The Use of Extracellular Membrane Vesicles for Immunization against Francisellosis in Nile Tilapia (Oreochromis niloticus) and Atlantic Cod (Gadus morhua L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria, Media and Growth Conditions
2.2. Isolation of Membrane Vesicles
2.3. Toxicity, Immunization and Challenge Trial in Tilapia
2.4. Toxicity, Immunization and Challenge in Atlantic Cod
2.5. Bacteriology
2.6. RNA Isolation and Quantitative Real-Time PCR
2.7. Serum Collection and ELISA
2.8. Quantification of Bacterial Burden in Tilapia
2.9. Statistical Analysis
3. Results
3.1. Fo MV Toxicity and Immunization Trial in Tilapia
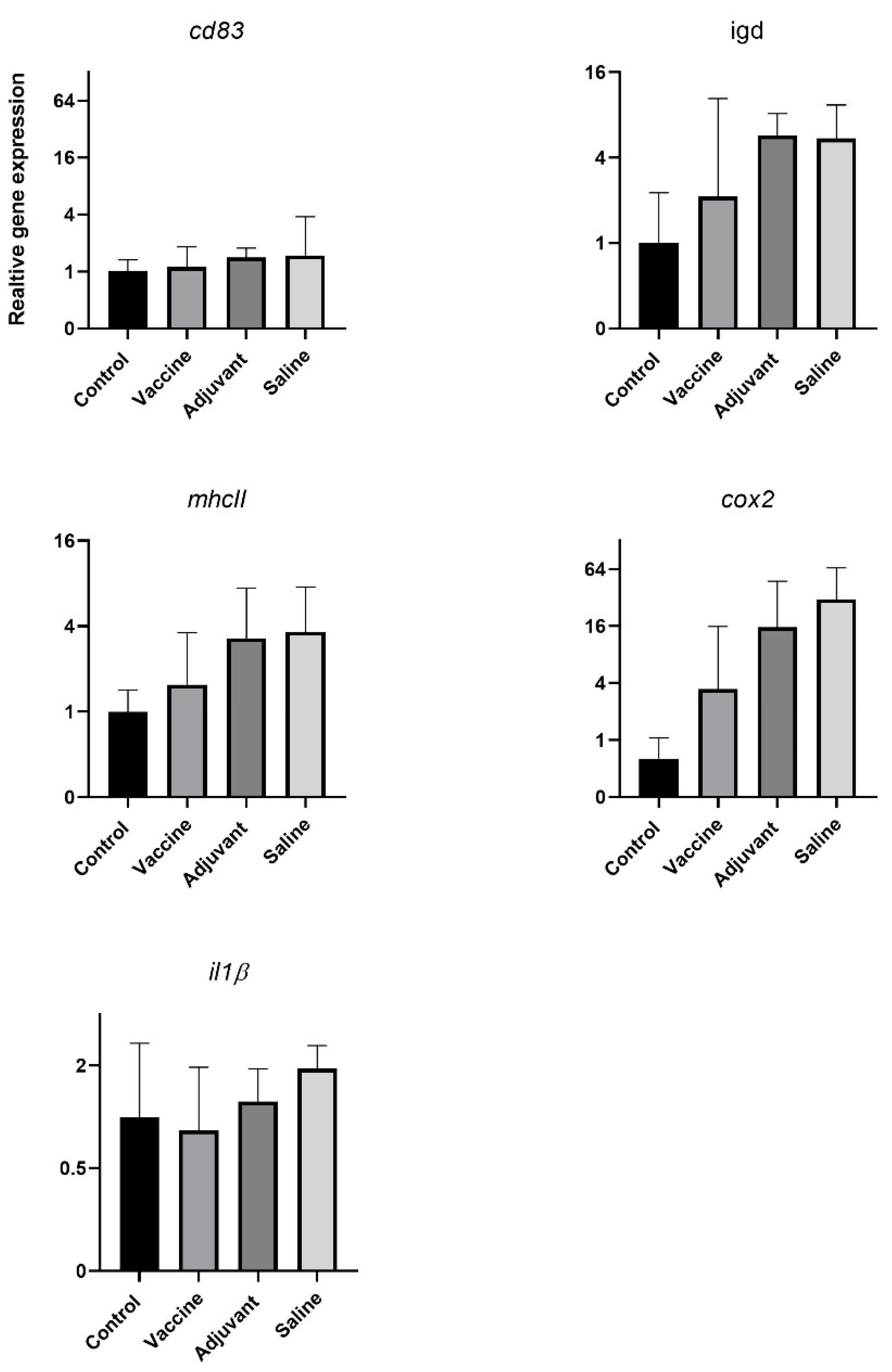
3.1.1. High Concentration of Fo MVs Are Not Toxic and Elicit a Limited Immune Response in Tilapia
3.1.2. MV Immunization Did Not Protect Tilapia From Fo Infection
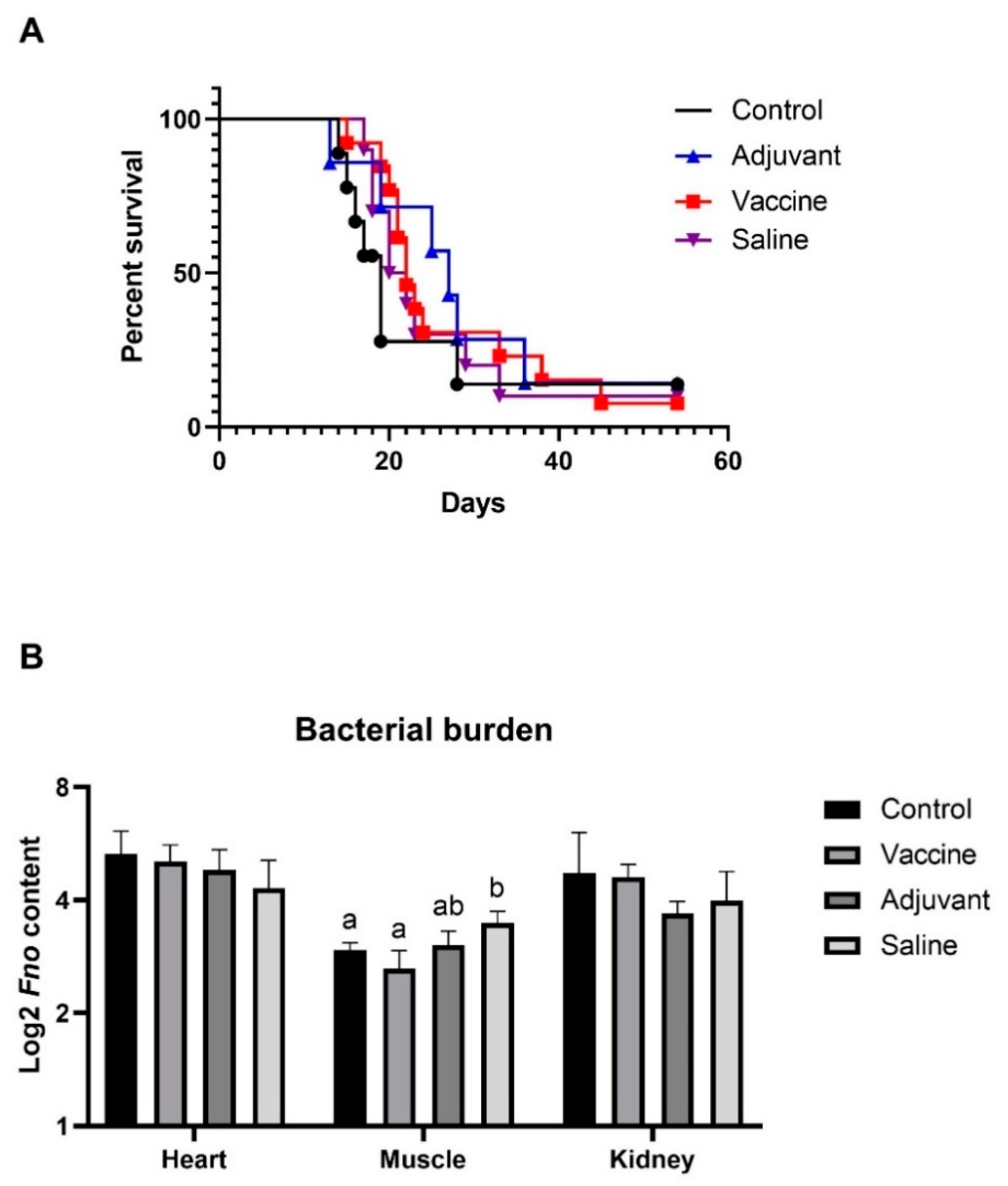
3.2. Fnn MV Toxicity and Immunization Trial in Atlantic Cod
Immunization with MVs Did Not Induce Immunity against Fnn Infection
3.3. Increased IgM level in Fnn Immunized Atlantic Cod before and 30 Days Postchallenge
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Name | Sequence 5′−3′ | Reference |
|---|---|---|
| CD83_Tilapia_fw | CCGGTGTCCAGTACCTTGCAGTGA | [55] |
| CD83_Tilapia_rv | GCTGGACAATCTGCCAGCACCAG | |
| IgD_Tilapia_fw | AACACCACCCTGTCCCTGAAT | [56] |
| IgD_Tilapia_rv | GGGTGAAAACCACATTCCAGC | |
| Tnfα_Tilapia_fw | TCATGGCTGGTATAACGCGA | This study |
| Tnfα_Tilapia_rv | GTACGGCCTTCATCGGTTTC | |
| Il-12_Tilapia_fw | GGAAGCACGGCAGCAGAATA | [57] |
| Il-12_Tilapia_rv | AACTTGAGGGAGAAGTAGGAATGG | |
| IFN- γ_Tilapia_fw | AGCGGCTGACTGAACTCAATTGAAG | [57] |
| IFN-γ_Tilapia_rv | GTCACAGTTTTCAGCTGTATAGGG | |
| β-actin_Tilapia_fw | CAGGATGCAGAAGGAGATCACA | [58] |
| β-actin_Tilapia_rv | CGATCCAGACGGAGTATTTACG | |
| IgM_Tilapia_fw | AGGCACAACGGTCACTGTCA | [59] |
| IgM_Tilapia_rv | GCAAGGCAGCCAAGAGTGAC | |
| MHCIIb_Tilapia_fw | GAGGAACAAGCTCGCCATCG | [59] |
| MHCIIb_Tilapia_rv | AGTCGTGCTCTGACCTCGAG | |
| COX2_Tilapia_fw | AGCAGCCAGAAGGAAGGCGG | [60] |
| COX2_Tilapia_rv | GACTGAGTTGCAGTTCTCTTAGTGTGC | |
| Il-1β_Tilapia_fw | TGCTGAGCACAGAATTCCAG | [61] |
| IL-1β_Tilapia_rv | GCTGTGGAGAAGAACCAAGC | |
| Ef1α_Cod_fw | ATGTGAGCGGTGTGGCAATC | [62] |
| Ef1α_Cod_rv | TCATCATCCTGAACCACCCTG | |
| Ubi_Cod_fw | GGCCGCAAAGATGCAGAT | [63] |
| Ubi_Cod_rv | CTGGGCTCGACCTCAAGAGT | |
| IL1β_Cod_fw | GGAGAACACGGACGACCTGA | [62] |
| IL1β_Cod_rv | CGCACCATGTCACTGTCCTT | |
| IL6_Cod_fw | TGAAGAAGGAGTACCCCGACAAT | [48] |
| IL6_Cod_rv | GGTGCCTCATCTTTTCCTCAATG | |
| IL8_Cod_fw | GGTTTGTTCAATGATGGGCTGTT | [62] |
| IL8_Cod_rv | GACCTTGCCTCCTCATGGTAATACT | |
| IL10_Cod_fw | CCTATAAAGCCATCGGCGAGTTA | [62] |
| IL10_Cod_rv | TGAAGTCGTCGTTTTGAACCAAG | |
| INF-γ_Cod_fw | TGGTCTGCATGTCAGTTTGTCTG | [62] |
| INF-γ_Cod_rv | TTCTGTGGATGTTGTTGGCTAAGA | |
| IGM-LC_Cod_fw | CACTACAGCTGGAGCAGCAC | [64] |
| IGM-LC_Cod_rv | CCATGCTGGAGCCTCTCTAC | |
| IGM-HC_Cod_fw | GGTGAGGTGTTATCCGTGCT | [64] |
| IGM-HC_Cod_rv | GCAGATAAACGGATGGAGGA | |
| Fo fw | CATGGGAAACAAATTCAAAAGGA | [37] |
| Fo rv | GGAGAGATTTCTTTTTTAGAGGAGCT |

References
- McCoy, G.W.; Chapin, C.W. Further Observations on a Plague-Like Disease of Rodents with a Preliminary Note on the Causative Agent, Bacterium Tularense. J. Infect. Dis. 1912, 10, 61–72. Available online: http://www.jstor.org/stable/30071893 (accessed on 7 June 2020). [CrossRef]
- Olsen, A.B.; Mikalsen, J.; Rode, M.; Alfjorden, A.; Hoel, E.; Straum-Lie, K.; Haldorsen, R.; Colquhoun, D.J. A systemic granulomatous inflammatory disease in wild Atlantic cod, Gadus morhua associated with a bacterium of the genus Francisella. J. Fish Dis. 2006, 29, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Nylund, A.; Ottem, K.F.; Watanabe, K.; Karlsbakk, E.; Krossøy, B. Francisella sp. (Family Francisellaceae) causing mortality in Norwegian cod (Gadus morhua) farming. Arch. Microbiol. 2006, 185, 383–392. [Google Scholar] [CrossRef]
- Mikalsen, J.; Colquhoun, D.J. Francisella asiatica sp. nov. isolated from farmed tilapia (Oreochromis sp.) and elevation of Francisella philomiragia subsp. noatunensis to species rank as Francisella noatunensis comb. nov., sp. nov. Int. J. Syst. Evol. Microbiol. 2009. [Google Scholar] [CrossRef] [PubMed]
- Svensson, K.; Larsson, P.; Johansson, D.; Byström, M.; Forsman, M.; Johansson, A. Evolution of Subspecies of Francisella tularensis. J. Bacteriol. 2005, 187, 3903–3908. Available online: http://jb.asm.org/content/187/11/3903.abstract (accessed on 18 August 2020). [CrossRef] [Green Version]
- Mauel, M.J.; Miller, D.L.; Frazier, K.; Liggett, A.D.; Styer, L.; Montgomery-Brock, D.; Brock, J. Characterization of a piscirickettsiosis-like disease in Hawaiian tilapia. Dis. Aquat. Organ. 2003, 53, 249–255. Available online: https://www.int-res.com/abstracts/dao/v53/n3/p249-255/ (accessed on 18 August 2020). [CrossRef]
- Jeffery, K.; Stone, D.; Feist, S.; Verner-Jeffreys, D. An outbreak of disease caused by Francisella sp. in Nile tilapia Oreochromis niloticus at a recirculation fish farm in the UK. Dis. Aquat. Org. 2010, 91, 161–165. [Google Scholar] [CrossRef] [Green Version]
- Birkbeck, T.H.; Feist, S.W.; Verner-Jeffreys, D.W. Francisella infections in fish and shellfish. J. Fish Dis. 2011, 34, 173–187. [Google Scholar] [CrossRef]
- Furevik, A.; Pettersen, E.F.; Colquhoun, D.; Wergeland, H.I. The intracellular lifestyle of Francisella noatunensis in Atlantic cod (Gadus morhua L.) leucocytes. Fish Shellfish Immunol. 2011, 30, 488–494. Available online: http://www.sciencedirect.com/science/article/pii/S105046481000361X (accessed on 19 March 2020). [CrossRef]
- Soto, E.; Kidd, S.; Gaunt, P.S.; Endris, R. Efficacy of florfenicol for control of mortality associated with Francisella noatunensis subsp. orientalis in Nile tilapia, Oreochromis niloticus (L.). J. Fish Dis. 2013, 36, 411–418. [Google Scholar] [CrossRef]
- Leal, C.A.G.; Tavares, G.C.; Figueiredo, H.C.P. Outbreaks and genetic diversity of Francisella noatunensis subsp orientalis isolated from farm-raised Nile tilapia (Oreochromis niloticus) in Brazil. Genet. Mol. Res. 2014, 13, 5704–5712. [Google Scholar] [CrossRef] [PubMed]
- Soto, E.; Primus, A.E.; Pouder, D.B.; George, R.H.; Gerlach, T.J.; Cassle, S.E.; Johnson, T.; Boyd, S.; Handsel, T.; Yanong, R.P.E. Identification of Francisella noatunensis in novel host species french grunt (Haemulon flavolineatum) and caesar grunt (Haemulon carbonarium). J. Zoo Wildl. Med. 2014, 45, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Colquhoun, D.J.; Duodu, S. Francisella infections in farmed and wild aquatic organisms. Vet. Res. 2011, 42, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Tung, M.-C.; Tsai, J.-F.; Wang, P.-C.; Chen, R.-S.; Lin, S.-C.; Adams, A.; Chen, S.-P. Systematic granulomas caused by a rickettsia-like organism in Nile tilapia, Oreochronuis niloticus (L.), from southern Taiwan. J. Fish Dis. 1994, 17, 591–599. [Google Scholar] [CrossRef]
- Chern, R.; Chao, C. Outbreaks of a Disease Caused by Rickettsia-like Organism in Cultured Tilapias in Taiwan. Fish Pathol. 1994, 29, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Mauel, M.J.; Miller, D.L.; Styer, E.; Pouder, D.B.; Yanong, R.P.E.; Goodwin, A.E.; Schwedler, T.E. Occurrence of Piscirickettsiosis-Like Syndrome in Tilapia in the Continental United States. J. Vet. Diagn. Investig. 2005, 17, 601–605. [Google Scholar] [CrossRef] [Green Version]
- Mauel, M.J.; Soto, E.; Moralis, J.A.; Hawke, J. A Piscirickettsiosis-like Syndrome in Cultured Nile Tilapia in Latin America with Francisella spp. as the Pathogenic Agent. J. Aquat. Anim. Health 2007, 19, 27–34. [Google Scholar] [CrossRef]
- Cowley, S.C.; Elkins, K.L. Immunity to Francisella. Front. Microbiol. 2011, 2, 26. [Google Scholar] [CrossRef] [Green Version]
- Muller, M.; Ilardi, P.; Avendaño-Herrera, R. Francisella sp. and Infectious Pancreatic Necrosis Virus (IPNV) pathogens of Atlantic salmon (Salmo salar) farmed in Chile. Arch. Med. Vet. 2011, 43, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Schrøder, M.B.; Ellingsen, T.; Mikkelsen, H.; Norderhus, E.A.; Lund, V. Comparison of antibody responses in Atlantic cod (Gadus morhua L.) to Vibrio anguillarum, Aeromonas salmonicida and Francisella sp. Fish Shellfish Immunol. 2009, 27, 112–119. Available online: http://www.sciencedirect.com/science/article/pii/S1050464808002854 (accessed on 19 August 2020). [CrossRef]
- Soto, E.; Wiles, J.; Elzer, P.; Macaluso, K.; Hawke, J.P. Attenuated Francisella asiatica iglC mutant induces protective immunity to francisellosis in tilapia. Vaccine 2011, 29, 593–598. Available online: http://www.sciencedirect.com/science/article/pii/S0264410X10008510 (accessed on 8 February 2020). [CrossRef] [PubMed]
- Lampe, E.O.; Tandberg, J.; Rishovd, A.-L.; Winther-Larsen, H.C. Francisella noatunensis ssp. noatunensis iglC deletion mutant protects adult zebrafish challenged with acute mortality dose of wild-type strain. Dis. Aquat. Org. 2017, 123, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Munang’Andu, H.M.; Paul, J.; Evensen, Ø. An Overview of Vaccination Strategies and Antigen Delivery Systems for Streptococcus agalactiae Vaccines in Nile Tilapia (Oreochromis niloticus). Vaccines 2016, 4, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lampe, E.O.; Zingmark, C.; Tandberg, J.I.; Thrane, I.M.P.; Brudal, E.; Sjöstedt, A.; Winther-Larsen, H.C. Francisella noatunensis subspecies noatunensis clpB deletion mutant impairs development of francisellosis in a zebrafish model. Vaccine 2017, 35, 7264–7272. Available online: http://www.sciencedirect.com/science/article/pii/S0264410X17315517 (accessed on 20 December 2019). [CrossRef] [PubMed]
- Bladen, H.A.; Waters, J.F. Electron microscopic study of some strains of Bacteroids. J. Bacteriol. 1963, 86, 1339–1344. Available online: http://jb.asm.org/content/86/6/1339.abstract (accessed on 30 June 2020). [CrossRef] [Green Version]
- Brudal, E.; Lampe, E.O.; Reubsaet, L.; Roos, N.; Hegna, I.K.; Thrane, I.M.; Koppang, E.O.; Winther-Larsen, H.C. Vaccination with outer membrane vesicles from Francisella noatunensis reduces development of francisellosis in a zebrafish model. Fish Shellfish Immunol. 2015, 42, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Lagos, L.; Tandberg, J.I.; Repnik, U.; Boysen, P.; Ropstad, E.; Varkey, D.R.; Paulsen, I.T.; Winther-Larsen, H.C. Characterization and Vaccine Potential of Membrane Vesicles Produced by Francisella noatunensis subsp. orientalis in an Adult Zebrafish Model. Clin. Vaccine Immunol. 2017, 24, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Cruz, C.; Carrión, O.; Delgado, L.; Martinez, G.; López-Iglesias, C.; Mercade, E. New Type of Outer Membrane Vesicle Produced by the Gram-Negative Bacterium Shewanella vesiculosa M7T: Implications for DNA Content. Appl. Environ. Microbiol. 2013, 79, 1874–1881. [Google Scholar] [CrossRef] [Green Version]
- Holst, J.; Feiring, B.; Næss, L.M.; Norheim, G.; Kristiansen, P.; Høiby, E.A.; Bryn, K.; Oster, P.; Costantino, P.; Taha, M.-K.; et al. The concept of “tailor-made”, protein-based, outer membrane vesicle vaccines against meningococcal disease. Vaccine 2005, 23, 2202–2205. Available online: http://www.sciencedirect.com/science/article/pii/S0264410X05000630 (accessed on 12 April 2020). [CrossRef]
- Van De Waterbeemd, B.; Streefland, M.; Van Der Ley, P.; Zomer, B.; Van Dijken, H.; Martens, D.; Wijffels, R.; Van Der Pol, L. Improved OMV vaccine against Neisseria meningitidis using genetically engineered strains and a detergent-free purification process. Vaccine 2010, 28, 4810–4816. Available online: http://www.sciencedirect.com/science/article/pii/S0264410X10006183 (accessed on 12 April 2020). [CrossRef]
- Bin Park, S.; Bin Jang, H.; Nho, S.W.; Cha, I.S.; Hikima, J.-I.; Ohtani, M.; Aoki, T.; Jung, T.S. Outer Membrane Vesicles as a Candidate Vaccine against Edwardsiellosis. PLoS ONE 2011, 6, e17629. [Google Scholar] [CrossRef]
- Soto, E.; Hawke, J.P.; Fernandez, D.; Morales, J.A. Francisella sp., an emerging pathogen of tilapia, Oreochromis niloticus (L.), in Costa Rica. J. Fish Dis. 2009, 32, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Brudal, E.; Winther-Larsen, H.C.; Colquhoun, D.J.; Duodu, S. Evaluation of reference genes for reverse transcription quantitative PCR analyses of fish-pathogenic Francisella strains exposed to different growth conditions. BMC Res. Notes 2013, 6, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brudal, E.; Ulanova, L.S.; Lampe, E.O.; Rishovd, A.-L.; Griffiths, G.; Winther-Larsen, H.C. Establishment of Three Francisella Infections in Zebrafish Embryos at Different Temperatures. Infect. Immunol. 2014, 82, 2180–2194. Available online: https://iai.asm.org/content/82/6/2180 (accessed on 20 December 2019). [CrossRef] [Green Version]
- Prentice, E.F.; Flagg, T.A.; Mccutcheon, C.S.; Brastow, D.F.; Cross, D.C. Equipment, Methods, and an Automated Data-Entry Station for PIT Tagging. Am. Fish Soc. Symp. 1990, 7, 335–340. [Google Scholar]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3. [Google Scholar] [CrossRef] [Green Version]
- Duodu, S.; Larsson, P.; Sjödin, A.; Soto, E.; Forsman, M.; Colquhoun, D.J. Real-time PCR assays targeting unique DNA sequences of fish-pathogenic Francisella noatunensis subspecies noatunensis and orientalis. Dis. Aquat. Org. 2012, 101, 225–234. [Google Scholar] [CrossRef] [Green Version]
- González, S.; Caballero, E.; Soria, Y.; Cobas, K.; Granadillo, M.; Pajón, R. Immunization with Neisseria meningitidis outer membrane vesicles prevents bacteremia in neonatal mice. Vaccine 2006, 24, 1633–1643. Available online: http://www.sciencedirect.com/science/article/pii/S0264410X05010170 (accessed on 20 December 2019). [CrossRef]
- Roberts, R.; Moreno, G.; Bottero, D.; Gaillard, M.E.; Fingermann, M.; Graieb, A.; Rumbo, M.; Hozbor, D. Outer membrane vesicles as acellular vaccine against pertussis. Vaccine 2008, 26, 4639–4646. Available online: http://www.sciencedirect.com/science/article/pii/S0264410X08008694 (accessed on 21 December 2019). [CrossRef]
- Gogos, C.A.; Drosou, E.; Bassaris, H.P.; Skoutelis, A. Pro- versus Anti-inflammatory Cytokine Profile in Patients with Severe Sepsis: A Marker for Prognosis and Future Therapeutic Options. J. Infect. Dis. 2000, 181, 176–180. [Google Scholar] [CrossRef]
- Jantrakajorn, S.; Wongtavatchai, J. FrancisellaInfection in Cultured Tilapia in Thailand and the Inflammatory Cytokine Response. J. Aquat. Anim. Health 2016, 28, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.L.; Napier, B.A.; Sampson, T.R.; Llewellyn, A.C.; Schroeder, M.R.; Weiss, D.S. Subversion of Host Recognition and Defense Systems by Francisella spp. Microbiol. Mol. Biol. Rev. 2012, 76, 383–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steiner, D.J.; Furuya, Y.; Metzger, D.W. Host-pathogen interactions and immune evasion strategies in Francisella tularensis pathogenicity. Infect. Drug Resist. 2014, 7, 239–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahin, K.; Shinn, A.P.; Metselaar, M.; Paredes, J.G.R.; Monaghan, S.J.; Thompson, K.D.; Hoare, R.; Adams, A. Efficacy of an inactivated whole-cell injection vaccine for nile tilapia, Oreochromis niloticus (L), against multiple isolates of Francisella noatunensis subsp. orientalis from diverse geographical regions. Fish Shellfish Immunol. 2019, 89, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Kondo, M.; Nakatsuka, Y.; Kawai, K.; Oshima, S.-I. Stationary phase culture supernatant containing membrane vesicles induced immunity to rainbow trout Oncorhynchus mykiss fry syndrome. Vaccine 2007, 25, 561–569. Available online: http://www.sciencedirect.com/science/article/pii/S0264410X06009091 (accessed on 8 February 2020). [CrossRef]
- Vojtech, L.N.; Sanders, G.E.; Conway, C.; Ostland, V.; Hansen, J.D. Host Immune Response and Acute Disease in a Zebrafish Model of Francisella Pathogenesis. Infect. Immun. 2008, 77, 914–925. [Google Scholar] [CrossRef] [Green Version]
- Asare, R.; Abu Kwaik, Y. Exploitation of host cell biology and evasion of immunity by Francisella tularensis. Front. Microbiol. 2011, 1, 145. [Google Scholar] [CrossRef] [Green Version]
- Bakkemo, K.R.; Mikkelsen, H.; Bordevik, M.; Torgersen, J.; Winther-Larsen, H.C.; Vanberg, C.; Olsen, R.; Johansen, L.-H.; Seppola, M. Intracellular localisation and innate immune responses following Francisella noatunensis infection of Atlantic cod (Gadus morhua) macrophages. Fish Shellfish Immunol. 2011, 31, 993–1004. Available online: https://www.sciencedirect.com/science/article/pii/S1050464811003251 (accessed on 30 June 2020). [CrossRef]
- Ellingsen, T.; Inami, M.; Gjessing, M.C.; Van Nieuwenhove, K.; Larsen, R.; Seppola, M.; Lund, V.; Schrøder, M.B. Francisella noatunensis in Atlantic cod (Gadus morhua L.); waterborne transmission and immune responses. Fish Shellfish Immunol. 2011, 31, 326–333. Available online: http://www.sciencedirect.com/science/article/pii/S1050464811002051 (accessed on 18 January 2020). [CrossRef]
- Gjessing, M.C.; Falk, K.; Weli, S.C.; Koppang, E.O.; Kvellestad, A. A sequential study of incomplete Freund’s adjuvant-induced peritonitis in Atlantic cod. Fish Shellfish Immunol. 2012, 32, 141–150. Available online: http://www.sciencedirect.com/science/article/pii/S1050464811004062 (accessed on 25 October 2020). [CrossRef]
- Solbakken, M.H.; Jentoft, S.; Reitan, T.; Mikkelsen, H.; Gregers, T.F.; Bakke, O.; Jakobsen, K.S.; Seppola, M. Disentangling the immune response and host-pathogen interactions in Francisella noatunensis infected Atlantic cod. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 30, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Star, B.; Nederbragt, A.J.; Jentoft, S.; Grimholt, U.; Malmstrøm, M.; Gregers, T.F.; Rounge, T.B.; Paulsen, J.; Solbakken, M.H.; Sharma, A.; et al. The genome sequence of Atlantic cod reveals a unique immune system. Nat. Cell Biol. 2011, 477, 207–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamers, C.H.J.; De Hass, M.J.H.; van Muiswinkel, W.B. Humoral response and memory formation in carp after injection of Aeromonashydrophila bacterin. Dev. Comp. Immunol. 1985, 9, 65–75. [Google Scholar] [CrossRef]
- Ye, J.; Kaattari, I.M.; Ma, C.; Kaattari, S. The teleost humoral immune response. Fish Shellfish Immunol. 2013, 35, 1719–1728. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.-M. Development and characterization of a cell line from tilapia head kidney with melanomacrophage characteristics. Fish Shellfish Immunol. 2016, 49, 442–449. Available online: https://www.sciencedirect.com/science/article/pii/S1050464816300134 (accessed on 17 June 2020). [CrossRef]
- Bei, W.; Wang, P.; Wu, Z.-H.; Lu, Y.; Wang, Z.-L.; Jian, J. Molecular Cloning and Expression Analysis of IgD in Nile Tilapia (Oreochromis niloticus) in Response to Streptococcus agalactiae Stimulus. Int. J. Mol. Sci. 2016, 17, 348. Available online: https://www.mdpi.com/1422-0067/17/3/348 (accessed on 30 June 2020). [CrossRef] [Green Version]
- Hamdan, A.M.; El-Sayed, A.; Mahmoud, M.M. Effects of a novel marine probiotic, Lactobacillus plantarum AH 78, on growth performance and immune response of Nile tilapia (Oreochromis niloticus). J. Appl. Microbiol. 2016, 120, 1061–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.-Z.; He, A.-Y.; Chen, L.; Limbu, S.M.; Wang, Y.-W.; Zhang, M.-L.; Du, Z.-Y. Molecular characterization and immune response to lipopolysaccharide (LPS) of the suppressor of cytokine signaling (SOCS)-1, 2 and 3 genes in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2016, 50, 160–167. Available online: https://www.sciencedirect.com/science/article/pii/S1050464816300274 (accessed on 15 March 2020). [CrossRef]
- Gallage, S.; Katagiri, T.; Endo, M.; Futami, K.; Endo, M.; Maita, M. Influence of moderate hypoxia on vaccine efficacy against Vibrio anguillarum in Oreochromis niloticus (Nile tilapia). Fish Shellfish Immunol. 2016, 51, 271–281. Available online: https://www.sciencedirect.com/science/article/pii/S1050464816300675 (accessed on 15 March 2020). [CrossRef]
- Chuang, W.-L.; Pan, B.S. Anti-Stress Effects ofGlycine tomentellaHayata in Tilapia: Inhibiting COX-2 Expression and Enhancing EPA Synthesis in Erythrocyte Membrane and Fish Growth. J. Agric. Food Chem. 2011, 59, 9532–9541. [Google Scholar] [CrossRef]
- Pirarat, N.; Pinpimai, K.; Endo, M.; Katagiri, T.; Ponpornpisit, A.; Chansue, N.; Maita, M. Modulation of intestinal morphology and immunity in nile tilapia (Oreochromis niloticus) by Lactobacillus rhamnosus GG. Res. Vet. Sci. 2011, 91, e92–e97. Available online: https://www.sciencedirect.com/science/article/pii/S0034528811001020 (accessed on 15 March 2020). [CrossRef] [PubMed]
- Seppola, M.; Larsen, A.N.; Steiro, K.; Robertsen, B.; Jensen, I. Characterisation and expression analysis of the interleukin genes, IL-1β, IL-8 and IL-10, in Atlantic cod (Gadus morhua L.). Mol. Immunol. 2008, 45, 887–897. Available online: http://www.sciencedirect.com/science/article/pii/S0161589007006827 (accessed on 17 June 2020). [CrossRef] [PubMed]
- Olsvik, P.A.; Søfteland, L.; Lie, K.K. Selection of reference genes for qRT-PCR examination of wild populations of Atlantic cod Gadus morhua. BMC Res. Notes 2008, 1, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Casanova, J.C.; Hamoutene, D.; Samuelson, S.; Burt, K.; King, T.L.; Lee, K. The immune response of juvenile Atlantic cod (Gadus morhua L.) to chronic exposure to produced water. Mar. Environ. Res. 2010, 70, 26–34. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mertes, V.; Bekkelund, A.K.; Lagos, L.; Ciani, E.; Colquhoun, D.; Haslene-Hox, H.; Sletta, H.; Sørum, H.; Winther-Larsen, H.C. The Use of Extracellular Membrane Vesicles for Immunization against Francisellosis in Nile Tilapia (Oreochromis niloticus) and Atlantic Cod (Gadus morhua L.). Vaccines 2021, 9, 34. https://doi.org/10.3390/vaccines9010034
Mertes V, Bekkelund AK, Lagos L, Ciani E, Colquhoun D, Haslene-Hox H, Sletta H, Sørum H, Winther-Larsen HC. The Use of Extracellular Membrane Vesicles for Immunization against Francisellosis in Nile Tilapia (Oreochromis niloticus) and Atlantic Cod (Gadus morhua L.). Vaccines. 2021; 9(1):34. https://doi.org/10.3390/vaccines9010034
Chicago/Turabian StyleMertes, Verena, Alexander Kashulin Bekkelund, Leidy Lagos, Elia Ciani, Duncan Colquhoun, Hanne Haslene-Hox, Håvard Sletta, Henning Sørum, and Hanne Cecilie Winther-Larsen. 2021. "The Use of Extracellular Membrane Vesicles for Immunization against Francisellosis in Nile Tilapia (Oreochromis niloticus) and Atlantic Cod (Gadus morhua L.)" Vaccines 9, no. 1: 34. https://doi.org/10.3390/vaccines9010034
APA StyleMertes, V., Bekkelund, A. K., Lagos, L., Ciani, E., Colquhoun, D., Haslene-Hox, H., Sletta, H., Sørum, H., & Winther-Larsen, H. C. (2021). The Use of Extracellular Membrane Vesicles for Immunization against Francisellosis in Nile Tilapia (Oreochromis niloticus) and Atlantic Cod (Gadus morhua L.). Vaccines, 9(1), 34. https://doi.org/10.3390/vaccines9010034





