Plant-Based Drugs and Vaccines for COVID-19
Abstract
1. Introduction
2. The COVID-19 Pandemic
3. Vaccines and Antibodies under Clinical Trials
4. Potential Drug Therapies Against COVID-19
4.1. Remdesivir
4.2. Favipiravir
4.3. Lopinavir/Ritonavir
4.4. Convalescent Plasma (CP) Therapy
5. Herbal Therapies Against COVID-19
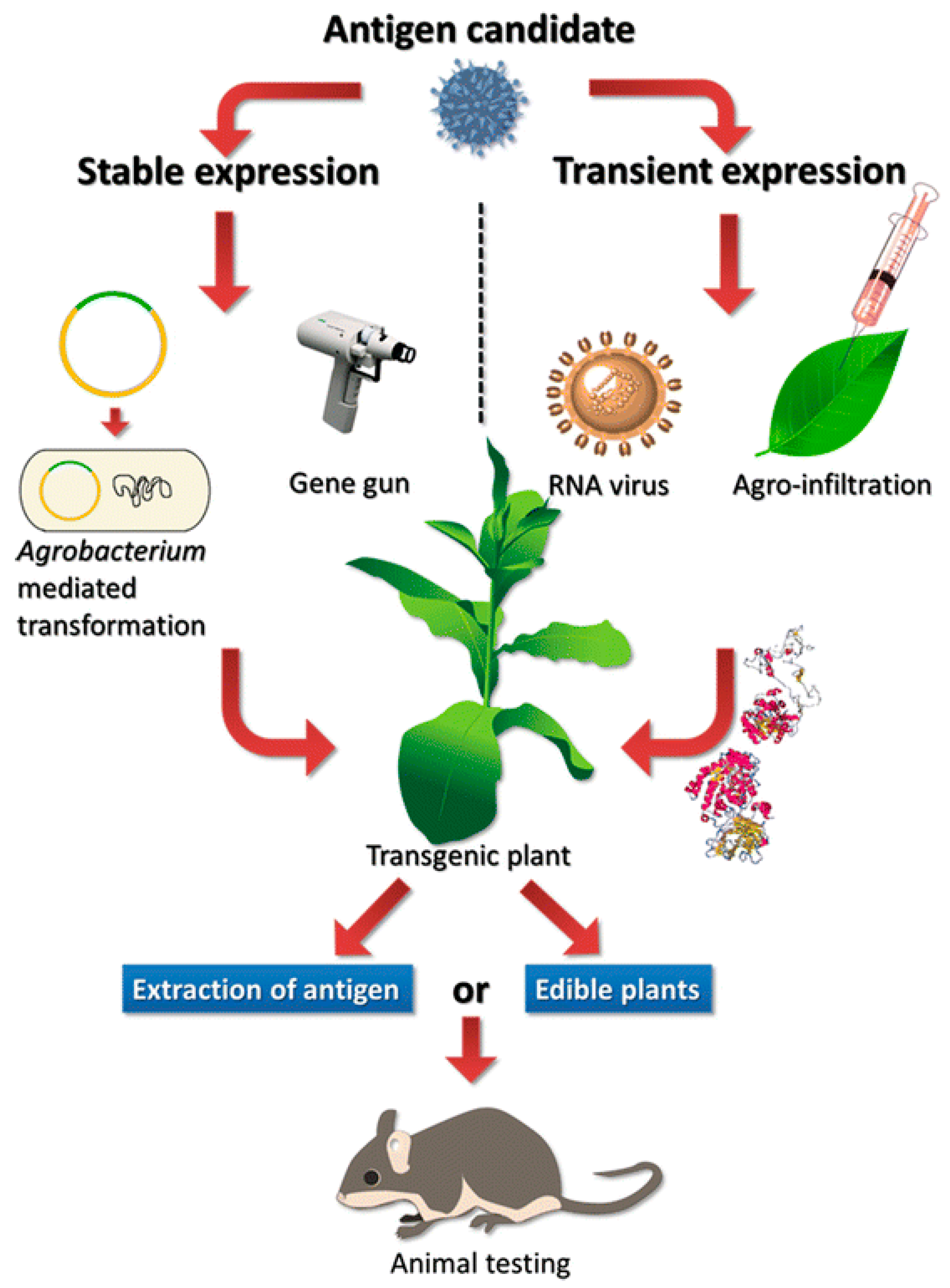
6. Plant Molecular Pharming to Combat COVID-19
Funding
Conflicts of Interest
References
- Dent, S.D.; Xia, D.; Wastling, J.M.; Neuman, B.W.; Britton, P.; Maier, H.J. The proteome of the infectious bronchitis virus Beau-R virion. J. Gen. Virol. 2015, 96, 3499–3506. [Google Scholar] [CrossRef]
- Masters, P.S. The Molecular Biology of Coronaviruses. Adv. Appl. Microbiol. 2006, 66, 193–292. [Google Scholar] [CrossRef]
- Ksiazek, T.G.; Erdman, D.; Goldsmith, C.S.; Zaki, S.R.; Peret, T.; Emery, S.; Tong, S.; Urbani, C.; Comer, J.A.; Lim, W.; et al. A Novel Coronavirus Associated with Severe Acute Respiratory Syndrome. N. Engl. J. Med. 2003, 348, 1953–1966. [Google Scholar] [CrossRef] [PubMed]
- Sahin, A.R.; Erdogan, A.; Agaoglu, P.M.; Dineri, Y.; Cakirci, A.Y.; Senel, M.E.; Okyay, R.A.; Tasdogan, A.M. 2019 Novel Coronavirus (COVID-19) Outbreak: A Review of the Current Literature. Eurasian J. Med. Oncol. 2020, 4, 1–7. [Google Scholar] [CrossRef]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Methods Mol. Biol. 2015, 1282, 1–23. [Google Scholar] [PubMed]
- Woo, P.C.; Lau, S.K.; Lam, C.S.; Lau, C.C.; Tsang, A.K.; Lau, J.H.; Bai, R.; Teng, J.L.; Tsang, C.C.; Wang, M.; et al. Discovery of Seven Novel Mammalian and Avian Coronaviruses in the Genus Deltacoronavirus Supports Bat Coronaviruses as the Gene Source of Alphacoronavirus and Betacoronavirus and Avian Coronaviruses as the Gene Source of Gammacoronavirus and Deltacoronavirus. J. Virol. 2012, 86, 3995–4008. [Google Scholar] [CrossRef]
- Kahn, J.S.; McIntosh, K. History and Recent Advances in Coronavirus Discovery. Pediatr. Infect. Dis. J. 2005, 24, S223–S227. [Google Scholar] [CrossRef]
- Geller, C.; Varbanov, M.; Duval, R.E. Human Coronaviruses: Insights into Environmental Resistance and Its Influence on the Development of New Antiseptic Strategies. Viruses 2012, 4, 3044–3068. [Google Scholar] [CrossRef]
- Drosten, C.; Günther, S.; Preiser, W.; Van Der Werf, S.; Brodt, H.R.; Becker, S.; Rabenau, H.; Panning, M.; Kolesnikova, L.; Fouchier, R.A.M.; et al. Identification of a Novel Coronavirus in Patients with Severe Acute Respiratory Syndrome. N. Engl. J. Med. 2003, 348, 1967–1976. [Google Scholar] [CrossRef]
- Zaki, A.; Van Boheemen, S.; Bestebroer, T.; Osterhaus, A.; Fouchier, R. Isolation of a Novel Coronavirus from a Man with Pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef]
- Tan, W.; Zhao, X.; Ma, X.; Wang, W.; Niu, P.; Xu, W.; Gao, G.F.; Wu, G. A Novel Coronavirus Genome Identified in a Cluster of Pneumonia CasesWuhan, China 2019−2020. China CDC Wkly. 2020, 2, 61–62. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Muth, D.; Niemeyer, D.; Drosten, C. Hosts and Sources of Endemic Human Coronaviruses. Adv. Appl. Microbiol. 2018, 100, 163–188. [Google Scholar] [CrossRef]
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Rajnik, M.; Cuomo, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation and Treatment Coronavirus (COVID-19). StatPearls Publishing. 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554776/ (accessed on 24 December 2020).
- WHO. Novel Coronavirus—Japan (ex-China). 2020. Available online: https://www.who.int/csr/don/17-january-2020-novel-coronavirus-japan-ex-china/en/ (accessed on 24 December 2020).
- CDC. First Travel-Related Case of 2019 Novel Coronavirus Detected in United States. Available online: https://www.cdc.gov/media/releases/2020/p0121-novel-coronavirus-travel-case.html (accessed on 23 January 2020).
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Bei Li, B.; Huang, C.-L.; et al. Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. BioRxiv 2020. [Google Scholar] [CrossRef]
- Xu, X.; Wang, P.; Feng, J.; Zhou, H.; Li, X.; Zhong, W.; Hao, P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020, 63, 457–460. [Google Scholar]
- Hu, D.; Zhu, C.; Ai, L.; He, T.; Wang, Y.; Ye, F.; Yang, L.; Ding, C.; Zhu, X.; Lv, R.; et al. Genomic characterization and infectivity of a novel SARS-like coronavirus in Chinese bats. Emerg. Microbes Infect. 2018, 7, 154. [Google Scholar] [CrossRef]
- Wang, C.; Horby, P.W.; Hayden, F.G.; Gao, G.F. A novel coronavirus outbreak of global health concern. Lancet 2020, 395, 470–473. [Google Scholar] [CrossRef]
- Munster, V.J.; Koopmans, M.; Van Doremalen, N.; Van Riel, D.; De Wit, E. A Novel Coronavirus Emerging in China—Key Questions for Impact Assessment. N. Engl. J. Med. 2020, 382, 692–694. [Google Scholar] [CrossRef]
- Paules, C.I.; Marston, H.D.; Fauci, A.S. Coronavirus Infections—More Than Just the Common Cold. JAMA 2020, 323, 707. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Fac. Opin. Post Publ. Peer Rev. Biomed. Lit. 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Chan, J.F.-W.; Yuan, S.; Kok, K.-H.; To, K.K.-W.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.-Y.; Poon, R.W.-S.; et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef]
- Ghebreyesus, T.A. WHO–Director-General’s Opening Remarks at the Media Briefing on COVID-19. 2020. Available online: www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-COVID-19---11-march-2020 (accessed on 24 December 2020).
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, function and antigenicity of the SARS-CoV-2 spike glycoprotein. BioRxiv 2020, 2, 956581. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J. Virol. 2020, 94, e00127-20. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yi, Y.; Luo, X.; Xiong, N.; Liu, Y.; Li, S.; Sun, R.; Wang, Y.; Hu, B.; Chen, W.; et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med. Virol. 2020, 92, 1518–1524. [Google Scholar] [CrossRef]
- Chan, S.K.; Du, P.; Ignacio, C.; Mehta, S.; Newton, I.G.; Steinmetz, N.F. Biomimetic Virus-Like Particles as Severe Acute Respiratory Syndrome Coronavirus 2 Diagnostic Tools. ACS Nano 2020. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Wei, M.; Yuan, J.; Liu, Y.; Fu, T.; Yu, X.; Zhang, Z.-J. Novel Coronavirus Infection in Hospitalized Infants Under 1 Year of Age in China. JAMA 2020, 323, 1313. [Google Scholar] [CrossRef]
- Huang, F.; Li, Y.; Leung, E.L.-H.; Liu, X.; Liu, K.; Wang, Q.; Lan, Y.; Li, X.; Yu, H.; Cui, L.; et al. A review of therapeutic agents and Chinese herbal medicines against SARS-COV-2 (COVID-19). Pharmacol. Res. 2020, 158, 104929. [Google Scholar] [CrossRef] [PubMed]
- Phan, L.T.; Nguyen, T.V.; Luong, Q.C.; Nguyen, T.V.; Nguyen, H.T.; Le, H.Q.; Nguyen, T.T.; Cao, T.M.; Pham, Q.D. Importation and Human-to-Human Transmission of a Novel Coronavirus in Vietnam. N. Engl. J. Med. 2020, 382, 872–874. [Google Scholar] [CrossRef] [PubMed]
- Bouadma, L.; Lescure, F.-X.; Lucet, J.-C.; Yazdanpanah, Y.; Timsit, J.-F. Severe SARS-CoV-2 infections: Practical considerations and management strategy for intensivists. Intensiv. Care Med. 2020, 46, 579–582. [Google Scholar] [CrossRef] [PubMed]
- FDA. Coronavirus Treatment Acceleration Program (CTAP). Available online: https://www.fda.gov/drugs/coronavirus-COVID-19-drugs/coronavirus-treatment-acceleration-program-ctap (accessed on 20 August 2020).
- Clover Produces Subunit Vaccine Candidate S-Trimer for Coronavirus. Available online: https://www.bioworld.com/articles/433055-clover-produc-es-subunit-vaccine-candidate-s-trimer-for-coronavirus (accessed on 20 December 2020).
- Chinese COVID-19 Vaccine Set for Human Trials in Australia. Available online: http://www.xinhuanet.com/english/2020-04/28/c_139014641.htm (accessed on 20 December 2020).
- Oxford University’s Covid-19 Vaccine Shows Promise in Animal Study. Available online: https://www.pharmaceutical-technology.com/news/oxford-university-covid-19-vaccine-monkey-data/ (accessed on 20 December 2020).
- Everything you Need to Know about the Oxford University Vaccine and Other Efforts to Beat Coronavirus. Available online: https://www.marketwatch.com/story/everything-you-need-to-know-about-the-oxford-university-vaccine-and-other-efforts-to-beat-coronavirus-2020-05-14 (accessed on 20 December 2020).
- NIH Clinical Trial of Investigational Vaccine for COVID-19 Begins. Available online: https://www.nih.gov/news-events/news-releases/nih-clinical-trial-investigational-vaccine-covid-19-begins (accessed on 20 December 2020).
- A Clinical Trial of a Plasmid DNA Vaccine for COVID-19 [Covigenix VAX-001] in Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT04591184 (accessed on 20 December 2020).
- Potential Therapeutic Options for COVID-19: Current Status, Challenges, and Future Perspectives. Available online: https://www.frontiersin.org/articles/10.3389/fphar.2020.572870/full (accessed on 20 December 2020).
- Roivant Doses First Patient in Pivotal BREATHE Clinical Trial Evaluating Gimsilumab in COVID-19 Patients for the Prevention and Treatment of Acute Respiratory Distress Syndrome. Available online: https://roivant.com/roivant-doses-first-patient-in-pivotal-breathe-clinical-trial-evaluating-gimsilumab-in-covid-19-patients.html (accessed on 20 December 2020).
- Comirnaty (BNT162b2) Vaccine. Available online: https://www.precisionvaccinations.com/vaccines/bnt162-sars-cov-2-vaccine (accessed on 20 December 2020).
- AdCOVID SARS-CoV-2 Vaccine. Available online: https://www.precisionvaccinations.com/vaccines/adcovid-sars-cov-2-vaccine (accessed on 20 December 2020).
- AdCOVID SARS-CoV-2 Vaccine. Available online: https://onlinelibrary.wiley.com/doi/10.1002/adtp.202000172 (accessed on 20 December 2020).
- Medicago Develops a Plant-Based Vaccine for Coronavirus. Available online: https://www.pmi.com/media-center/news/medicago-develops-a-plant-based-vaccine-for-coronavirus (accessed on 20 December 2020).
- Cincinnati Company Could Have Therapeutic Option For COVID-19. Available online: https://www.forbes.com/sites/lizengel/2020/03/14/cincinnati-startup-could-have-therapeutic-option-for-covid-19/#4262a5597d27 (accessed on 20 December 2020).
- Tiziana Life Sciences plc To Expedite Development of its Fully Human Anti-Interleukin-6-Receptor Monoclonal Antibody, a Potential Treatment of Certain Patients Infected with Coronavirus COVID-19. Available online: https://www.tizianalifesciences.com/news-item?s=2020-03-11-tiziana-life-sciences-plc-to-expedite-development-of-its-fully-human-anti-interleukin-6-receptor-monoclonal-antibody-a-potential-treatment-of-certain-patients-infected-with-coronavirus-covid-19 (accessed on 20 December 2020).
- INO-4800 DNA Coronavirus Vaccine. Available online: https://www.precisionvaccinations.com/vaccines/ino-4800-dna-coronavirus-vaccine (accessed on 20 December 2020).
- Israeli Team Developing Coronavirus Vaccine Raises $12M For New Startup, To Begin Summer Trials. Available online: https://nocamels.com/2020/04/israeli-coronavirus-vaccine-raises-12m-migvax-ourcrowd/ (accessed on 20 December 2020).
- TNX-1800 (Coronavirus Vaccine). Available online: https://www.tonixpharma.com/pipeline/tnx-1800-coronavirus-vaccine (accessed on 20 December 2020).
- Vaxart Taking COVID-19 Tablet Vaccine into Clinic. Available online: https://bioprocessintl.com/bioprocess-insider/global-markets/vaxart-taking-covid-19-tablet-vaccine-into-clinic/ (accessed on 20 December 2020).
- Vaxart Announces First Quarter 2020 Financial Results and Provides Corporate Update. Available online: https://www.globenewswire.com/news-release/2020/05/12/2032226/0/en/Vaxart-Announces-First-Quarter-2020-Financial-Results-and-Provides-Corporate-Update.html (accessed on 20 December 2020).
- Safety and Immunogenicity of an rAd26 and rAd5 Vector-Based Heterologous Prime-Boost COVID-19 Vaccine in Two Formulations: Two Open, Non-Randomised Phase 1/2 Studies from Russia. Available online: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31866-3/fulltext (accessed on 20 December 2020).
- Moscow Opens Dozens of COVID-19 Vaccination Centres, Prioritizes High-Risk Groups. Available online: https://www.cbc.ca/news/world/russia-vaccine-covid-19-coronavirus-1.5829964 (accessed on 20 December 2020).
- Mulangu, S.; Dodd, L.E.; Davey, R.T.; Mbaya, O.T.; Proschan, M.; Mukadi, D.; Manzo, M.L.; Nzolo, D.; Oloma, A.T.; Ibanda, A.; et al. A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. N. Engl. J. Med. 2019, 381, 2293–2303. [Google Scholar] [CrossRef]
- Zhou, B.; Zhong, N.; Guan, Y. Treatment with Convalescent Plasma for Influenza A (H5N1) Infection. N. Engl. J. Med. 2007, 357, 1450–1451. [Google Scholar] [CrossRef]
- Grein, J.; Ohmagari, N.; Shin, D.; Diaz, G.; Asperges, E.; Castagna, A.; Feldt, T.; Green, G.; Green, M.L.; Lescure, F.-X.; et al. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N. Engl. J. Med. 2020, 382, 2327–2336. [Google Scholar] [CrossRef]
- Furuta, Y.; Komeno, T.; Nakamura, T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad. Ser. B 2017, 93, 449–463. [Google Scholar] [CrossRef]
- Furuta, Y.; Takahashi, K.; Kuno-Maekawa, M.; Sangawa, H.; Uehara, S.; Kozaki, K.; Nomura, N.; Egawa, H.; Shiraki, K. Mechanism of Action of T-705 against Influenza Virus. Antimicrob. Agents Chemother. 2005, 49, 981–986. [Google Scholar] [CrossRef]
- Bai, C.-Q.; Mu, J.-S.; Kargbo, D.; Song, Y.-B.; Niu, W.-K.; Nie, W.-M.; Kanu, A.; Liu, W.-W.; Wang, Y.-P.; Dafae, F.; et al. Clinical and Virological Characteristics of Ebola Virus Disease Patients Treated with Favipiravir (T-705)—Sierra Leone, 2014. Clin. Infect. Dis. 2016, 63, 1288–1294. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Cai, Q.; Yang, M.; Liu, D.; Chen, J.; Shu, D.; Xia, J.; Liao, X.; Gu, Y.; Cai, Q.; Yang, Y.; et al. Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study. Engineering 2020. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, Y.; Huang, J.; Ying, P.; Cheng, Z.; Wu, J.; Chen, S.; Zhang, Y.; Chen, B.; Lu, M.; et al. Favipiravir versus Arbidol for COVID-19: A randomized clinical trial. MedRxiv 2020. [Google Scholar] [CrossRef]
- Jian-ya, G. Clinical characteristics of 51 patients discharged from hospital with COVID-19 in Chongqing, China. MedRxiv 2020. [Google Scholar] [CrossRef]
- Qin, X.; Qiu, S.; Yuan, Y.; Zong, Y.; Tuo, Z.; Li, J.; Liu, J. Clinical Characteristics and Treatment of Patients Infected with COVID-19 in Shishou, China. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef]
- Cheng, Y.; Wong, R.; Soo, Y.O.Y.; Wong, W.S.; Lee, C.K.; Ng, M.H.L.; Chan, P.; Wong, K.C.; Leung, C.B.; Cheng, G. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 24, 44–46. [Google Scholar] [CrossRef]
- Hung, I.F.; To, K.K.; Lee, C.-K.; Lee, K.-L.; Chan, K.; Yan, W.-W.; Liu, R.; Watt, C.-L.; Chan, W.-M.; Lai, K.-Y.; et al. Convalescent Plasma Treatment Reduced Mortality in Patients With Severe Pandemic Influenza A (H1N1) 2009 Virus Infection. Clin. Infect. Dis. 2011, 52, 447–456. [Google Scholar] [CrossRef]
- Ko, J.-H.; Seok, H.; Cho, S.Y.; Ha, Y.E.; Baek, J.Y.; Kim, S.H.; Kim, Y.-J.; Park, J.K.; Chung, C.R.; Kang, E.-S.; et al. Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: A single centre experience. Antivir. Ther. 2018, 23, 617–622. [Google Scholar] [CrossRef]
- Mair-Jenkins, J.; Saavedra-Campos, M.; Baillie, J.K.; Cleary, P.; Khaw, F.-M.; Likm, W.S.; Makki, S.; Rooney, K.D. Convalescent Plasma Study Group, The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta-analysis. J. Infect. Dis. 2015, 211, 80–90. [Google Scholar] [CrossRef]
- Shen, C.; Wang, Z.; Zhao, F.; Yang, Y.; Li, J.; Yuan, J.; Wang, F.; Li, D.; Yang, M.; Xing, L.; et al. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA 2020, 323, 1582. [Google Scholar] [CrossRef] [PubMed]
- Duan, K.; Liu, B.; Li, C.; Zhang, H.; Yu, T.; Qu, J.; Zhou, M.; Chen, L.; Meng, S.; Hu, Y.; et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. USA 2020, 117, 9490–9496. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, V.; Delghandi, P.S.; Moallem, S.A.; Karimi, G. Safety and toxicity of silymarin, the major constituent of milk thistle extract: An updated review. Phytotherapy Res. 2019, 33, 1627–1638. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Witek-Krowiaket, A.; Skrzypczak, D.; Mikula, K.; Młynarz, Z. Phytochemicals containing biologically active polyphenols as an effective agent against COVID-19-inducing coronavirus. J. Funct. Foods 2020, 73, 104146. [Google Scholar] [CrossRef] [PubMed]
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem. 2017, 220, 153–161. [Google Scholar] [CrossRef]
- Ma, L.; Yao, L. Antiviral Effects of Plant-Derived Essential Oils and Their Components: An Updated Review. Molecules 2020, 25, 2627. [Google Scholar] [CrossRef]
- Ryu, Y.B.; Jeong, H.J.; Kim, J.H.; Kim, M.Y.; Park, J.-Y.; Kim, D.; Nguyen, T.T.H.; Park, S.J.; Chang, J.S.; Park, K.H.; et al. Biflavonoids from Torreya nucifera displaying SARS-CoV 3CL (pro) inhibition. Bioorganic Med. Chem. 2010, 18, 7940–7947. [Google Scholar] [CrossRef]
- Gebrelibanos, M.; Periyasamy, G.; Sintayehu, B. Senna occidentalis seed: Is it health risk or potential medicine? Int. J. Pharmacogn. 2014, 1, 161–167. [Google Scholar]
- Monkheang, P. Species diversity, usages, molecular markers and barcode of medicinal Senna species (Fabaceae, Caesalpinioideae) in Thailand. J. Med. Plants Res. 2011, 5, 6173–6181. [Google Scholar] [CrossRef]
- Hennebelle, T.; Weniger, B.; Joseph, H.; Sahpaz, S.; Bailleul, F. Senna alata. Fitoter. 2009, 80, 385–393. [Google Scholar] [CrossRef]
- Kayembe, J.S.; Taba, K.M.; Ntumba, K.; Kazadi, T.K. In vitro Antimalarial Activity of 11 Terpenes Isolated from Ocimum gratissimum and Cassia alata Leaves. Screening of Their Binding Affinity with Haemin. J. Plant Stud. 2012, 1, 168–172. [Google Scholar] [CrossRef]
- Kayembe, J.S.; Taba, K.M.; Ntumba, K. In vitro anti-malarial activity of 20 quinones isolated from four plants used by traditional healers in the Democratic Republic of Congo. J. Med. Plants Res. 2010, 4, 991–994. [Google Scholar]
- Oladeji, O.S.; Adelowo, F.E.; Oluyori, A.P.; Bankole, D.T. Ethnobotanical Description and Biological Activities of Senna alata. Evid. Based Complement. Altern. Med. 2020, 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Panyod, S.; Ho, C.-T.; Sheen, L.-Y. Dietary therapy and herbal medicine for COVID-19 prevention: A review and perspective. J. Tradit. Complement. Med. 2020, 10, 420–427. [Google Scholar] [CrossRef]
- Runfeng, L.; Yunlong, H.; Jicheng, H.; Weiqi, P.; Qinhai, M.; Yongxia, S.; Chufang, L.; Jin, Z.; Zhenhua, J.; Haiming, J.; et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol. Res. 2020, 156, 104761. [Google Scholar] [CrossRef]
- Luo, H.; Tang, Q.L.; Shang, Y.X.; Liang, S.B.; Yang, M.; Robinson, N.; Liu, J.P. Can Chinese Medicine Be Used for Prevention of Corona Virus Disease 2019 (COVID-19)? A Review of Historical Classics, Research Evidence and Current Prevention Programs. Chin. J. Integr. Med. 2020, 26, 243–250. [Google Scholar] [CrossRef]
- Chen, C.-J.; Michaelis, M.; Hsu, H.-K.; Tsai, C.-C.; Yang, K.D.; Wu, Y.-C.; Cinatl, J.; Doerr, H.W. Toona sinensis Roem tender leaf extract inhibits SARS coronavirus replication. J. Ethnopharmacol. 2008, 120, 108–111. [Google Scholar] [CrossRef]
- Bailly, C.; Vergoten, G. Glycyrrhizin: An alternative drug for the treatment of COVID-19 infection and the associated respiratory syndrome? Pharmacol. Ther. 2020, 214, 107618. [Google Scholar] [CrossRef]
- Fuzimoto, A.D.; Isidoro, C. The antiviral and coronavirus-host protein pathways inhibiting properties of herbs and natural compounds—Additional weapons in the fight against the COVID-19 pandemic? J. Tradit. Complement. Med. 2020, 10, 405–419. [Google Scholar] [CrossRef]
- Mani, J.S.; Johnson, J.B.; Steel, J.C.; Broszczak, D.A.; Neilsen, P.M.; Walsh, K.B.; Naiker, M. Natural product-derived phytochemicals as potential agents against coronaviruses: A review. Virus Res. 2020, 284, 197989. [Google Scholar] [CrossRef]
- Wen, C.-C.; Shyur, L.-F.; Jan, J.-T.; Liang, P.-H.; Kuo, C.-J.; Arulselvan, P.; Wu, J.-B.; Kuo, S.-C.; Yang, N.-S. Traditional Chinese medicine herbal extracts of Cibotium barometz, Gentiana scabra, Dioscorea batatas, Cassia tora, and Taxillus chinensis inhibit SARS-CoV replication. J. Tradit. Complement. Med. 2011, 1, 41–50. [Google Scholar] [CrossRef]
- Park, J.-Y.; Kim, J.H.; Kwon, J.M.; Kwon, H.-J.; Jeong, H.J.; Kim, Y.M.; Kim, D.; Lee, W.S.; Ryu, Y.B. Dieckol, a SARS-CoV 3CLpro inhibitor, isolated from the edible brown algae Ecklonia cava. Bioorganic Med. Chem. 2013, 21, 3730–3737. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Buyel, J.F. Molecular farming—The slope of enlightenment. Biotechnol. Adv. 2020, 40, 107519. [Google Scholar] [CrossRef] [PubMed]
- Capell, T.; Twyman, R.M.; Armario-Najera, V.; Ma, J.K.; Schillberg, S.; Christou, P. Potential applications of plant biotechnology against SARS-CoV-2. Trends Plant Sci. 2020, 25, 635–643. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Shavandi, A.; Raie, D.S.; Sangeetha, J.; Soleimani, M.; Hajibehzad, S.S.; Thangadurai, D.; Hospet, R.; Popoola, J.O.; Arzani, A.; et al. Plant molecular farming: Production of metallic nanoparticles and therapeutic proteins using green factories. Green Chem. 2019, 21, 1845–1865. [Google Scholar] [CrossRef]
- Aboul-Ata, A.A.E.; Vitti, A.; Nuzzaci, M.; El-Attar, A.K.; Piazzolla, G.; Tortorella, C.; Harandi, A.M.; Olson, O.; Wright, S.A.I.; Piazolla, P.; et al. Plant-based vaccines: Novel and low-cost possible route for mediterranean innovative vaccination strategies. Adv. Virus Res. 2014, 89, 1–37. [Google Scholar]
- Saxena, J.; Rawat, S. Edible vaccines. Adv. Biotechnol. 2014, 207–226. [Google Scholar]
- Paul, M.; Ma, J.K. Plant-made pharmaceuticals: Leading products and production platforms. Biotechnol. Appl. Biochem. 2011, 58, 58–67. [Google Scholar] [CrossRef]
- Grabowski, G.A.; Golembo, M.; Shaaltiel, Y. Taliglucerase alfa: An enzyme replacement therapy using plant cell expression technology. Mol. Genet. Metab. 2014, 112, 1–8. [Google Scholar] [CrossRef]
- McDonald, K.; Holtz, R.B. From Farm to Finger Prick—A Perspective on How Plants Can Help in the Fight Against COVID-19. Front. Bioeng. Biotechnol. 2020, 8, 782. [Google Scholar] [CrossRef]
- Krenek, P.; Šamajová, O.; Luptovciak, I.; Doskocilova, A.; Komis, G.; Šamaj, J. Transient plant transformation mediated by Agrobacterium tumefaciens: Principles, methods and applications. Biotechnol. Adv. 2015, 33, 1024–1042. [Google Scholar] [CrossRef]
- Rosales-Mendoza, S.; Márquez-Escobar, V.A.; González-Ortega, O.; Nieto-Gómez, R.; Arévalo-Villalobos, J.I. What Does Plant-Based Vaccine Technology Offer to the Fight against COVID-19? Vaccines 2020, 8, 183. [Google Scholar] [CrossRef] [PubMed]
- Pillet, S.; Couillard, J.; Trépanier, S.; Poulin, J.-F.; Yassine-Diab, B.; Guy, B.; Ward, B.J.; Landry, N. Immunogenicity and safety of a quadrivalent plant-derived virus like particle influenza vaccine candidate—Two randomized Phase II clinical trials in 18 to 49 and ≥50 years old adults. PLoS ONE 2019, 14, e0216533. [Google Scholar] [CrossRef] [PubMed]
- Rybicki, E. History and Promise of Plant-Made Vaccines for Animals. In Prospects of Plant-Based Vaccines in Veterinary Medicine; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2018; pp. 1–22. [Google Scholar]
- Makay, C. Algae Tasked with Making COVID-19 Kits. 2020. Available online: https://phys.org/news/2020-04-algae-tasked-covid-kits.html (accessed on 24 December 2020).
- Specht, E.A.; Mayfield, S. Algae-based oral recombinant vaccines. Front. Microbiol. 2014, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Gretler, C. Tobacco-Based Coronavirus Vaccine Poised for Human Tests Bloomberg, May 15. Available online: https://www.bloomberg.com/news/articles/2020-05-15/cigarette-maker-s-coronavirus-vaccine-poised-for-human-tests (accessed on 24 December 2020).
- Palca, J. Tobacco Plants Contribute Key Ingredient For COVID-19 Vaccine. 2020. Available online: https://www.npr.org/sections/health-shots/2020/10/15/923210562/tobacco-plants-contribute-key-ingredient-for-covid-19-vaccine#:~:text=Historically%2C%20tobacco%20plants%20are%20responsible,be%20used%20in%20a%20vaccine (accessed on 20 December 2020).
- Mullan, K. Tobacco Giant BAT Says it Could be Making 1 to 3 Million COVID-19 Vaccines a Week by June. 2020. Available online: https://www.derryjournal.com/news/people/tobacco-giant-bat-says-it-could-be-making-1-3-million-covid-19-vaccines-week-june-2526933 (accessed on 24 December 2020).
- Nogrady, B. How SARS-CoV-2 Tests Work and What’s Next in COVID-19 Diagnostics. The Scientist March 3. 2020. Available online: https://www.the-scientist.com/news-opinion/how-sars-cov-2-tests-work-and-whats-next-in-covid-19-diagnostics-67210 (accessed on 24 December 2020).
- Chung, Y.H.; Cai, H.; Steinmetz, N.F. Viral nanoparticles for drug delivery, imaging, immunotherapy, and theranostic applications. Adv. Drug Deliv. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Ramirez, M.A.; Soto, F.; Wang, C.; Rueda, R.; Shukla, S.; Silva-Lopez, C.; Kupor, D.; McBride, D.A.; Pokorski, J.K.; Nourhani, A.; et al. Built-In Active Microneedle Patch with Enhanced Autonomous Drug Delivery. Adv. Mater. 2020, 32, e1905740. [Google Scholar] [CrossRef]
- Zhang, W.; Bailey-Elkin, B.A.; Knaap, R.C.M.; Khare, B.; Dalebout, T.J.; Johnson, G.G.; Van Kasteren, P.B.; McLeish, N.J.; Gu, J.; He, W.; et al. Potent and selective inhibition of pathogenic viruses by engineered ubiquitin variants. PLoS Pathog. 2017, 13, e1006372. [Google Scholar] [CrossRef]
- Clemente, V.; D’Arcy, P.; Bazzaro, M. Deubiquitinating Enzymes in Coronaviruses and Possible Therapeutic Opportunities for COVID-19. Int. J. Mol. Sci. 2020, 21, 3492. [Google Scholar] [CrossRef]
- Hefferon, K.L. DNA Virus Vectors for Vaccine Production in Plants: Spotlight on Geminiviruses. Vaccines 2014, 2, 642–653. [Google Scholar] [CrossRef]
- Rattanapisit, K.; Shanmugaraj, B.; Manopwisedjaroen, S.; Purwono, P.B.; Siriwattananon, K.; Khorattanakulchai, N.; Hanittinan, O.; Boonyayothin, W.; Thitithanyanont, A.; Ferguson-Smith, A.C.; et al. Rapid production of SARS-CoV-2 receptor binding domain (RBD) and spike specific monoclonal antibody CR3022 in Nicotiana benthamiana. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Tusé, D.; Nandi, S.; McDonald, K.A.; Buyel, J.F. The Emergency Response Capacity of Plant-Based Biopharmaceutical Manufacturing-What It Is and What It Could Be. Front. Plant Sci. 2020, 11, 594019. [Google Scholar] [CrossRef] [PubMed]
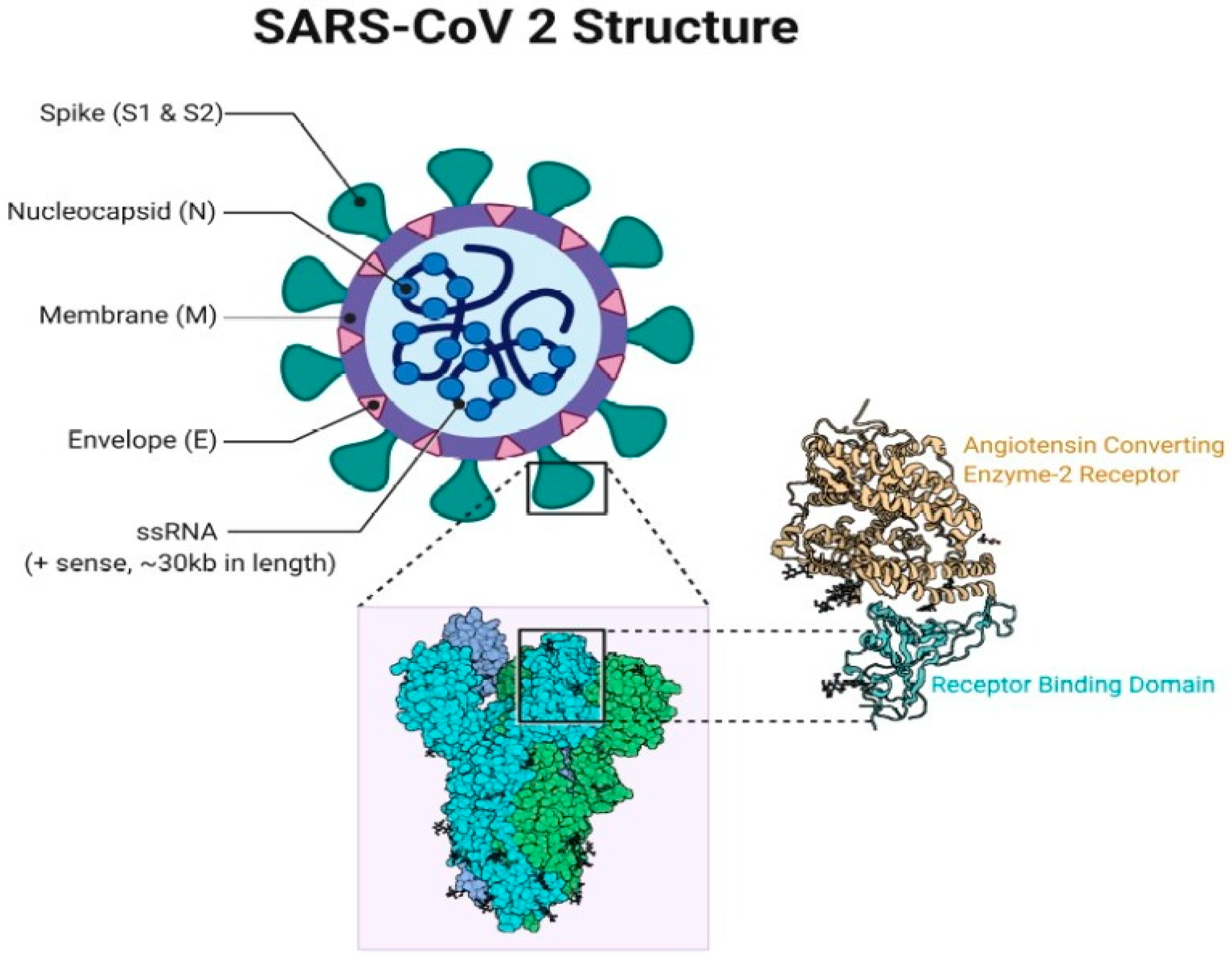


| Name of Vaccine | Nature | Company/Institution, Country | Reference |
|---|---|---|---|
| Recombinant subunit vaccine | It is a trimeric Spike-protein subunit vaccine expressed in mammalian cell system. The company claims to have preserved the original trimeric viral spike protein | Clover, China | [39,40] |
| ChAdOx1 nCoV-19 | Weakened version of a common cold virus (adenovirus) in which Spike glycoprotein from SARS-CoV2 has been added | Oxford University, UK | [41,42] |
| mRNA-1273 vaccine | mRNA (messenger RNA) has been used to make this vaccine. It directs body cells to express a viral protein that would elicit an immune response. Promising results received in animals | Kaiser Permanente Washington Health Research Institute (KPWHRI), USA | [43] |
| Covigenix | It is a DNA-based vaccine that will directly induce a plasmid to encode the antigen against which an immune response is required. Phase 1 clinical trials underway | Entos Pharmaceuticals, Inc., Canada | [44] |
| Gimsilumab | It is based on Monoclonal antibodies that selectively inhibit granulocyte-macrophage colony-stimulating factor (GM-CSF). Phase 2 clinical trials underway | Roivant Sciences Ltd., USA | [45,46] |
| BNT162 vaccine | It is based on a nucleoside-modified RNA expressed in lipid nanoparticles that encodes the viral spike protein to elicit an immune response | Precision Vax LLC, Germany | [47] |
| Adcovid | It is based on the expression of receptor-binding domain (RBD) of the SARS-CoV-2 spike protein. Provides benefits of single dose efficacy, intranasal administration, and convenient storage conditions. Phase 1 clinical trials are underway | University of Alabama, UK | [48] |
| TJM2 vaccine | Consists of a neutralizing antibody that has a high affinity for human GM-CSF. Binding to GM-CSF results in inhibition of inflammatory responses to reduce disease complications. Phase 1 trials are underway | I-Mab Biopharma, China | [49] |
| Coronavirus-Like Particle COVID-19 vaccine (CoVLP) | VLPs of spike (S) glycoprotein of SARS-CoV2 have been produced in plant system. Phase 2 clinical trials are underway | Medicago, Canada | [50] |
| AT-100 | Engineered version of a human recombinant protein that reduces inflammation and infection in the body | Airway Therapeutics Inc., USA | [51] |
| TZLS-501 | Monoclonal antibody targeting the receptor for IL-6 to reduce cytokine storm and prevent exaggerated immune response. | UK/US combine company | [52] |
| INO-4800 | It is a DNA based vaccine containing the plasmid pGX9501, which encodes the Spike glycoprotein of SARS-CoV-2. | INOVIO, China | [53] |
| Avian Coronavirus Infectious Bronchitis Virus (IBV) vaccine | By-product of the IBV vaccine, consists of a protein vector that secretes a soluble chimeric protein carrying the viral antigen into tissue resulting in production of antibodies | Migal Research Institute, Israel | [54] |
| TNX-1800 | Live modified horsepox virus vaccine; consists of modified horsepox virus that expresses the SARS-CoV2 protein | Tonix Pharmaceuticals, USA | [55] |
| Vaxart’s coronavirus vaccine | The vaccine consists of a viral vector that carries genes for two SARS-CoV-2 proteins, the nucleocapsid and spike. The vector carries both genes to the cell along with a strong adjuvant that elicits an immune response to the viral proteins. | Vaxart, USA | [56,57] |
| Russian vaccine (Sputnik V) | It is an adenoviral-based vaccine that uses weakened virus to generate an immune response | The Gamaleya Center, Russia | [58,59] |
| Common Name | Scientific Name | Chinese Name |
|---|---|---|
| Weeping forsythia | Forsythia suspensa (Thunb.) Vahl | Lián qiáo |
| Chinese ephedra | Ephedra sinica Stapf | Cǎo má huáng |
| Japanese honeysuckle | Lonicera japonica Thunb. | Rěndōng |
| Woad | Isatis indigotica Fortune | Sōng lán |
| Mint | Mentha haplocalyx Briq. | Bò hé |
| Thick-stemmed wood fern | Dryopteris crassirhizoma Nakai | Cū jīng lín máo jué |
| Golden Root | Rhodiola rosea L. | Hóng jǐng tiān |
| Gypsum | Gypsum Fibrosum | Shí gāo |
| Patchouli | Pogostemon cablin (Blanco) Benth. | Guǎng huò xiāng |
| Chinese Rhubarb | Rheum palmatum L. | Zhǎng yè dà huáng |
| Fish Mint | Houttuynia cordata Thunb. | Yú xīng cǎo |
| Licorice | Glycyrrhiza uralensis Fisch. | Gāncǎo |
| Siberian Apricot | Armeniaca sibirica (L.) Lam. | Shān xìng |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmood, N.; Nasir, S.B.; Hefferon, K. Plant-Based Drugs and Vaccines for COVID-19. Vaccines 2021, 9, 15. https://doi.org/10.3390/vaccines9010015
Mahmood N, Nasir SB, Hefferon K. Plant-Based Drugs and Vaccines for COVID-19. Vaccines. 2021; 9(1):15. https://doi.org/10.3390/vaccines9010015
Chicago/Turabian StyleMahmood, Nasir, Sarah Bushra Nasir, and Kathleen Hefferon. 2021. "Plant-Based Drugs and Vaccines for COVID-19" Vaccines 9, no. 1: 15. https://doi.org/10.3390/vaccines9010015
APA StyleMahmood, N., Nasir, S. B., & Hefferon, K. (2021). Plant-Based Drugs and Vaccines for COVID-19. Vaccines, 9(1), 15. https://doi.org/10.3390/vaccines9010015






