sujets Eligibles à la Vaccination Contre la Grippe Saisonnière; Haut Conseil de la Santé Publique: Paris, France, 2010; Available online: https://www.hcsp.fr/Explore.cgi/avisrapportsdomaine?clefr=179 (accessed on 14 December 2018).
- WHOCC—ATC/DDD Index. Available online: https://www.whocc.no/atc_ddd_index/ (accessed on 31 January 2019).
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Bannay, A.; Chaignot, C.; Blotière, P.-O.; Weill, A.; Ricordeau, P.; Alla, F. Score de Charlson à partir des données du Sniiram chaînées au PMSI: Faisabilité et valeur pronostique sur la mortalité à un an. Rev. DÉpidémiologie Santé Publique 2013, 61, S9. [Google Scholar] [CrossRef]
- Bannay, A.; Chaignot, C.; Blotière, P.-O.; Basson, M.; Weill, A.; Ricordeau, P.; Alla, F. The Best Use of the Charlson Comorbidity Index With Electronic Health Care Database to Predict Mortality. Med. Care 2016, 54, 188–194. [Google Scholar] [CrossRef] [PubMed]
- HCSP. Infections Invasives A Pneumocoque: Recommandations Vaccinales Pour les Personnes à Risque; Haut Conseil de la Santé Publique: Paris, France, 2013; Available online: https://www.hcsp.fr/Explore.cgi/avisrapportsdomaine?clefr=355 (accessed on 17 September 2018).
- Codes Géographiques et Codes Pays | Publication ATIH. Available online: https://www.atih.sante.fr/codes-geographiques-et-codes-pays (accessed on 28 May 2018).
- Rey, G.; Jougla, E.; Fouillet, A.; Hémon, D. Ecological association between a deprivation index and mortality in France over the period 1997–2001: Variations with spatial scale, degree of urbanicity, age, gender and cause of death. BMC Public Health 2009, 9, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alemu, A.; Singh, M.; Blumberg, C.; Richards, J.; Oaks, M.; Thompson, M. Multiple Myeloma Vaccination Patterns in a Large Health System: A Pilot Study. J. Patient-Cent. Res. Rev. 2017, 4, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Moulis, G.; Lapeyre-Mestre, M.; Mahévas, M.; Montastruc, J.-L.; Sailler, L. Need for an improved vaccination rate in primary immune thrombocytopenia patients exposed to rituximab or splenectomy. A nationwide population-based study in France. Am. J. Hematol. 2015, 90, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Couderc, S. Risque Infectieux des Medicaments Biologiques Versus Traitements Systemiques Conventionnels Dans le Psoriasis Modere a Severe = Infectious Risk of Biological Drugs Versus Conventional Systemic Treatements in Moderate to Severe Psoriasis, Limoges, 2015. Available online: http://aurore.unilim.fr/ori-oai-search/notice/view/unilim-ori-53243 (accessed on 6 May 2019).
- Alemu, A.; Richards, J.O.; Oaks, M.K.; Thompson, M.A. Vaccination in Multiple Myeloma: Review of Current Literature. Clin. Lymphoma Myeloma Leuk. 2016, 16, 495–502. [Google Scholar] [CrossRef]
- Moulis, G.; Palmaro, A.; Montastruc, J.-L.; Godeau, B.; Lapeyre-Mestre, M.; Sailler, L. Epidemiology of incident immune thrombocytopenia: A nationwide population-based study in France. Blood 2014, 124, 3308–3315. [Google Scholar] [CrossRef] [Green Version]
- Moulis, G.; Sailler, L.; Adoue, D.; Lapeyre-Mestre, M. Pharmacoepidemiology of Immune Thrombocytopenia: Protocols of FAITH and CARMEN studies. Therapie 2014, 69, 437–448. [Google Scholar] [CrossRef]
- INPES—Calendrier des Vaccinations. Available online: http://inpes.santepubliquefrance.fr/10000/themes/vaccination/calendrier/calendrier-vaccination.asp (accessed on 7 May 2019).
- Blanchard-Rohner, G.; Pollard, A.J. Sustaining immunity after immunization against encapsulated bacteria. Hum. Vaccin. 2008, 4, 309–312. [Google Scholar] [CrossRef]
- HCSP. Vaccination des Personnes Immunodéprimées ou Aspléniques. Recommandations Actualisées; Haut Conseil de la Santé Publique: Paris, France, 2014; Available online: https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=504 (accessed on 6 February 2018).
- Bahuaud, M.; Bodilis, H.; Malphettes, M.; Maugard Landre, A.; Matondo, C.; Bouscary, D.; Batteux, F.; Launay, O.; Fermand, J.-P. Immunogenicity and persistence of the 13-valent Pneumococcal Conjugate Vaccine (PCV13) in patients with untreated Smoldering Multiple Myeloma (SMM): A pilot study. Heliyon 2017, 3, e00441. [Google Scholar] [CrossRef]

| Characteristics | Total Population | Primary Outcome | p-Value | |
|---|---|---|---|---|
| Not All Recommended Vaccines | Influenza and SP and Hib | |||
| Number of subjects, n (%) | 22,831 | 22,679 (99.3) | 152 (0.7) | |
| Age (years), median (IQR) | 74 (64–82) | 74 (64–82) | 66 (62–75) | <0.0001 |
| Females, n (%) | 11,034 (48.3) | 10,964 (48.3) | 70 (46.1) | 0.5731 |
| Charlson Comorbidity Index, n (%) | 0.0418 | |||
| 0 | 11,632 (50.9) | 11,540 (50.9) | 92 (60.5) | |
| 1–2 | 6737 (29.5) | 6696 (29.5) | 41 (27.0) | |
| 3–4 | 2119 (9.3) | 2113 (9.3) | 6 (3.9) | |
| ≥5 | 2343 (10.3) | 2330 (10.3) | 13 (8.6) | |
| SP vaccination recommendation not related to MM, n (%) | 11,099 (48.6) | 11,028 (48.6) | 71 (46.7) | 0.6376 |
| Fdep99 deprivation index, n (%) | 0.4765 | |||
| 1st quintile | 4213 (18.4) | 4180 (18.4) | 33 (21.6) | |
| 2nd quintile | 4067 (17.8) | 4040 (17.8) | 27 (17.8) | |
| 3rd quintile | 4145 (18.2) | 4117 (18.1) | 28 (18.4) | |
| 4th quintile | 4281 (18.8) | 4254 (18.8) | 27 (17.8) | |
| 5th quintile | 4264 (18.7) | 4233 (18.7) | 31 (20.4) | |
| Unknown | 1861 (8.1) | 1855 (8.2) | 6 (4.0) | |
| Complementary universal health insurance (CMU-C), n (%) | 1285 (5.6) | 1279 (5.6) | 6 (4.0) | 0.3669 |
| Geographic area, n (%) | 0.9821 | |||
| Urban | 13,008 (57.0) | 12,921 (57.0) | 87 (57.2) | |
| Rural | 4491 (19.7) | 4462 (19.7) | 29 (19.1) | |
| Unknown | 5332 (23.3) | 5296 (23.3) | 36 (23.7) | |
| Number of medical visits, median (IQR) | 13 (8–19) | 13 (8–19) | 12.5 (8–17) | 0.1336 |
| Patients with at least one hospital stay, n (%) | 8579 (37.6) | 8528 (37.6) | 51 (33.6) | 0.3041 |
| Number of drugs used, excluding vaccines, median (IQR) | 17 (11–24) | 17 (11–24) | 17 (12.5–23) | 0.5273 |
| Vaccinated patients, n (%) | 9587 (42.0) | 9946 (41.9) | 91 (59.9) | <0.0001 |
| Characteristics | Total Population | Secondary Outcome | p-Value | |
|---|---|---|---|---|
| No SP or Hib | SP or Hib | |||
| Number of subjects, n (%) | 22,831 | 20,466 (89.6) | 2365 (10.4) | |
| Age (years), median (IQR) | 74 (64–82) | 75 (64–82) | 67 (60–77) | <0.0001 |
| Females, n (%) | 11,034 (48.3) | 9965 (48.7) | 1069 (45.2) | 0.0013 |
| Charlson Comorbidity Index, n (%) | <0.0001 | |||
| 0 | 11,632 (50.9) | 10,249 (50.1) | 1383 (58.5) | |
| 1–2 | 6737 (29.5) | 6086 (29.7) | 651 (27.5) | |
| 3–4 | 2119 (9.3) | 1984 (9.7) | 135 (5.7) | |
| ≥5 | 2343 (10.3) | 2147 (10.5) | 196 (8.3) | |
| SP vaccination recommendation not related to MM, n (%) | 11,099 (48.6) | 10,090 (49.3) | 1009 (42.7) | <0.0001 |
| Fdep99 deprivation index, n (%) | <0.0001 | |||
| 1st quintile | 4213 (18.4) | 3722 (18.2) | 491 (20.8) | |
| 2nd quintile | 4067 (17.8) | 3609 (17.6) | 458 (19.4) | |
| 3rd quintile | 4145 (18.2) | 3677 (18.0) | 468 (19.8) | |
| 4th quintile | 4281 (18.8) | 3832 (18.7) | 449 (19.0) | |
| 5th quintile | 4264 (18.7) | 3863 (18.9) | 401 (16.9) | |
| Unknown | 1861 (8.1) | 1763 (8.6) | 98 (4.1) | |
| Complementary universal health insurance, n (%) | 1285 (5.6) | 1158 (5.7) | 127 (5.4) | 0.5648 |
| Geographic area, n (%) | 0.0003 | |||
| Urban | 13,008 (57.0) | 11,745 (57.4) | 1263 (53.4) | |
| Rural | 4491 (19.7) | 4011 (19.6) | 480 (20.3) | |
| Unknown | 5332 (23.3) | 4710 (23.0) | 622 (26.3) | |
| Number of medical visits, median (IQR) | 13 (8–19) | 13 (8–19) | 12.5 (8–18) | 0.0025 |
| Patients with at least one hospital stay, n (%) | 8579 (37.6) | 7811 (38.2) | 768 (32.5) | <0.0001 |
| Number of drugs used, median (IQR) | 18 (12–25) | 18 (12–24) | 18 (12–25) | 0.0015 |
| Number of drugs used, excluding vaccines, median (IQR) | 17 (11–24) | 17 (11–24) | 18 (12–24) | 0.0017 |
| Vaccinated patients, n (%) | 9587 (42.0) | 8550 (41.8) | 1037 (43.9) | 0.0533 |
| Vaccinated Patients, n (%) | Time after MM Diagnosis | ||
|---|---|---|---|
| 0–12 Months | 12–24 Months | 0–24 Months | |
| Against influenza | 6517 (28.5) | 5960 (26.1) | 8000 (35.1) |
| Against S.p. | 1353 (5.9) | 1149 (5.0) | 2350 (10.3) |
| Against H.i.b. | 199 (0.9) | 125 (0.6) | 316 (1.4) |
| Characteristics | Crude OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value |
|---|---|---|---|---|
| Age (year) | 0.97 (0.96–0.98) | <0.0001 | 0.98 (0.97–0.99) | 0.0132 |
| Female gender | 0.91 (0.66–1.26) | 0.5732 | 1.00 (0.73–1.39) | 0.9853 |
| Charlson Comorbidity Index | 0.0507 | – | – | |
| 0 | 1 | |||
| 1–2 | 0.77 (0.53–1.11) | |||
| 3–4 | 0.36 (0.16–0.82) | |||
| ≥5 | 0.70 (0.39–1.25) | |||
| SP vaccination recommended | 0.93 (0.67–1.28) | 0.6377 | – | – |
| Fdep99 deprivation index | 0.5024 | – | – | |
| 1st quintile | 1 | |||
| 2nd quintile | 0.85 (0.51–1.41) | |||
| 3rd quintile | 0.86 (0.52–1.43) | |||
| 4th quintile | 0.80 (0.48–1.34) | |||
| 5th quintile | 0.93 (0.57–1.52) | |||
| Unknown | 0.41 (0.17–0.98) | |||
| Complementary universal health insurance | 0.69 (0.30–1.56) | 0.3697 | – | – |
| Geographic area | 0.9824 | – | – | |
| Urban | 1 | |||
| Rural | 0.97 (0.63–1.47) | |||
| Unknown | 1.01 (0.68–1.49) | |||
| Number of medical visits during observation | 0.99 (0.98–1.01) | 0.4251 | – | – |
| Patients with at least one hospital stay during observation | 0.84 (0.60–1.18) | 0.3048 | – | – |
| Number of nonvaccine drugs used during observation | 1.01 (0.99–1.02) | 0.4004 | – | – |
| Patients vaccinated during observation | 2.07 (1.50–2.87) | <0.0001 | 3.00 (2.11–4.25) | <0.0001 |
| HSV-VZV prophylaxis during study | 5.87 (3.94–8.76) | <0.0001 | 3.15 (1.93–5.14) | <0.0001 |
| P. Jirovecii prophylaxis during study | 5.11 (3.67–7.11) | <0.0001 | 2.55 (1.70–3.80) | <0.0001 |
| Characteristics | Crude OR (95% CI) | p Value | Adjusted OR (95% CI) | p Value |
|---|---|---|---|---|
| Age (year) | 0.970 (0.967–0.974) | <0.0001 | 0.98 (0.97–0.99) | <0.0001 |
| Female gender | 0.87 (0.80–0.95) | 0.0013 | 0.91(0.83–0.99) | 0.0324 |
| Charlson Comorbidity Index | <0.0001 | 0.0448 | ||
| 0 | 1 | 1 | ||
| 1–2 | 0.79 (0.72–0.88) | 1.00 (0.90–1.11) | ||
| 3–4 | 0.50 (0.42–0.61) | 0.83 (0.68–1.00) | ||
| ≥5 | 0.68 (0.58–0.79) | 0.84 (0.71–0.98) | ||
| SP vaccination recommended | 0.77 (0.70–0.84) | <0.0001 | – | – |
| Fdep99 deprivation index | <0.0001 | <0.0001 | ||
| 1st quintile | 1 | 1 | ||
| 2nd quintile | 0.96 (0.84–1.10) | 0.97 (0.85–1.11) | ||
| 3rd quintile | 0.97 (0.84–1.10) | 1.03 (0.90–1.18) | ||
| 4th quintile | 0.89 (0.78–1.02) | 0.96 (0.84–1.11) | ||
| 5th quintile | 0.79 (0.69–0.91) | 0.87 (0.75–1.00) | ||
| Unknown | 0.42 (0.34–0.53) | 0.54 (0.43–0.68) | ||
| Complementary universal health insurance | 0.95 (0.78–1.14) | 0.5648 | – | – |
| Geographic area | 0.0003 | – | – | |
| Urban | 1 | |||
| Rural | 1.11 (1.00–1.24) | |||
| Unknown | 1.23 (1.11–1.36) | |||
| Number of medical visits during observation | 1.00 (0.99–1.00) | 0.2455 | 0.99 (0.98–1.00) | 0.0226 |
| Patients with at least one hospital stay during observation | 0.78 (0.71–0.85) | <0.0001 | 0.89 (0.81–0.99) | 0.0256 |
| Number of nonvaccine drugs used during observation | 1.01 (1.00–1.01) | 0.0003 | 1.01 (1.00–1.02) | 0.0002 |
| Patients vaccinated during observation | 1.09 (1.00–1.19) | 0.0534 | 1.39 (1.26–1.53) | <0.0001 |
| HSV-VZV prophylaxis during study | 3.01 (2.75–3.29) | <0.0001 | 2.11 (1.89–2.36) | <0.0001 |
| P. Jirovecii prophylaxis during study | 2.44 (2.23–2.66) | <0.0001 | 1.27 (1.14–1.41) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tournaire, G.; Conte, C.; Perrot, A.; Lapeyre-Mester, M.; Despas, F. Vaccination during the First Diagnosis of Multiple Myeloma: A Cohort Study of the French National Health Insurance Database. Vaccines 2020, 8, 722. https://doi.org/10.3390/vaccines8040722
Tournaire G, Conte C, Perrot A, Lapeyre-Mester M, Despas F. Vaccination during the First Diagnosis of Multiple Myeloma: A Cohort Study of the French National Health Insurance Database. Vaccines. 2020; 8(4):722. https://doi.org/10.3390/vaccines8040722
Chicago/Turabian StyleTournaire, Guilhem, Cécile Conte, Aurore Perrot, Maryse Lapeyre-Mester, and Fabien Despas. 2020. "Vaccination during the First Diagnosis of Multiple Myeloma: A Cohort Study of the French National Health Insurance Database" Vaccines 8, no. 4: 722. https://doi.org/10.3390/vaccines8040722
APA StyleTournaire, G., Conte, C., Perrot, A., Lapeyre-Mester, M., & Despas, F. (2020). Vaccination during the First Diagnosis of Multiple Myeloma: A Cohort Study of the French National Health Insurance Database. Vaccines, 8(4), 722. https://doi.org/10.3390/vaccines8040722






