Comparative Immunological Study in Mice of Inactivated Influenza Vaccines Used in the Russian Immunization Program
Abstract
:1. Introduction
2. Materials and Methods Used
2.1. Inactivated Split-Virion Influenza Vaccines (IIVs)
- U3—Ultrix® (IIV3, FORT LLC, Russia);
- U4—Ultrix® Quadri (IIV4, FORT LLC, Russia);
- SGF—SOVIGRIPP® (IIV3, FORT LLC, Russia);
- SGU—SOVIGRIPP® (IIV3, NPO Microgen JSC, Russia);
- VG—VAXIGRIP® (IIV3, Sanofi Pasteur C.A., France).
- Influenza A virus (H1N1): A/Brisbane/02/2018 (H1N1)pdm09-like virus (A/Brisbane/02/2018, IVR-190);
- Influenza A virus (H3N2): A/Kansas/14/2017 (H3N2)-like virus (A/Kansas/14/2017, NYMC X-327);
- Influenza B virus (Victoria lineage): B/Colorado/06/2017-like strain (B/Maryland/15/2016, NYMC BX-69A).
- Influenza B virus (Yamagata lineage): B/Phuket/3073/2013-like virus (B/Phuket/3073/2013 wild type).
2.2. Viruses and Antigens, Antisera
2.3. Study Design, Immunization, and Challenge of Mice
2.4. Replication of Influenza Viruses in Lungs
2.5. SRD Assay
2.6. HAI Assay
2.7. MN Assay
2.8. ELISA
2.9. Neuraminidase Isolation
2.10. Dot Blotting
2.11. Statistics
3. Results
3.1. Determining Vaccine-Specific Activity in SRD
3.2. Determining Immune Response in HAI Assay
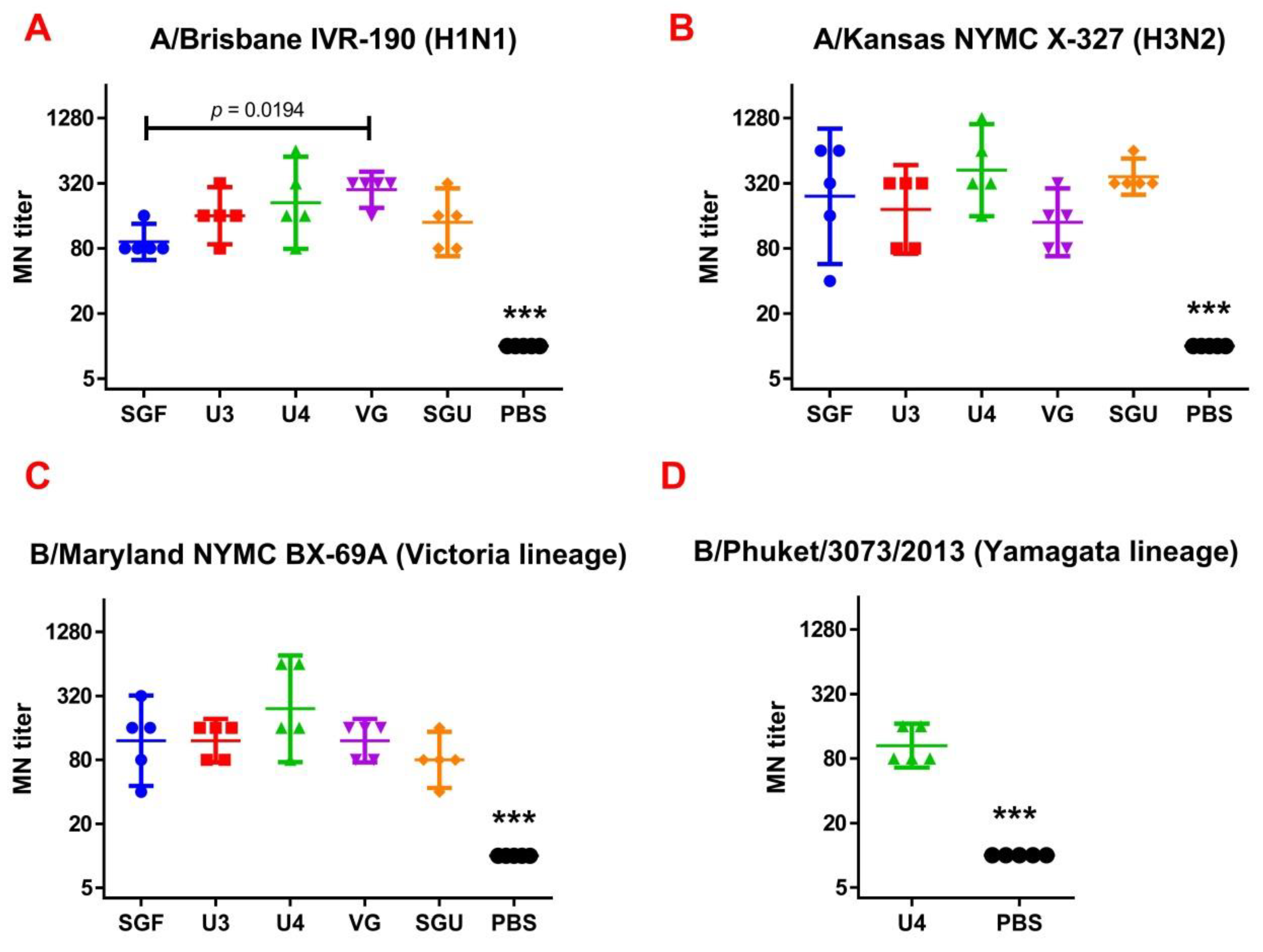
3.3. Determining Immune Response in MN Assay
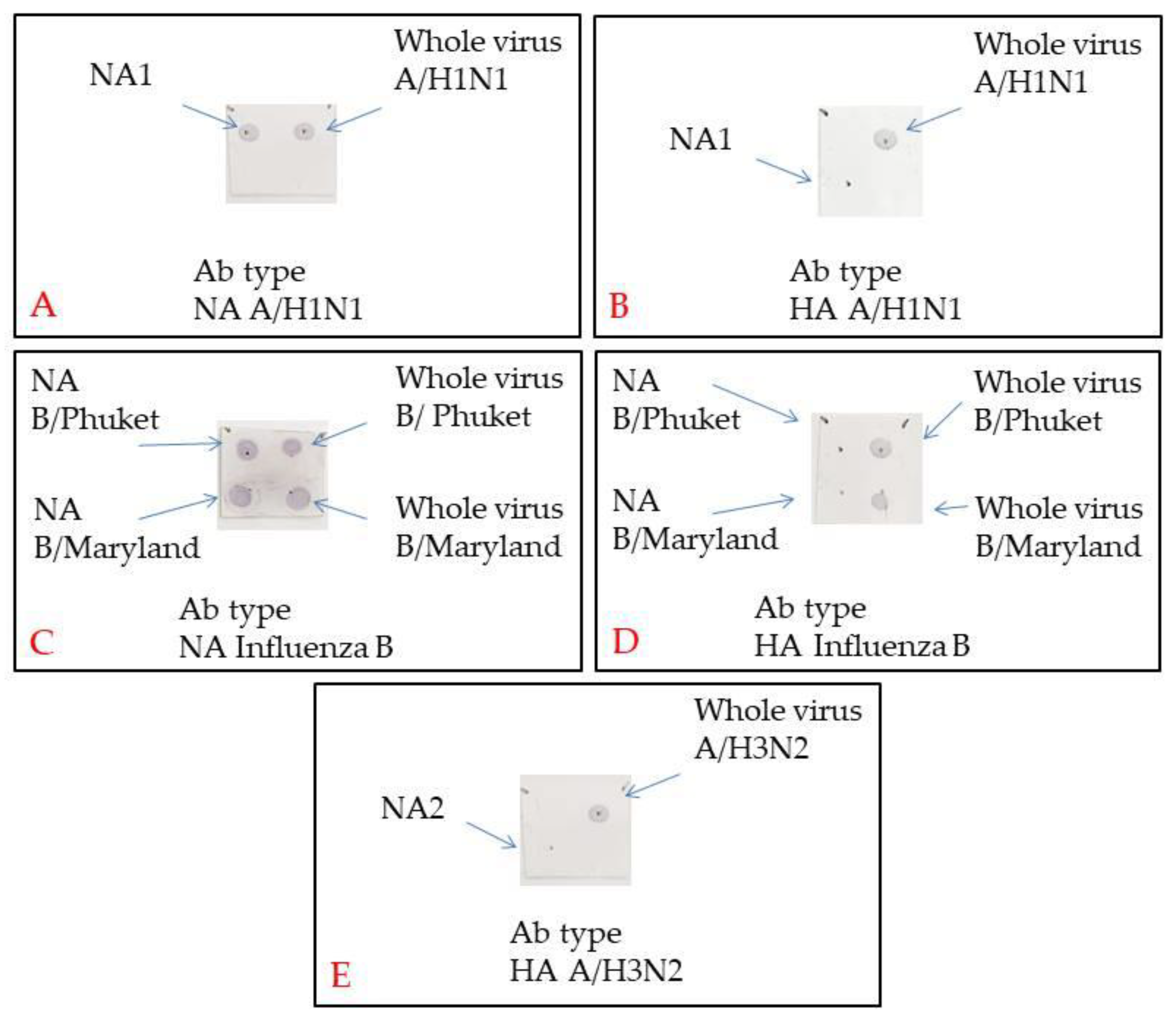
3.4. Neuraminidase Isolation
3.5. Determining NA Immune Response in ELISA
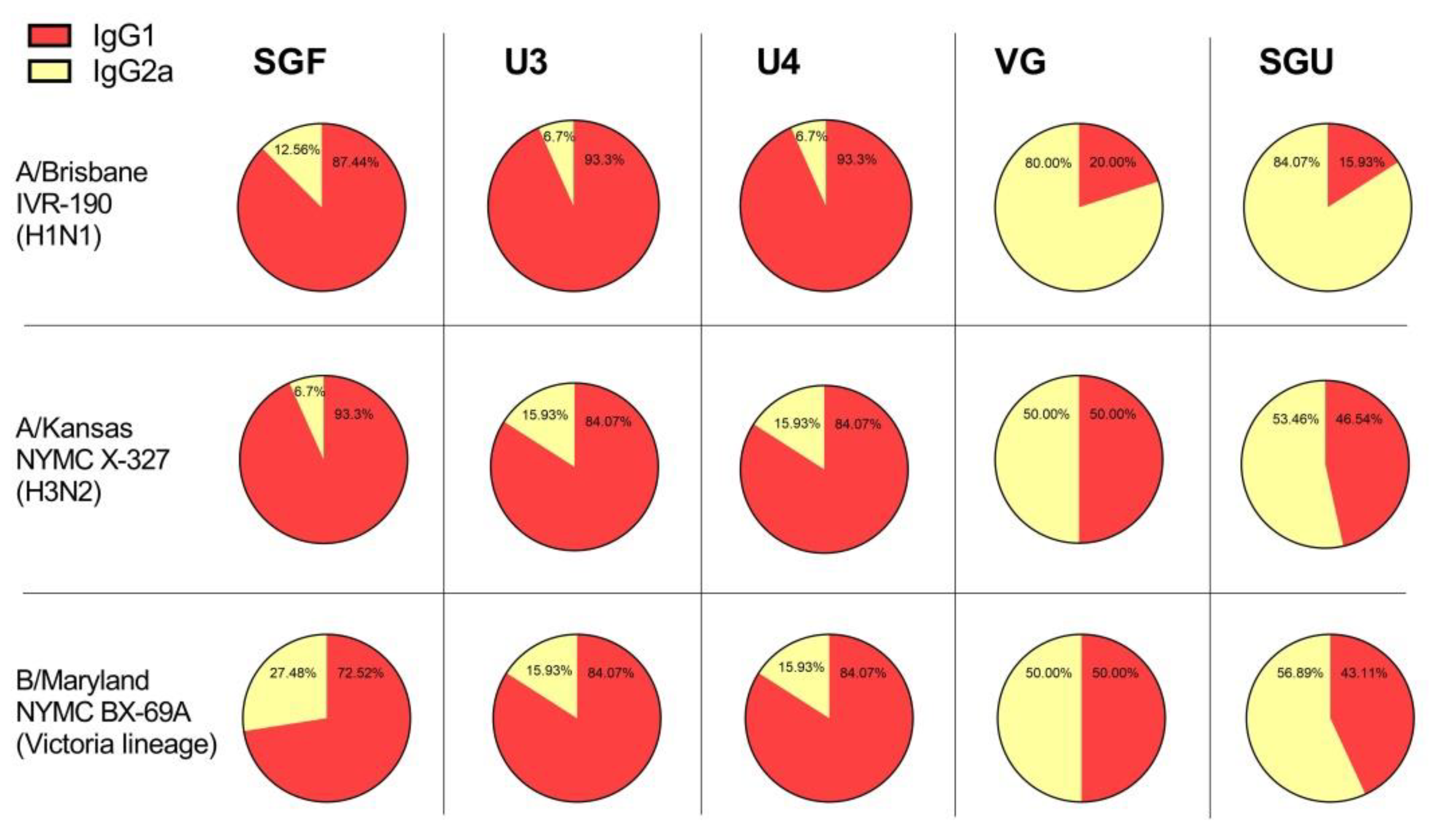
3.6. Determining IgG and Specific IgG1 and IgG2a Subclasses in ELISA after Second Immunization
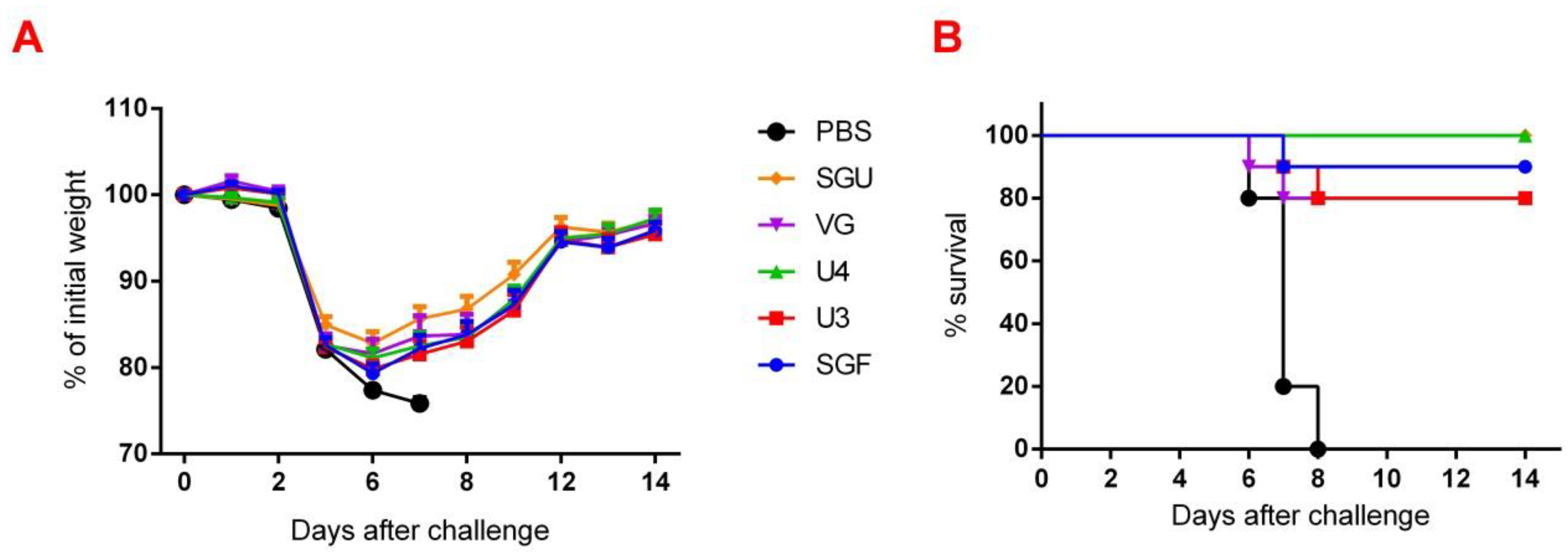
3.7. Estimating the IIV Protective Effect on a Lethal IV Infection Model
3.8. Estimating the IIV Protective Effect in IV Reproduction in the Lungs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Grohskopf, L.A.; Alyanak, E.; Broder, K.R.; Walter, E.B.; Fry, A.M.; Jernigan, D.B. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2019–2020 Influenza Season. MMWR. Recomm. Rep. 2019, 68, 1–21. [Google Scholar]
- World Health Organization. Influenza (Seasonal) Fact Sheet. Available online: https://www.who.int/newsroom/fact-sheets/detail/influenza-(seasonal) (accessed on 6 November 2018).
- Chen, J.-R.; Liu, Y.-M.; Tseng, Y.-C.; Ma, C. Better influenza vaccines: An industry perspective. J. Biomed. Sci. 2020, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- WHO Global Influenza Surveillance Network. Manual for the Laboratory Diagnosis and Virological Surveillance of Influenza; World Health Organization: Geneva, Switzerland, 2011; ISBN 978 9241548090:43-62. Available online: http://whqlibdoc.who.int/publications/2011/9789241548090_eng.pdf (accessed on 6 November 2018).
- EMA. Guideline on influenza vaccines. In Non-Clinical and Clinical Module; EMA/CHMP/VWP/457259/2014; European Medicines Agency: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Trombetta, C.M.; Montomoli, E. Influenza immunology evaluation and correlates of protection: A focus on vaccines. Expert Rev. Vaccines 2016, 15, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Eichelberger, M.C.; Monto, A.S. Neuraminidase, the Forgotten Surface Antigen, Emerges as an Influenza Vaccine Target for Broadened Protection. J. Infect. Dis. 2019, 219, S75–S80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shtyrya, Y.A.; Mochalova, L.V.; Bovin, N.V. Influenza virus neuraminidase: Structure and function. Acta Naturae 2009, 1, 26–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krammer, F.; Fouchier, R.A.; Eichelberger, M.C.; Webby, R.J.; Shaw-Saliba, K.; Wan, H.; Wilson, P.C.; Compans, R.W.; Skountzou, I.; Monto, A.S. NAction! How Can Neuraminidase-Based Immunity Contribute to Better Influenza Virus Vaccines? mBio 2018, 9, e02332-17. [Google Scholar] [CrossRef] [Green Version]
- Giurgea, L.T.; Morens, D.M.; Taubenberger, J.K.; Memoli, M.J. Influenza Neuraminidase: A Neglected Protein and Its Potential for a Better Influenza Vaccine. Vaccines 2020, 8, 409. [Google Scholar] [CrossRef]
- Wohlbold, T.J.; Nachbagauer, R.; Xu, H.; Tan, G.S.; Hirsh, A.; Brokstad, K.A.; Cox, R.J.; Palese, P.; Krammer, F. Vaccination with adjuvanted recombinant neuraminidase induces broad heterologous, but not heterosubtypic, cross-protection against influenza virus infection in mice. mBio 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.E.; Sun, X.; Bai, Y.; Liu, Y.V.; Massare, M.J.; Pearce, M.B.; Belser, J.A.; Maines, T.R.; Creager, H.M.; Glenn, G.M.; et al. Neuraminidase-based recombinant virus-like particles protect against lethal avian influenza A(H5N1) virus infection in ferrets. Virology 2017, 509, 90–97. [Google Scholar] [CrossRef]
- Monto, A.S.; Kendal, A.P. Effect of neuraminidase antibody on Hong Kong influenza. Lancet 1973, 623–625. [Google Scholar] [CrossRef] [Green Version]
- Spellberg, B.; Edwards, J.E., Jr. Type 1/Type 2 immunity in infectious diseases. Clin. Infect. Dis. 2001, 32, 76–102. [Google Scholar] [CrossRef] [PubMed]
- Delves, P.J.; Roitt, I.M. The immune system. First of two parts. N. Engl. J. Med. 2000, 343, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Delves, P.J.; Roitt, I.M. The immune system. Second of two parts. N. Engl. J. Med. 2000, 343, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, L.A.; Kotlyarov, R.Y.; Shuklina, M.A.; Blochina, E.A.; Sergeeva, M.V.; Potapchuk, M.V.; Kovaleva, A.A.; Ravin, N.V.; Tsybalova, L.M. Influence of the Linking Order of Fragments of HA2 and M2e of the influenza A Virus to Flagellin on the Properties of Recombinant Proteins. Acta Nat. 2018, 10, 85–94. [Google Scholar] [CrossRef]
- Stepanova, L.A.; Kotlyarov, R.Y.; Kovaleva, A.A.; Potapchuk, M.V.; Korotkov, A.V.; Sergeeva, M.V.; Kasianenko, M.A.; Kuprianov, V.V.; Ravin, N.V.; Tsybalova, L.M.; et al. Protection against multiple influenza a virus strains induced by candidate recombinant vaccine based on heterologous M2e peptides linked to flagellin. PLoS ONE 2015, 10, e0119520. [Google Scholar] [CrossRef]
- Stöhr, K.; Bucher, D.; Colgate, T.; Wood, J. Influenza virus surveillance, vaccine strain selection, and manufacture. Methods Mol. Biol. 2012, 865, 147–162. [Google Scholar]
- Sicca, F.; Martinuzzi, D.; Montomoli, E.; Huckriede, A. Comparison of influenza-specific neutralizing antibody titers determined using different assay readouts and hemagglutination inhibition titers: Good correlation but poor agreement. Vaccine 2020, 38, 2527–2541. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Candiano, G.; Bruschi, M.; Musante, L.; Santucci, L.; Ghiggeri, G.M.; Carnemolla, B.; Orecchia, P.; Zardi, L.; Righetti, P.G. Blue silver: A very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis 2004, 25, 1327–1333. [Google Scholar] [CrossRef]
- Timoshicheva, T.A.; Zabrodskaya, Y.A.; Ramsay, E.; Amosova, I.V. Use of hexon as an antigen for the production of monoclonal antibodies capable of detecting multiple adenovirus types. Biologicals 2019, 58, 44–49. [Google Scholar] [CrossRef]
- Groves, H.T.; McDonald, J.U.; Pinky, L.; Ekaterina, K.; Paul, K.; John, M.; Joanna, E.; Catherine, T.; Ruth, E.; Lauren, P.; et al. Mouse Models of Influenza Infection with Circulating Strains to Test Seasonal Vaccine Efficacy. Front. Immunol. 2018, 9, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monto, A.S.; Petrie, J.G.; Cross, R.T.; Johnson, E.; Liu, M.; Zhong, W.; Levine, M.; Katz, J.M.; Ohmit, S.E. Antibody to Influenza Virus Neuraminidase: An Independent Correlate of Protection. J. Infect. Dis. 2015, 212, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Marcelin, G.; Sandbulte, M.R.; Webby, R.J. Contribution of antibody production against neuraminidase to the protection afforded by influenza vaccines. Rev. Med. Virol. 2012, 22, 267–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagadesh, A.; Salam, A.A.; Mudgal, P.P.; Arunkumar, G. Influenza virus neuraminidase (NA): A target for antivirals and vaccines. Arch. Virol. 2016, 161, 2087–2094. [Google Scholar] [CrossRef]
- Chen, J.; Fang, F.; Li, X.; Chang, H.; Chen, Z. Protection against influenza virus infection in BALB/c mice immunised with a single dose of neuraminidase-expressing DNAs by electroporation. Vaccine 2005, 23, 4322–4328. [Google Scholar] [CrossRef]
- Job, E.R.; Schotsaert, M.; Ibañez, L.I.; Smet, A.; Ysenbaert, T.; Roose, K.; Dai, M.; de Haan, C.A.M.; Kleanthous, H.; Vogel, T.U.; et al. Antibodies Directed toward Neuraminidase N1 Control Disease in a Mouse Model of Influenza. J. Virol. 2018, 92, e01584-17. [Google Scholar] [CrossRef] [Green Version]
- Couch, R.B.; Atmar, R.L.; Keitel, W.A.; Quarles, J.M.; Wells, J.; Arden, N.; Niño, D. Randomized comparative study of the serum antihemagglutinin and antineuraminidase antibody responses to six licensed trivalent influenza vaccines. Vaccine 2012, 31, 190–195. [Google Scholar] [CrossRef] [Green Version]
- Westgeest, K.B.; Bestebroer, T.M.; Spronken, M.I.; Gao, J.; Couzens, L.; Osterhaus, A.D.; Eichelberger, M.; Fouchier, R.A.; de Graaf, M. Optimization of an enzyme-linked lectin assay suitable for rapid antigenic characterization of the neuraminidase of human influenza A(H3N2) viruses. J. Virol. Methods 2015, 217, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Cate, T.R.; Rayford, Y.; Niño, D.; Winokur, P.; Brady, R.; Belshe, R.; Chen, W.; Atmar, R.L.; Couch, R.B. A high dosage influenza vaccine induced significantly more neuraminidase antibody than standard vaccine among elderly subjects. Vaccine 2010, 28, 2076–2079. [Google Scholar] [CrossRef] [Green Version]
- Hocart, M.J.; Mackenzie, J.S.; Stewart, G.A. The Immunoglobulin G Subclass Responses of Mice to Influenza A Virus: The Effect of Mouse Strain, and the Neutralizing Abilities of Individual Protein A-purified Subclass Antibodies. J. Gen. Virol. 1989, 70, 2439–2448. [Google Scholar] [CrossRef]
- Moran, T.M.; Park, H.; Fernandez-Sesma, A.; Schulman, J.L. Th2 Responses to Inactivated Influenza Virus Can Be Converted to Th1 Responses and Facilitate Recovery from Heterosubtypic Virus Infection. J. Infect. Dis. 1999, 180, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Hovden, A.O.; Cox, R.J.; Haaheim, L.R. Whole influenza virus vaccine is more immunogenic than split influenza virus vaccine and induces primarily an IgG2a response in BALB/c mice. Scand. J. Immunol. 2005, 62, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.J.; Hovden, A.O.; Brokstad, K.A.; Szyszko, E.; Madhun, A.S.; Haaheim, L.R. The humoral immune response and protective efficacy of vaccination with inactivated split and whole influenza virus vaccines in BALB/c mice. Vaccine 2006, 24, 6585–6587. [Google Scholar] [CrossRef] [PubMed]
- Hauge, S.; Madhun, A.S.; Cox, R.J.; Brokstad, K.A.; Haaheim, L.R. A comparison of the humoral and cellular immune responses at different immunological sites after split influenza virus vaccination of mice. Scand. J. Immunol. 2007, 65, 14–21. [Google Scholar] [CrossRef]
- Baz, M.; Samant, M.; Zekki, H.; Tribout-Jover, P.; Plante, M.; Lanteigne, A.M.; Hamelin, M.E.; Mallett, C.; Papadopoulou, B.; Boivin, G. Effects of Different Adjuvants in the Context of Intramuscular and Intranasal Routes on Humoral and Cellular Immune Responses Induced by Detergent-Split A/H3N2 Influenza Vaccines in Mice. Clin. Vaccine Immunol. 2012, 19, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Huber, V.C.; McKeon, R.M.; Brackin, M.N.; Miller, L.A.; Keating, R.; Brown, S.A.; Makarova, N.; Perez, D.R.; MacDonald, G.H.; McCullers, J.A. Distinct contributions of vaccine-induced immunoglobulin G1 (IgG1) and IgG2a antibodies to protective immunity against influenza. Clin. Vacc. Immunol. 2006, 13, 981–990. [Google Scholar] [CrossRef] [Green Version]
- Boravleva, E.Y.; Lunitsin, A.V.; Kaplun, A.P.; Bykova, N.V.; Krasilnikov, I.V.; Gambaryan, A.S. Immune Response and Protective Efficacy of Inactivated and Live Influenza Vaccines Against Homologous and Heterosubtypic Challenge. Biochemistry 2020, 85, 553–566. [Google Scholar] [CrossRef]
- Song, L.; Xiong, D.; Hu, M.; Kang, X.; Pan, Z.; Jiao, X. Immunopotentiation of Different Adjuvants on Humoral and Cellular Immune Responses Induced by HA1-2 Subunit Vaccines of H7N9 Influenza in Mice. PLoS ONE 2016, 11, e0150678. [Google Scholar] [CrossRef]
- Smith, L.R.; Wodal, W.; Crowe, B.A.; Kerschbaum, A.; Bruehl, P.; Schwendinger, M.G.; Savidis-Dacho, H.; Sullivan, S.M.; Shlapobersky, M.; Hartikka, J.; et al. Preclinical evaluation of Vaxfectin®-adjuvanted Vero cell-derived seasonal split and pandemic whole virus influenza vaccines. Hum. Vaccines Immunother. 2013, 9, 1333–1345. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, N.; Beerli, R.R.; Bauer, M.; Jegerlehner, A.; Dietmeier, K.; Maudrich, M.; Pumpens, P.; Saudan, P.; Bachmann, M.F. Universal vaccine against influenza virus: Linking TLR signaling to anti-viral protection. Eur. J. Immunol. 2012, 42, 863–869. [Google Scholar] [CrossRef]
- Tarasov, M.; Shanko, A.; Kordyukova, L.; Katlinski, A. Characterization of Inactivated Influenza Vaccines Used in the Russian National Immunization Program. Vaccines 2020, 8, 488. [Google Scholar] [CrossRef] [PubMed]




| A(H1N1) Normative/SRD | A(H3N2) Normative/SRD | B/Victoria Normative/SRD | B/Yamagata Normative/SRD | |||||
|---|---|---|---|---|---|---|---|---|
| U3 | 15.2 | 17.9 | 15.1 | 14.6 | 13.4 | 13.5 | - | - |
| U4 | 15.5 | 16.0 | 15.6 | 16.8 | 15.7 | 14.9 | 15.2 | 15.9 |
| VG | 15 | 31.3 | 15 | 19.0 | 15 | 18.2 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by FORT LLC. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shanko, A.; Shuklina, M.; Kovaleva, A.; Zabrodskaya, Y.; Vidyaeva, I.; Shaldzhyan, A.; Fadeev, A.; Korotkov, A.; Zaitceva, M.; Stepanova, L.; et al. Comparative Immunological Study in Mice of Inactivated Influenza Vaccines Used in the Russian Immunization Program. Vaccines 2020, 8, 756. https://doi.org/10.3390/vaccines8040756
Shanko A, Shuklina M, Kovaleva A, Zabrodskaya Y, Vidyaeva I, Shaldzhyan A, Fadeev A, Korotkov A, Zaitceva M, Stepanova L, et al. Comparative Immunological Study in Mice of Inactivated Influenza Vaccines Used in the Russian Immunization Program. Vaccines. 2020; 8(4):756. https://doi.org/10.3390/vaccines8040756
Chicago/Turabian StyleShanko, Andrei, Marina Shuklina, Anna Kovaleva, Yana Zabrodskaya, Inna Vidyaeva, Aram Shaldzhyan, Artem Fadeev, Alexander Korotkov, Marina Zaitceva, Liudmila Stepanova, and et al. 2020. "Comparative Immunological Study in Mice of Inactivated Influenza Vaccines Used in the Russian Immunization Program" Vaccines 8, no. 4: 756. https://doi.org/10.3390/vaccines8040756
APA StyleShanko, A., Shuklina, M., Kovaleva, A., Zabrodskaya, Y., Vidyaeva, I., Shaldzhyan, A., Fadeev, A., Korotkov, A., Zaitceva, M., Stepanova, L., Tsybalova, L., Kordyukova, L., & Katlinski, A. (2020). Comparative Immunological Study in Mice of Inactivated Influenza Vaccines Used in the Russian Immunization Program. Vaccines, 8(4), 756. https://doi.org/10.3390/vaccines8040756







