Immunogenicity of Plant-Produced Human Papillomavirus (HPV) Virus-Like Particles (VLPs)
Abstract
:1. Introduction
2. Materials and Methods
2.1. HPV Recombinant Plasmids
2.2. Transformation of Agrobacterium
2.3. Agrobacterium-Mediated Transient Expression in N. benthamiana
2.4. Extraction and Purification of VLPs
2.5. SDS-PAGE and Western Blot Analysis
2.6. Transmission Electron Microscopy (TEM)
2.7. Quantification of Vaccine Candidates
2.8. Immunization of Mice
2.9. Detection of Anti-L1 Antibodies in Mice Sera by Indirect Enzyme-Linked Immunosorbent Assay (ELISA)
2.9.1. Pre-absorption of Mice Sera
2.9.2. Detection of Anti-L1 Antibodies in Mice Sera
2.10. Pseudovirion-Based Neutralization Assays
3. Results
3.1. Expression and Purification of HPV L1 VLPs
3.2. Determination of Anti-L1 Titres
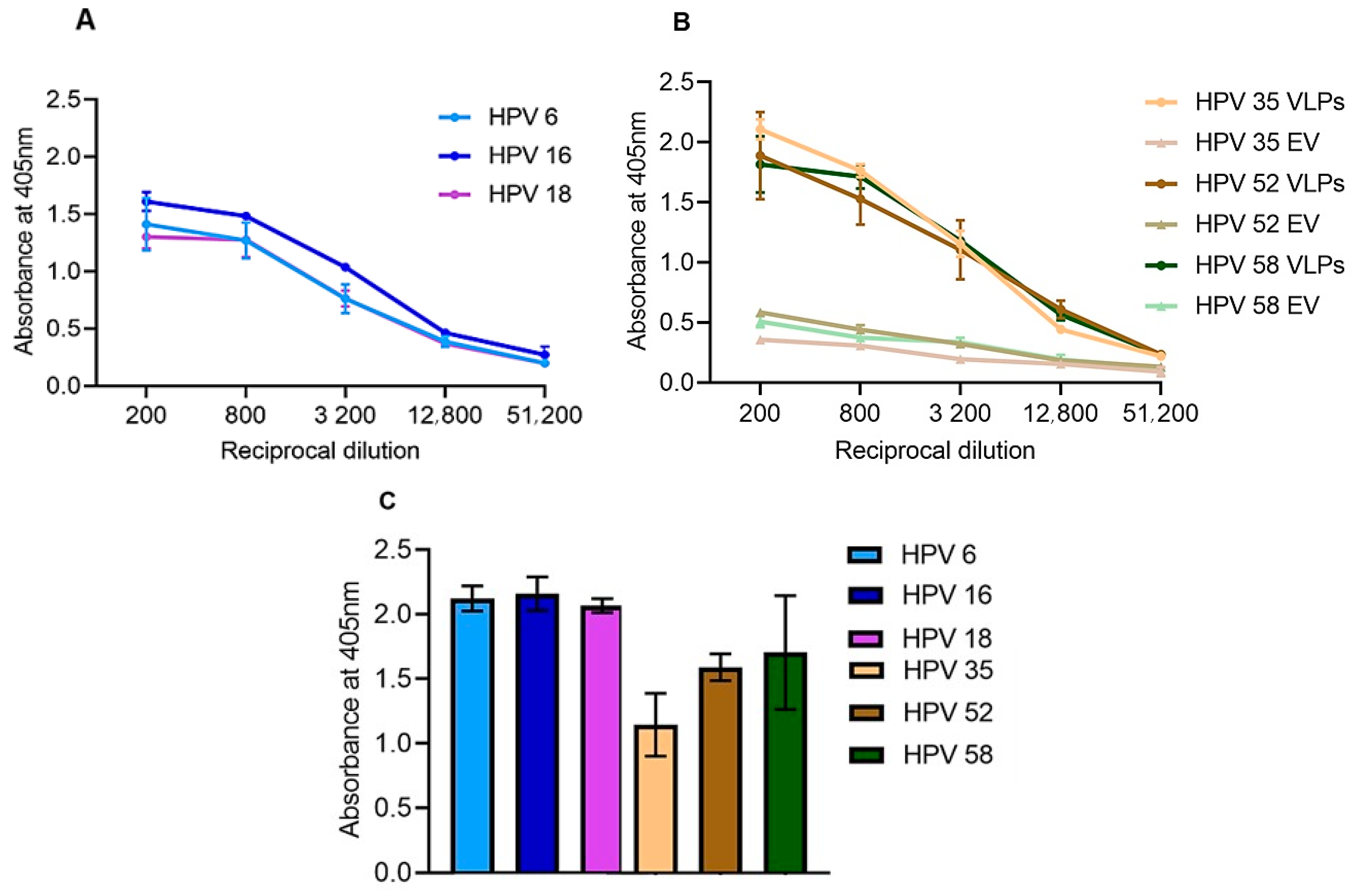
3.2.1. ELISA Analysis
3.2.2. Detection of Anti-L1 Neutralizing Antibodies in Mice Sera
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jordan, T.; Barcellona, C.; Basore, D.; Clark, C.; Guo, Z.; Isern, S.; Nand, K.; Rabasa, G.; Shoemaker, T.; Werner, G. HPV VLPs as Scaffolds for Vaccine Design. Biophys. J. 2019, 116, 58a. [Google Scholar] [CrossRef] [Green Version]
- Chabeda, A.; van Zyl, A.R.; Rybicki, E.P.; Hitzeroth, I.I. Substitution of Human Papillomavirus Type 16 L2 Neutralising Epitopes Into L1 Surface Loops: The Effect on Virus-Like Particle Assembly and Immunogenicity. Front. Plant Sci. 2019, 10, 779. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.O.; Speiser, D.E.; Knuth, A.; Bachmann, M.F. Virus-like particles for vaccination against cancer. Wiley Interdiscip. Rev. 2019, 12, e1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohsen, M.O.; Gomes, A.C.; Vogel, M.; Bachmann, M.F. Interaction of viral capsid-derived virus-like particles (VLPs) with the innate immune system. Vaccines 2018, 6, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pineo, C.B.; Hitzeroth, I.I.; Rybicki, E.P. Immunogenic assessment of plant-produced human papillomavirus type 16 L1/L2 chimaeras. Plant Biotechnol. J. 2013, 11, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Giannini, S.L.; Hanon, E.; Moris, P.; Van Mechelen, M.; Morel, S.; Dessy, F.; Fourneau, M.A.; Colau, B.; Suzich, J.; Losonksy, G. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine 2006, 24, 5937–5949. [Google Scholar] [CrossRef]
- Schiller, J.T.; Castellsagué, X.; Villa, L.L.; Hildesheim, A. An update of prophylactic human papillomavirus L1 virus-like particle vaccine clinical trial results. Vaccine 2008, 26, K53–K61. [Google Scholar] [CrossRef] [Green Version]
- Petrosky, E.; Bocchini Jr, J.A.; Hariri, S.; Chesson, H.; Curtis, C.R.; Saraiya, M.; Unger, E.R.; Markowitz, L.E. Use of 9-valent human papillomavirus (HPV) vaccine: Updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR 2015, 64, 300. [Google Scholar]
- Zhao, Q.; Potter, C.S.; Carragher, B.; Lander, G.; Sworen, J.; Towne, V.; Abraham, D.; Duncan, P.; Washabaugh, M.W.; Sitrin, R.D. Characterization of virus-like particles in GARDASIL® by cryo transmission electron microscopy. Hum. Vaccines Immunother. 2014, 10, 734–739. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Lai, H. Plant-derived virus-like particles as vaccines. Hum. Vaccines Immunother. 2013, 9, 26–49. [Google Scholar] [CrossRef]
- McKee, S.J.; Bergot, A.S.; Leggatt, G.R. Recent progress in vaccination against human papillomavirus-mediated cervical cancer. Rev. Med Virol. 2015, 25, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Biemelt, S.; Sonnewald, U.; Galmbacher, P.; Willmitzer, L.; Müller, M. Production of human papillomavirus type 16 virus-like particles in transgenic plants. J. Virol. 2003, 77, 9211–9220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginsburg, O.; Bray, F.; Coleman, M.P.; Vanderpuye, V.; Eniu, A.; Kotha, S.R.; Sarker, M.; Huong, T.T.; Allemani, C.; Dvaladze, A. The global burden of women’s cancers: A grand challenge in global health. Lancet 2017, 389, 847–860. [Google Scholar] [CrossRef]
- Bruni, L.; Diaz, M.; Barrionuevo-Rosas, L. Global estimates of human papillomavirus coverage by country and income level: A systematic review. Lancet Glob. Health 2016, 4, e453–e463. [Google Scholar] [CrossRef] [Green Version]
- Chido-Amajuoyi, O.G.; Domgue, J.F.; Obi-Jeff, C.; Schmeler, K.; Shete, S. A call for the introduction of gender-neutral HPV vaccination to national immunisation programmes in Africa. Lancet Glob. Health 2019, 7, e20–e21. [Google Scholar] [CrossRef] [Green Version]
- Rybicki, E.P. Plant-made vaccines for humans and animals. Plant Biotechnol. J. 2010, 8, 620–637. [Google Scholar] [CrossRef]
- Fischer, R.; Stoger, E.; Schillberg, S.; Christou, P.; Twyman, R.M. Plant-based production of biopharmaceuticals. Curr. Opin. Plant Biol. 2004, 7, 152–158. [Google Scholar] [CrossRef]
- Marsian, J.; Lomonossoff, G.P. Molecular pharming—VLPs made in plants. Curr. Opin. Plant Biol. 2016, 37, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Fischer, R.; Schillberg, S.; Hellwig, S.; Twyman, R.M.; Drossard, J. GMP issues for recombinant plant-derived pharmaceutical proteins. Biotechnol. Adv. 2012, 30, 434–439. [Google Scholar] [CrossRef]
- Zahin, M.; Joh, J.; Khanal, S.; Husk, A.; Mason, H.; Warzecha, H.; Ghim, S.-j.; Miller, D.M.; Matoba, N.; Jenson, A.B. Scalable production of HPV16 L1 protein and VLPs from tobacco leaves. PLoS ONE 2016, 11, e0160995. [Google Scholar] [CrossRef] [Green Version]
- De la Rosa, G.P.; Monroy-García, A.; de Lourdes Mora-García, M.; Peña, C.G.R.; Hernández-Montes, J.; Weiss-Steider, B.; Lim, M.A.G. An HPV 16 L1-based chimeric human papilloma virus-like particles containing a string of epitopes produced in plants is able to elicit humoral and cytotoxic T-cell activity in mice. Virol. J. 2009, 6, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-San Millán, A.; Ortigosa, S.M.; Hervás-Stubbs, S.; Corral-Martínez, P.; Seguí-Simarro, J.M.; Gaétan, J.; Coursaget, P.; Veramendi, J. Human papillomavirus L1 protein expressed in tobacco chloroplasts self-assembles into virus-like particles that are highly immunogenic. Plant Biotechnol. J. 2008, 6, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Maclean, J.; Koekemoer, M.; Olivier, A.; Stewart, D.; Hitzeroth, I.; Rademacher, T.; Fischer, R.; Williamson, A.-L.; Rybicki, E. Optimization of human papillomavirus type 16 (HPV-16) L1 expression in plants: Comparison of the suitability of different HPV-16 L1 gene variants and different cell-compartment localization. J. Gen. Virol. 2007, 88, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Varsani, A.; Williamson, A.-L.; Stewart, D.; Rybicki, E.P. Transient expression of Human papillomavirus type 16 L1 protein in Nicotiana benthamiana using an infectious tobamovirus vector. Virus Res. 2006, 120, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-L.; Li, W.-S.; Lei, T.; Zheng, J.; Zhang, Z.; Yan, X.-F.; Wang, Z.-Z.; Wang, Y.-L.; Si, L.-S. Expression of human papillomavirus type 16 L1 protein in transgenic tobacco plants. Acta Biochim. Biophys. Sin. 2005, 37, 153–158. [Google Scholar] [CrossRef]
- Varsani, A.; Williamson, A.-L.; Rose, R.; Jaffer, M.; Rybicki, E.P. Expression of Human papillomavirus type 16 major capsid protein in transgenic Nicotiana tabacum cv. Xanthi. Arch. Virol. 2003, 148, 1771–1786. [Google Scholar] [CrossRef]
- Kohl, T.O.; Hitzeroth, I.I.; Christensen, N.D.; Rybicki, E.P. Expression of HPV-11 L1 protein in transgenic Arabidopsis thaliana and Nicotiana tabacum. BMC Biotechnol. 2007, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Warzecha, H.; Mason, H.S.; Lane, C.; Tryggvesson, A.; Rybicki, E.; Williamson, A.-L.; Clements, J.D.; Rose, R.C. Oral immunogenicity of human papillomavirus-like particles expressed in potato. J. Virol. 2003, 77, 8702–8711. [Google Scholar] [CrossRef] [Green Version]
- van Zyl, A.R.; Saxena, P.; Lavoie, P.O.; D’Aoust, M.A.; Rybicki, E.P.; Hitzeroth, I. Expression and assembly of virus-like particles of Human Papillomavirus (HPV) in Nicotiana benthamiana. Manuscript in preparation.
- Shen, W.-J.; Forde, B.G. Efficient transformation of Agrobacterium spp. by high voltage electroporation. Nucleic Acids Res. 1989, 17, 8385. [Google Scholar]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989. [Google Scholar]
- Longet, S.; Schiller, J.T.; Bobst, M.; Jichlinski, P.; Nardelli-Haefliger, D. A murine genital-challenge model is a sensitive measure of protective antibodies against human papillomavirus infection. J. Virol. 2011, 85, 13253–13259. [Google Scholar] [CrossRef] [Green Version]
- Cangussu, A.S.R.; Mariúba, L.A.M.; Lalwani, P.; Pereira, K.D.E.; Astolphi-Filho, S.; Orlandi, P.P.; Epiphanio, S.; Viana, K.F.; Ribeiro, M.F.B.; Silva, H.M. A hybrid protein containing MSP1a repeats and Omp7, Omp8 and Omp9 epitopes protect immunized BALB/c mice against anaplasmosis. Vet. Res. 2018, 49, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vleeschauwer, A.R.; Zhou, X.; Lefebvre, D.J.; Garnier, A.; Watier, F.; Pignon, C.; Lacour, S.A.; Zientara, S.; Bakkali-Kassimi, L.; De Clercq, K. A canine adenovirus type 2 vaccine vector confers protection against foot-and-mouth disease in guinea pigs. Vaccine 2018, 36, 2193–2198. [Google Scholar] [CrossRef] [PubMed]
- Rybicki, E.; von Wechmar, M.; Burger, J. The “Lunchbox” Immunoabsorbent Technique. Available online: http://www.mcb.uct.ac.za/mcb/resources/molbio_techniques/lunchbox/ (accessed on 11 November 2020).
- Buck, C.B.; Pastrana, D.V.; Lowy, D.R.; Schiller, J.T. Generation of HPV pseudovirions using transfection and their use in neutralization assays. In Human Papillomaviruses; Springer: Berlin/Heidelberg, Germany, 2005; pp. 445–462. [Google Scholar]
- Christensen, N.D.; Dillner, J.; Eklund, C.; Carter, J.J.; Wipf, G.C.; Reed, C.A.; Cladel, N.M.; Galloway, D.A. Surface conformational and linear epitopes on HPV-16 and HPV-18 L1 virus-like particles as defined by monoclonal antibodies. Virology 1996, 223, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.F.; Brownlow, M.; Brown, M.; Kowalski, R.; Esser, M.T.; Ruiz, W.; Barr, E.; Brown, D.R.; Bryan, J.T. Antibodies from women immunized with Gardasil® cross-neutralize HPV 45 pseudovirions. Hum. Vaccines 2007, 3, 109–115. [Google Scholar] [CrossRef]
- Einstein, M.H.; Baron, M.; Levin, M.J.; Chatterjee, A.; Edwards, R.P.; Zepp, F.; Carletti, I.; Dessy, F.J.; Trofa, A.F.; Schuind, A. Comparison of the immunogenicity and safety of Cervarix™ and Gardasil® human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum. Vaccines 2009, 5, 705–719. [Google Scholar] [CrossRef] [Green Version]
- Veerapen, V.P.; Van Zyl, A.R.; Wigdorovitz, A.; Rybicki, E.P.; Meyers, A.E. Novel expression of immunogenic foot-and-mouth disease virus-like particles in Nicotiana benthamiana. Virus Res. 2018, 244, 213–217. [Google Scholar] [CrossRef]
- Sharp, J.M.; Doran, P.M. Characterization of monoclonal antibody fragments produced by plant cells. Biotechnol. Bioeng. 2001, 73, 338–346. [Google Scholar] [CrossRef] [Green Version]
- De Neve, M.; De Loose, M.; Jacobs, A.; Van Houdt, H.; Kaluza, B.; Weidle, U.; Van Montagu, M.; Depicker, A. Assembly of an antibody and its derived antibody fragment inNicotiana andArabidopsis. Transgenic Res. 1993, 2, 227–237. [Google Scholar] [CrossRef]
- Doran, P.M. Foreign protein degradation and instability in plants and plant tissue cultures. Trends Biotechnol. 2006, 24, 426–432. [Google Scholar] [CrossRef]
- Duwadi, K.; Chen, L.; Menassa, R.; Dhaubhadel, S. Identification, characterization and down-regulation of cysteine protease genes in tobacco for use in recombinant protein production. PLoS ONE 2015, 10, e0130556. [Google Scholar] [CrossRef] [Green Version]
- Hao, L.; Hsiang, T.; Goodwin, P. Role of two cysteine proteinases in the susceptible response of Nicotiana benthamiana to Colletotrichum destructivum and the hypersensitive response to Pseudomonas syringae pv. tomato. Plant Sci. 2006, 170, 1001–1009. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, S.Y.; Lim, S.J.; Kim, J.Y.; Lee, S.J.; Kim, H.-J. One-step chromatographic purification of human papillomavirus type 16 L1 protein from Saccharomyces cerevisiae. Protein Expr. Purif. 2010, 70, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Kirnbauer, R.; Taub, J.; Greenstone, H.; Roden, R.; Dürst, M.; Gissmann, L.; Lowy, D.R.; Schiller, J.T. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J. Virol. 1993, 67, 6929–6936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mach, H.; Volkin, D.B.; Troutman, R.D.; Wang, B.; Luo, Z.; Jansen, K.U.; Shi, L. Disassembly and reassembly of yeast-derived recombinant human papillomavirus virus-like particles (HPV VLPs). J. Pharm. Sci. 2006, 95, 2195–2206. [Google Scholar] [CrossRef] [PubMed]
- Aljabali, A.A.; Berardi, A.; Evans, D.J. Nature’s nanoparticles: Using viruses as nanomedicines and for bioimaging. In Fundamentals of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2018; pp. 29–50. [Google Scholar]
- Fifis, T.; Gamvrellis, A.; Crimeen-Irwin, B.; Pietersz, G.A.; Li, J.; Mottram, P.L.; McKenzie, I.F.; Plebanski, M. Size-dependent immunogenicity: Therapeutic and protective properties of nano-vaccines against tumors. J. Immunol. 2004, 173, 3148–3154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breitburd, F.; Kirnbauer, R.; Hubbert, N.L.; Nonnenmacher, B.; Trin-Dinh-Desmarquet, C.; Orth, G.; Schiller, J.T.; Lowy, D.R. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J. Virol. 1995, 69, 3959–3963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrero, R. Human Papillomavirus (HPV) Vaccines: Limited Cross-Protection against Additional HPV Types. J. Infect. Dis. 2009, 199, 919–922. [Google Scholar] [CrossRef]
- Bernard, H.U.; Burk, R.D.; Chen, Z.; van Doorslaer, K.; zur Hausen, H.; de Villiers, E.M. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 2010, 401, 70–79. [Google Scholar] [CrossRef] [Green Version]
- Burk, R.D.; Chen, Z.; Harari, A.; Smith, B.C.; Kocjan, B.J.; Maver, P.J.; Poljak, M. Classification and nomenclature system for human Alphapapillomavirus variants: General features, nucleotide landmarks and assignment of HPV6 and HPV11 isolates to variant lineages. Acta Dermatovenerol. Alp. Pannonica Adriat. 2011, 20, 113–123. [Google Scholar]





| Vaccine Group | Vaccine Name | HPV L1 Content | Adjuvant | # of Mice | L1 Dose µg/100 µL | Total L1 Dose µg/100 µL |
|---|---|---|---|---|---|---|
| Group 1 | Gardasil® | 6, 11, 16, and 18 | Amorphous aluminum hydroxyphosphate sulfate | 5 | HPV 6–4 µg HPV 11–8 µg HPV 16–8 µg HPV 18–4 µg | 24 µg (1/5th human dose) |
| Group 2 | Plant-made VLPs | 35, 52, and 58 | MONTANIDE™ ISA 50 V2 | 5 | HPV 35–2 µg HPV 52–2 µg HPV58–2 µg | 6 µg |
| Group 3 | Purified empty vector | - | MONTANIDE™ ISA 50 V2 | 5 | - | - |
| Neutralization Titre | |||||
|---|---|---|---|---|---|
| HPV 16 | HPV 18 | HPV 35 | HPV 52 | HPV 58 | |
| Gardasil® sera | ≤64,000 | <64,000 | - | - | - |
| H16V5 | 5000 | - | - | - | - |
| H18.J3 | - | 10,000 | - | - | - |
| Plant-produced VLPs sera | - | - | ≤256,000 | ≤256,000 | <256,000 |
| H35Q8 | - | - | 500 | - | - |
| H52D11 | - | - | - | 100 | - |
| H58J6.3 | - | - | - | - | 20 000 |
| Empty Vector | NN | NN | NN | NN | NN |
| PsVs only | NN | NN | NN | NN | NN |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naupu, P.N.; van Zyl, A.R.; Rybicki, E.P.; Hitzeroth, I.I. Immunogenicity of Plant-Produced Human Papillomavirus (HPV) Virus-Like Particles (VLPs). Vaccines 2020, 8, 740. https://doi.org/10.3390/vaccines8040740
Naupu PN, van Zyl AR, Rybicki EP, Hitzeroth II. Immunogenicity of Plant-Produced Human Papillomavirus (HPV) Virus-Like Particles (VLPs). Vaccines. 2020; 8(4):740. https://doi.org/10.3390/vaccines8040740
Chicago/Turabian StyleNaupu, Paulina N., Albertha R. van Zyl, Edward P. Rybicki, and Inga I. Hitzeroth. 2020. "Immunogenicity of Plant-Produced Human Papillomavirus (HPV) Virus-Like Particles (VLPs)" Vaccines 8, no. 4: 740. https://doi.org/10.3390/vaccines8040740







