Immunotherapeutic Strategies for Neuroblastoma: Present, Past and Future
Abstract
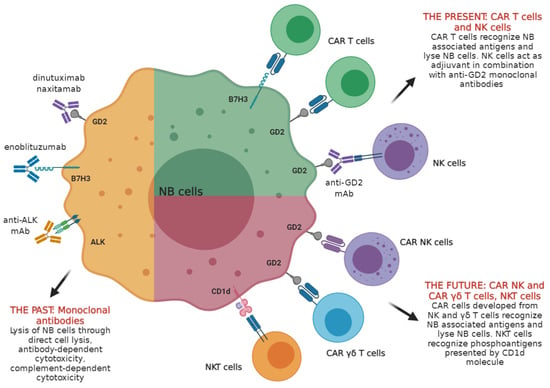
:1. General Features of Neuroblastoma
2. Conventional Therapies for High Risk Patients
3. Immunological Features of NB
4. Immunotherapeutic Approaches for Neuroblastoma
4.1. Antibodies Targeting NB in Clinical Settings: the GD2 Disialoganglioside Prototype and Other Tumor-Associated Antigens
4.2. Other NB Associated Antigens as Targets for Antibody-Mediated Immunotherapy
5. Adoptive Cell Therapy Based on NK Cells
6. Adoptive Cell Therapy Based on NKT Cells
7. CAR T Cells for Therapy of High Risk NB Patients
8. Future Prospects: CAR NK Cells and γδ T Cells
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maris, J.M. Recent advances in neuroblastoma. N. Engl. J. Med. 2010, 362, 2202–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolbert, V.P.; Matthay, K.K. Neuroblastoma: Clinical and biological approach to risk stratification and treatment. Cell Tissue Res. 2018, 372, 195–209. [Google Scholar] [CrossRef]
- London, W.B.; Castleberry, R.P.; Matthay, K.K.; Look, A.T.; Seeger, R.C.; Shimada, H.; Thorner, P.; Brodeur, G.; Maris, J.M.; Reynolds, C.P.; et al. Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children’s Oncology Group. J. Clin. Oncol. 2005, 23, 6459–6465. [Google Scholar] [CrossRef] [PubMed]
- Vo, K.T.; Matthay, K.K.; Neuhaus, J.; London, W.B.; Hero, B.; Ambros, P.F.; Nakagawara, A.; Miniati, D.; Wheeler, K.; Pearson, A.D.; et al. Clinical, biologic, and prognostic differences on the basis of primary tumor site in neuroblastoma: A report from the international neuroblastoma risk group project. J. Clin. Oncol. 2014, 32, 3169–3176. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.S.; Park, J.R. Neuroblastoma: Paradigm for precision medicine. Pediatr. Clin. N. Am. 2015, 62, 225–256. [Google Scholar] [CrossRef]
- Matthay, K.K.; Maris, J.M.; Schleiermacher, G.; Nakagawara, A.; Mackall, C.L.; Diller, L.; Weiss, W.A. Neuroblastoma. Nat. Rev. Dis. Prim. 2016, 2, 16078. [Google Scholar] [CrossRef]
- DuBois, S.G.; Kalika, Y.; Lukens, J.N.; Brodeur, G.M.; Seeger, R.C.; Atkinson, J.B.; Haase, G.M.; Black, C.T.; Perez, C.; Shimada, H.; et al. Metastatic sites in stage IV and IVS neuroblastoma correlate with age, tumor biology, and survival. J. Pediatr. Hematol. Oncol. 1999, 21, 181–189. [Google Scholar] [CrossRef]
- Morgenstern, D.A.; London, W.B.; Stephens, D.; Volchenboum, S.L.; Simon, T.; Nakagawara, A.; Shimada, H.; Schleiermacher, G.; Matthay, K.K.; Cohn, S.L.; et al. Prognostic significance of pattern and burden of metastatic disease in patients with stage 4 neuroblastoma: A study from the International Neuroblastoma Risk Group database. Eur. J. Cancer 2016, 65, 1–10. [Google Scholar] [CrossRef]
- Brodeur, G.; Seeger, R.; Schwab, M.; Varmus, H.; Bishop, J. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science 1984, 224, 1121–1124. [Google Scholar] [CrossRef]
- Seeger, R.C.; Brodeur, G.M.; Sather, H.; Dalton, A.; Siegel, S.E.; Wong, K.Y.; Hammond, D. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N. Engl. J. Med. 1985, 313, 1111–1116. [Google Scholar] [CrossRef]
- Matthay, K.K.; Shulkin, B.; Ladenstein, R.; Michon, J.; Giammarile, F.; Lewington, V.; Pearson, A.D.; Cohn, S.L. Criteria for evaluation of disease extent by (123)I-metaiodobenzylguanidine scans in neuroblastoma: A report for the International Neuroblastoma Risk Group (INRG) Task Force. Br. J. Cancer 2010, 102, 1319–1326. [Google Scholar] [CrossRef]
- Cohn, S.L.; Pearson, A.D.J.; London, W.B.; Monclair, T.; Ambros, P.F.; Brodeur, G.M.; Faldum, A.; Hero, B.; Iehara, T.; Machin, D.; et al. The International Neuroblastoma Risk Group (INRG) classification system: An INRG Task Force report. J. Clin. Oncol. 2009, 27, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.L.; Schmidt, M.L.; Cohn, S.L.; Maris, J.M.; London, W.B.; Buxton, A.; Stram, D.; Castleberry, R.P.; Shimada, H.; Sandler, A.; et al. Outcome after Reduced Chemotherapy for Intermediate-Risk Neuroblastoma. N. Engl. J. Med. 2010, 363, 1313–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubie, H.; De Bernardi, B.; Gerrard, M.; Canete, A.; Ladenstein, R.; Couturier, J.; Ambros, P.; Munzer, C.; Pearson, A.D.; Garaventa, A.; et al. Excellent outcome with reduced treatment in infants with nonmetastatic and unresectable neuroblastoma without MYCN amplification: Results of the prospective INES 99.1. J. Clin. Oncol. 2011, 29, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Strother, D.R.; London, W.B.; Schmidt, M.L.; Brodeur, G.M.; Shimada, H.; Thorner, P.; Collins, M.H.; Tagge, E.; Adkins, S.; Reynolds, C.P.; et al. Outcome after surgery alone or with restricted use of chemotherapy for patients with low-risk neuroblastoma: Results of Children’s Oncology Group study P9641. J. Clin. Oncol. 2012, 30, 1842–1848. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, D.A.; Pötschger, U.; Moreno, L.; Papadakis, V.; Owens, C.; Ash, S.; Pasqualini, C.; Luksch, R.; Garaventa, A.; Canete, A.; et al. Risk stratification of high-risk metastatic neuroblastoma: A report from the HR-NBL-1/SIOPEN study. Pediatr. Blood Cancer 2018, 65, 17. [Google Scholar] [CrossRef]
- Pinto, N.R.; Applebaum, M.A.; Volchenboum, S.L.; Matthay, K.K.; London, W.B.; Ambros, P.F.; Nakagawara, A.; Berthold, F.; Schleiermacher, G.; Park, J.R.; et al. Advances in Risk Classification and Treatment Strategies for Neuroblastoma. J. Clin. Oncol. 2015, 33, 3008–3017. [Google Scholar] [CrossRef]
- Di Giannatale, A.; Dias-Gastellier, N.; Devos, A.; Mc Hugh, K.; Boubaker, A.; Courbon, F.; Verschuur, A.; Ducassoul, S.; Malekzadeh, K.; Casanova, M.; et al. Phase II study of temozolomide in combination with topotecan (TOTEM) in relapsed or refractory neuroblastoma: A European Innovative Therapies for Children with Cancer-SIOP-European Neuroblastoma study. Eur. J. Cancer 2014, 50, 170–177. [Google Scholar] [CrossRef]
- Bagatell, R.; London, W.B.; Wagner, L.M.; Voss, S.D.; Stewart, C.F.; Maris, J.M.; Kretschmar, C.; Cohn, S.L. Phase II study of irinotecan and temozolomide in children with relapsed or refractory neuroblastoma: A Children’s Oncology Group study. J. Clin. Oncol. 2011, 29, 208–213. [Google Scholar] [CrossRef] [Green Version]
- Kushner, B.H.; Kramer, K.; Modak, S.; Cheung, N.-K.V. Irinotecan Plus Temozolomide for Relapsed or Refractory Neuroblastoma. J. Clin. Oncol. 2006, 24, 5271–5276. [Google Scholar] [CrossRef]
- London, W.B.; Frantz, C.N.; Campbell, L.A.; Seeger, R.C.; Brumback, B.A.; Cohn, S.L.; Matthay, K.K.; Castleberry, R.P.; Diller, L. Phase II randomized comparison of topotecan plus cyclophosphamide versus topotecan alone in children with recurrent or refractory neuroblastoma: A Children’s Oncology Group study. J. Clin. Oncol. 2010, 28, 3808–3815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berthold, F.; Boos, J.; Burdach, S.; Erttmann, R.; Henze, G.; Hermann, J.; Klingebiel, T.; Kremens, B.; Schilling, F.H.; Schrappe, M.; et al. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: A randomised controlled trial. Lancet Oncol. 2005, 6, 649–658. [Google Scholar] [CrossRef]
- Matthay, K.K.; Villablanca, J.G.; Seeger, R.C.; Stram, D.O.; Harris, R.E.; Ramsay, N.K.; Swift, P.; Shimada, H.; Black, C.T.; Brodeur, G.M.; et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N. Engl. J. Med. 1999, 341, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.; Cotterill, S.J.; Germond, S.M.; Imeson, J.; de Kraker, J.; Jones, D.R. High dose melphalan in the treatment of advanced neuroblastoma: Results of a randomised trial (ENSG-1) by the European Neuroblastoma Study Group. Pediatr. Blood Cancer 2005, 44, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; London, W.B.; Kreissman, S.G.; Chen, H.X.; Smith, M.; Anderson, B.; Villablanca, J.G.; Matthay, K.K.; et al. Anti-GD2 Antibody with GM-CSF, Interleukin-2, and Isotretinoin for Neuroblastoma. N. Engl. J. Med. 2010, 363, 1324–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayano, D.; Kinuya, S. Current Consensus on I-131 MIBG Therapy. Nucl. Med. Mol. Imaging 2018, 52, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Matthay, K.K.; Yanik, G.; Messina, J.; Quach, A.; Huberty, J.; Cheng, S.C.; Veatch, J.; Goldsby, R.; Brophy, P.; Kersun, L.S.; et al. Phase II study on the effect of disease sites, age, and prior therapy on response to iodine-131-metaiodobenzylguanidine therapy in refractory neuroblastoma. J. Clin. Oncol. 2007, 25, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.S.; Gains, J.E.; Moroz, V.; Wheatley, K.; Gaze, M.N. A systematic review of 131I-meta iodobenzylguanidine molecular radiotherapy for neuroblastoma. Eur. J. Cancer 2014, 50, 801–815. [Google Scholar] [CrossRef]
- Zhou, M.J.; Doral, M.Y.; DuBois, S.G.; Villablanca, J.G.; Yanik, G.A.; Matthay, K.K. Different outcomes for relapsed versus refractory neuroblastoma after therapy with (131)I-metaiodobenzylguanidine ((131)I-MIBG). Eur. J. Cancer 2015, 51, 2465–2472. [Google Scholar] [CrossRef] [Green Version]
- Park, J.A.; Cheung, N.-K.V. Targets and Antibody Formats for Immunotherapy of Neuroblastoma. J. Clin. Oncol. 2020, 38, 1836–1848. [Google Scholar] [CrossRef]
- Corrias, M.V.; Occhino, M.; Croce, M.; De Ambrosis, A.; Pistillo, M.P.; Bocca, P.; Pistoia, V.; Ferrini, S. Lack of HLA-class I antigens in human neuroblastoma cells: Analysis of its relationship to TAP and tapasin expression. Tissue Antigens 2001, 57, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Marimpietri, D.; Petretto, A.; Raffaghello, L.; Pezzolo, A.; Gagliani, C.; Tacchetti, C.; Mauri, P.; Melioli, G.; Pistoia, V. Proteome profiling of neuroblastoma-derived exosomes reveal the expression of proteins potentially involved in tumor progression. PLoS ONE 2013, 8, e75054. [Google Scholar] [CrossRef] [PubMed]
- Morandi, F.; Levreri, I.; Bocca, P.; Galleni, B.; Raffaghello, L.; Ferrone, S.; Prigione, I.; Pistoia, V. Human Neuroblastoma Cells Trigger an Immunosuppressive Program in Monocytes by Stimulating Soluble HLA-G Release. Cancer Res. 2007, 67, 6433–6441. [Google Scholar] [CrossRef] [Green Version]
- Morandi, F.; Rizzo, R.; Fainardi, E.; Rouas-Freiss, N.; Pistoia, V. Recent Advances in Our Understanding of HLA-G Biology: Lessons from a Wide Spectrum of Human Diseases. J. Immunol. Res. 2016, 2016, 4326495. [Google Scholar] [CrossRef] [Green Version]
- Raffaghello, L.; Prigione, I.; Airoldi, I.; Camoriano, M.; Morandi, F.; Bocca, P.; Gambini, C.; Ferrone, S.; Pistoia, V. Mechanisms of immune evasion of human neuroblastoma. Cancer Lett. 2005, 228, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef] [Green Version]
- Feichtinger, R.G.; Neureiter, D.; Mayr, J.A.; Zimmermann, F.A.; Berthold, F.; Jones, N.; Sperl, W.; Kofler, B. Loss of mitochondria in ganglioneuromas. Front. Biosci. 2011, 3, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Feichtinger, R.G.; Zimmermann, F.; Mayr, J.A.; Neureiter, D.; Hauser-Kronberger, C.; Schilling, F.H.; Jones, N.; Sperl, W.; Kofler, B. Low aerobic mitochondrial energy metabolism in poorly- or undifferentiated neuroblastoma. BMC Cancer 2010, 10, 149. [Google Scholar] [CrossRef] [Green Version]
- Morscher, R.J.; Aminzadeh-Gohari, S.; Feichtinger, R.G.; Mayr, J.A.; Lang, R.; Neureiter, D.; Sperl, W.; Kofler, B. Inhibition of Neuroblastoma Tumor Growth by Ketogenic Diet and/or Calorie Restriction in a CD1-Nu Mouse Model. PLoS ONE 2015, 10, e0129802. [Google Scholar] [CrossRef] [Green Version]
- Asgharzadeh, S.; Salo, J.A.; Ji, L.; Oberthuer, A.; Fischer, M.; Berthold, F.; Hadjidaniel, M.; Liu, C.W.; Metelitsa, L.S.; Pique-Regi, R.; et al. Clinical significance of tumor-associated inflammatory cells in metastatic neuroblastoma. J. Clin. Oncol. 2012, 30, 3525–3532. [Google Scholar] [CrossRef] [Green Version]
- Pelizzo, G.; Veschi, V.; Mantelli, M.; Croce, S.; Di Benedetto, V.; D’Angelo, P.; Maltese, A.; Catenacci, L.; Apuzzo, T.; Scavo, E.; et al. Microenvironment in neuroblastoma: Isolation and characterization of tumor-derived mesenchymal stromal cells. BMC Cancer 2018, 18, 1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanichapol, T.; Chutipongtanate, S.; Anurathapan, U.; Hongeng, S. Immune Escape Mechanisms and Future Prospects for Immunotherapy in Neuroblastoma. BioMed Res. Int. 2018, 2018, 1812535. [Google Scholar] [CrossRef] [PubMed]
- Lammie, G.; Cheung, N.; Gerald, W.; Rosenblum, M.; Cordoncardo, C. Ganglioside gd(2) expression in the human nervous-system and in neuroblastomas—An immunohistochemical study. Int. J. Oncol. 1993, 3, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Cheung, N.K. Disialoganglioside GD2 as a therapeutic target for human diseases. Expert Opin. Ther. Targets 2015, 19, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Heiner, J.P.; Miraldi, F.; Kallick, S.; Makley, J.; Neely, J.; Smith-Mensah, W.H.; Cheung, N.K. Localization of GD2-specific monoclonal antibody 3F8 in human osteosarcoma. Cancer Res. 1987, 47, 5377–5381. [Google Scholar] [PubMed]
- Ploessl, C.; Pan, A.; Maples, K.T.; Lowe, D.K. Dinutuximab: An Anti-GD2 Monoclonal Antibody for High-Risk Neuroblastoma. Ann. Pharmacother. 2016, 50, 416–422. [Google Scholar] [CrossRef]
- Kushner, B.H.; Cheung, N.K. GM-CSF enhances 3F8 monoclonal antibody-dependent cellular cytotoxicity against human melanoma and neuroblastoma. Blood 1989, 73, 1936–1941. [Google Scholar] [CrossRef] [Green Version]
- Ladenstein, R.; Pötschger, U.; Valteau-Couanet, D.; Luksch, R.; Castel, V.; Yaniv, I.; Laureys, G.; Brock, P.; Michon, J.M.; Owens, C.; et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): A multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1617–1629. [Google Scholar] [CrossRef]
- Munn, D.H.; Cheung, N.K. Antibody-dependent antitumor cytotoxicity by human monocytes cultured with recombinant macrophage colony-stimulating factor. Induction of efficient antibody-mediated antitumor cytotoxicity not detected by isotope release assays. J. Exp. Med. 1989, 170, 511–526. [Google Scholar] [CrossRef] [Green Version]
- Saarinen, U.M.; Coccia, P.F.; Gerson, S.L.; Pelley, R.; Cheung, N.K. Eradication of neuroblastoma cells in vitro by monoclonal antibody and human complement: Method for purging autologous bone marrow. Cancer Res. 1985, 45, 5969–5975. [Google Scholar]
- Kushner, B.H.; Cheung, I.Y.; Modak, S.; Basu, E.M.; Roberts, S.S.; Cheung, N.-K. Humanized 3F8 Anti-GD2 Monoclonal Antibody Dosing With Granulocyte-Macrophage Colony-Stimulating Factor in Patients With Resistant Neuroblastoma: A Phase 1 Clinical Trial. JAMA Oncol. 2018, 4, 1729–1735. [Google Scholar] [CrossRef] [Green Version]
- Mody, R.; Naranjo, A.; Van Ryn, C.; Yu, A.L.; London, W.B.; Shulkin, B.L.; Parisi, M.T.; Servaes, S.-E.N.; Diccianni, M.B.; Sondel, P.M.; et al. Irinotecan-temozolomide with temsirolimus or dinutuximab in children with refractory or relapsed neuroblastoma (COG ANBL1221): An open-label, randomised, phase 2 trial. Lancet. Oncol. 2017, 18, 946–957. [Google Scholar] [CrossRef] [Green Version]
- Voeller, J.; Sondel, P.M. Advances in Anti-GD2 Immunotherapy for Treatment of High-risk Neuroblastoma. J. Pediatr. Hematol. Oncol. 2019, 41, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Lin, J.-X.; Leonard, W.J. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 2013, 38, 13–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, M.; Reaman, G.H.; Hank, J.A.; Cairo, M.S.; Anderson, P.; Blazar, B.R.; Frierdich, S.; Sondel, P.M. A phase II trial of human recombinant lnterleukin-2 administered as a 4-day continuous infusion for children with refractory neuroblastoma, non-Hodgkin’s lymphoma, sarcoma, renal cell carcinoma, and malignant melanoma. A childrens cancer group study. Cancer 1995, 75, 2959–2965. [Google Scholar] [CrossRef]
- Kalwak, K.; Ussowicz, M.; Gorczyńska, E.; Turkiewicz, D.; Toporski, J.; Dobaczewski, G.; Latos-Grazyńska, E.; Ryczan, R.; Noworolska-Sauren, D.; Chybicka, A. Immunologic effects of intermediate-dose IL-2 i.v. after autologous hematopoietic cell transplantation in pediatric solid tumors. J. Interf. Cytokine Res. 2003, 23, 173–181. [Google Scholar] [CrossRef]
- Roper, M.; Smith, M.A.; Sondel, P.M.; Gillespie, A.; Reaman, G.H.; Hammond, G.D.; Levitt, D.; Rosolen, A.; Colamonici, O.R.; Neckers, L.M.; et al. A phase I study of interleukin-2 in children with cancer. Am. J. Pediatr. Hematol. Oncol. 1992, 14, 305–311. [Google Scholar] [CrossRef]
- Becher, B.; Tugues, S.; Greter, M. GM-CSF: From Growth Factor to Central Mediator of Tissue Inflammation. Immunity 2016, 45, 963–973. [Google Scholar] [CrossRef] [Green Version]
- Bresler, S.C.; Weiser, D.A.; Huwe, P.J.; Park, J.H.; Krytska, K.; Ryles, H.; Laudenslager, M.; Rappaport, E.F.; Wood, A.C.; McGrady, P.W.; et al. ALK mutations confer differential oncogenic activation and sensitivity to ALK inhibition therapy in neuroblastoma. Cancer Cell 2014, 26, 682–694. [Google Scholar] [CrossRef] [Green Version]
- Schulte, J.H.; Schulte, S.; Heukamp, L.C.; Astrahantseff, K.; Stephan, H.; Fischer, M.; Schramm, A.; Eggert, A. Targeted Therapy for Neuroblastoma: ALK Inhibitors. Klin. Padiatr. 2013, 225, 303–308. [Google Scholar] [CrossRef]
- Mossé, Y.P.; Lim, M.S.; Voss, S.D.; Wilner, K.; Ruffner, K.; Laliberte, J.; Rolland, D.; Balis, F.M.; Maris, J.M.; Weigel, B.J.; et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: A Children’s Oncology Group phase 1 consortium study. Lancet Oncol. 2013, 14, 472–480. [Google Scholar] [CrossRef] [Green Version]
- Camidge, D.R.; Doebele, R.C. Treating ALK-positive lung cancer--early successes and future challenges. Nat. Rev. Clin. Oncol. 2012, 9, 268–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doebele, R.C.; Pilling, A.B.; Aisner, D.L.; Kutateladze, T.G.; Le, A.T.; Weickhardt, A.J.; Kondo, K.L.; Linderman, D.J.; Heasley, L.E.; Franklin, W.A.; et al. Mechanisms of Resistance to Crizotinib in Patients with ALK Gene Rearranged Non–Small Cell Lung Cancer. Clin. Cancer Res. 2012, 18, 1472–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katayama, R.; Shaw, A.T.; Khan, T.M.; Mino-Kenudson, M.; Solomon, B.J.; Halmos, B.; Jessop, N.A.; Wain, J.C.; Yeo, A.T.; Benes, C.; et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung Cancers. Sci. Transl. Med. 2012, 4, 120ra117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sano, R.; Krytska, K.; Larmour, C.E.; Raman, P.; Martinez, D.; Ligon, G.F.; Lillquist, J.S.; Cucchi, U.; Orsini, P.; Rizzi, S.; et al. An antibody-drug conjugate directed to the ALK receptor demonstrates efficacy in preclinical models of neuroblastoma. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Loo, D.; Alderson, R.F.; Chen, F.Z.; Huang, L.; Zhang, W.; Gorlatov, S.; Burke, S.; Ciccarone, V.; Li, H.; Yang, Y.; et al. Development of an Fc-Enhanced Anti–B7-H3 Monoclonal Antibody with Potent Antitumor Activity. Clin. Cancer Res. 2012, 18, 3834–3845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caligiuri, M.A. Human natural killer cells. Blood 2008, 112, 461–469. [Google Scholar] [CrossRef]
- Vivier, E.; Raulet, D.H.; Moretta, A.; Caligiuri, M.A.; Zitvogel, L.; Lanier, L.L.; Yokoyama, W.M.; Ugolini, S. Innate or Adaptive Immunity? The Example of Natural Killer Cells. Science 2011, 331, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef]
- Brenner, M.K.; Heslop, H.; Krance, R.; Horowitz, M.; Strother, D.; Nuchtern, J.; Grilley, B.; Martingano, E.; Cooper, K. Phase I study of chemokine and cytokine gene-modified autologous neuroblastoma cells for treatment of relapsed/refractory neuroblastoma using an adenoviral vector. Hum. Gene Ther. 2000, 11, 1477–1488. [Google Scholar] [CrossRef]
- Handgretinger, R.; Baader, P.; Dopfer, R.; Klingebiel, T.; Reuland, P.; Treuner, J.; Reisfeld, R.A.; Niethammer, D. A phase I study of neuroblastoma with the anti-ganglioside GD2 antibody 14.G2a. Cancer Immunol. Immunother. 1992, 35, 199–204. [Google Scholar] [CrossRef]
- Kushner, B.H.; Kramer, K.; Cheung, N.K. Phase II trial of the anti-G(D2) monoclonal antibody 3F8 and granulocyte-macrophage colony-stimulating factor for neuroblastoma. J. Clin. Oncol. 2001, 19, 4189–4194. [Google Scholar] [CrossRef]
- Ozkaynak, M.F.; Sondel, P.M.; Krailo, M.D.; Gan, J.; Javorsky, B.; Reisfeld, R.A.; Matthay, K.K.; Reaman, G.H.; Seeger, R.C. Phase I study of chimeric human/murine anti-ganglioside G(D2) monoclonal antibody (ch14.18) with granulocyte-macrophage colony-stimulating factor in children with neuroblastoma immediately after hematopoietic stem-cell transplantation: A Children’s Cancer Group Study. J. Clin. Oncol. 2000, 18, 4077–4085. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, R.F.; Haight, A.E.; Hirschmann-Jax, C.; Yvon, E.S.; Rill, D.R.; Mei, Z.; Smith, S.C.; Inman, S.; Cooper, K.; Alcoser, P.; et al. Local and systemic effects of an allogeneic tumor cell vaccine combining transgenic human lymphotactin with interleukin-2 in patients with advanced or refractory neuroblastoma. Blood 2003, 101, 1718–1726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moretta, L.; Moretta, A. Killer immunoglobulin-like receptors. Curr. Opin. Immunol. 2004, 16, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Castriconi, R.; Dondero, A.; Corrias, M.V.; Lanino, E.; Pende, D.; Moretta, L.; Bottino, C.; Moretta, A. Natural killer cell-mediated killing of freshly isolated neuroblastoma cells: Critical role of DNAX accessory molecule-1-poliovirus receptor interaction. Cancer Res. 2004, 64, 9180–9184. [Google Scholar] [CrossRef] [Green Version]
- Castriconi, R.; Dondero, A.; Augugliaro, R.; Cantoni, C.; Carnemolla, B.; Sementa, A.R.; Negri, F.; Conte, R.; Corrias, M.V.; Moretta, L.; et al. Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc. Natl. Acad. Sci. USA 2004, 101, 12640–12645. [Google Scholar] [CrossRef] [Green Version]
- Castriconi, R.; Dondero, A.; Cilli, M.; Ognio, E.; Pezzolo, A.; De Giovanni, B.; Gambini, C.; Pistoia, V.; Moretta, L.; Moretta, A.; et al. Human NK cell infusions prolong survival of metastatic human neuroblastoma-bearing NOD/scid mice. Cancer Immunol. Immunother. 2007, 56, 1733–1742. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, H.W.; Sheard, M.A.; Sposto, R.; Somanchi, S.S.; Cooper, L.J.; Lee, D.A.; Seeger, R.C. Growth and activation of natural killer cells ex vivo from children with neuroblastoma for adoptive cell therapy. Clin. Cancer Res. 2013, 19, 2132–2143. [Google Scholar] [CrossRef] [Green Version]
- Barry, W.E.; Jackson, J.R.; Asuelime, G.E.; Wu, H.W.; Sun, J.; Wan, Z.; Malvar, J.; Sheard, M.A.; Wang, L.; Seeger, R.C.; et al. Activated Natural Killer Cells in Combination with Anti-GD2 Antibody Dinutuximab Improve Survival of Mice after Surgical Resection of Primary Neuroblastoma. Clin. Cancer Res. 2019, 25, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Tran, H.C.; Wan, Z.; Sheard, M.A.; Sun, J.; Jackson, J.R.; Malvar, J.; Xu, Y.; Wang, L.; Sposto, R.; Kim, E.S.; et al. TGFβR1 Blockade with Galunisertib (LY2157299) Enhances Anti-Neuroblastoma Activity of the Anti-GD2 Antibody Dinutuximab (ch14.18) with Natural Killer Cells. Clin. Cancer Res. 2017, 23, 804–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Federico, S.M.; McCarville, M.B.; Shulkin, B.L.; Sondel, P.M.; Hank, J.A.; Hutson, P.; Meagher, M.; Shafer, A.; Ng, C.Y.; Leung, W.; et al. A Pilot Trial of Humanized Anti-GD2 Monoclonal Antibody (hu14.18K322A) with Chemotherapy and Natural Killer Cells in Children with Recurrent/Refractory Neuroblastoma. Clin. Cancer Res. 2017, 23, 6441–6449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talleur, A.C.; Triplett, B.M.; Federico, S.; Mamcarz, E.; Janssen, W.; Wu, J.; Shook, D.; Leung, W.; Furman, W.L. Consolidation Therapy for Newly Diagnosed Pediatric Patients with High-Risk Neuroblastoma Using Busulfan/Melphalan, Autologous Hematopoietic Cell Transplantation, Anti-GD2 Antibody, Granulocyte-Macrophage Colony–Stimulating Factor, Interleukin-2, and Haploidentical Natural Killer Cells. Biol. Blood Marrow Transplant. 2017, 23, 1910–1917. [Google Scholar] [CrossRef]
- Nguyen, R.; Sahr, N.; Sykes, A.; McCarville, M.B.; Federico, S.M.; Sooter, A.; Cullins, D.; Rooney, B.; Janssen, W.E.; Talleur, A.C.; et al. Longitudinal NK cell kinetics and cytotoxicity in children with neuroblastoma enrolled in a clinical phase II trial. J. Immunother. Cancer 2020, 8, e000176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modak, S.; Le Luduec, J.B.; Cheung, I.Y.; Goldman, D.A.; Ostrovnaya, I.; Doubrovina, E.; Basu, E.; Kushner, B.H.; Kramer, K.; Roberts, S.S.; et al. Adoptive immunotherapy with haploidentical natural killer cells and Anti-GD2 monoclonal antibody m3F8 for resistant neuroblastoma: Results of a phase I study. Oncoimmunology 2018, 7, e1461305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinze, A.; Grebe, B.; Bremm, M.; Huenecke, S.; Munir, T.A.; Graafen, L.; Frueh, J.T.; Merker, M.; Rettinger, E.; Soerensen, J.; et al. The Synergistic Use of IL-15 and IL-21 for the Generation of NK Cells From CD3/CD19-Depleted Grafts Improves Their ex vivo Expansion and Cytotoxic Potential Against Neuroblastoma: Perspective for Optimized Immunotherapy Post Haploidentical Stem Cell Transplantation. Front. Immunol. 2019, 10, 2816. [Google Scholar] [CrossRef] [Green Version]
- Kronenberg, M.; Gapin, L. The unconventional lifestyle of NKT cells. Nat. Rev. Immunol. 2002, 2, 557–568. [Google Scholar] [CrossRef]
- Berzofsky, J.A.; Terabe, M. The contrasting roles of NKT cells in tumor immunity. Curr. Mol. Med. 2009, 9, 667–672. [Google Scholar] [CrossRef] [Green Version]
- Swann, J.; Crowe, N.Y.; Hayakawa, Y.; Godfrey, D.I.; Smyth, M.J. Regulation of antitumour immunity by CD1d-restricted NKT cells. Immunol. Cell Biol. 2004, 82, 323–331. [Google Scholar] [CrossRef]
- Swann, J.B.; Coquet, J.M.; Smyth, M.J.; Godfrey, D.I. CD1-restricted T cells and tumor immunity. Curr. Top. Microbiol. Immunol. 2007, 314, 293–323. [Google Scholar] [CrossRef]
- Dhodapkar, M.V.; Geller, M.D.; Chang, D.H.; Shimizu, K.; Fujii, S.-I.; Dhodapkar, K.M.; Krasovsky, J. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J. Exp. Med. 2003, 197, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Muhammad Ali Tahir, S.; Cheng, O.; Shaulov, A.; Koezuka, Y.; Bubley, G.J.; Wilson, S.B.; Balk, S.P.; Exley, M.A. Loss of IFN-γ Production by Invariant NK T Cells in Advanced Cancer. J. Immunol. 2001, 167, 4046–4050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanagisawa, K.; Seino, K.-i.; Ishikawa, Y.; Nozue, M.; Todoroki, T.; Fukao, K. Impaired Proliferative Response of Vα24 NKT Cells from Cancer Patients Against α-Galactosylceramide. J. Immunol. 2002, 168, 6494–6499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabry, M.; Lowdell, M.W. Killers at the crossroads: The use of innate immune cells in adoptive cellular therapy of cancer. STEM CELLS Transl. Med. 2020, 9, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Song, L.; Wei, J.; Courtney, A.N.; Gao, X.; Marinova, E.; Guo, L.; Heczey, A.; Asgharzadeh, S.; Kim, E.; et al. IL-15 protects NKT cells from inhibition by tumor-associated macrophages and enhances antimetastatic activity. J. Clin. Investig. 2012, 122, 2221–2233. [Google Scholar] [CrossRef] [Green Version]
- Gross, G.; Gorochov, G.; Waks, T.; Eshhar, Z. Generation of effector T cells expressing chimeric T cell receptor with antibody type-specificity. Transplant. Proc. 1989, 21, 127–130. [Google Scholar]
- Hartmann, J.; Schüßler-Lenz, M.; Bondanza, A.; Buchholz, C.J. Clinical development of CAR T cells—challenges and opportunities in translating innovative treatment concepts. EMBO Mol. Med. 2017, 9, 1183–1197. [Google Scholar] [CrossRef]
- Majzner, R.G.; Mackall, C.L. Clinical lessons learned from the first leg of the CAR T cell journey. Nat. Med. 2019, 25, 1341–1355. [Google Scholar] [CrossRef]
- Yazdanifar, M.; Barbarito, G.; Bertaina, A.; Airoldi, I. γδ T Cells: The Ideal Tool for Cancer Immunotherapy. Cells 2020, 9, 1305. [Google Scholar] [CrossRef]
- Yazdanifar, M.; Zhou, R.; Mukherjee, P. Emerging immunotherapeutics in adenocarcinomas: A focus on CAR-T cells. Curr. Trends Immunol. 2016, 17, 95–115. [Google Scholar] [PubMed]
- Allegra, A.; Innao, V.; Gerace, D.; Vaddinelli, D.; Musolino, C. Adoptive immunotherapy for hematological malignancies: Current status and new insights in chimeric antigen receptor T cells. Blood Cells Mol. Dis. 2016, 62, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Chavez, J.C.; Bachmeier, C.; Kharfan-Dabaja, M.A. CAR T-cell therapy for B-cell lymphomas: Clinical trial results of available products. Ther. Adv. Hematol. 2019, 10, 2040620719841581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Li, W.; Huang, K.; Zhang, Y.; Kupfer, G.; Zhao, Q. Chimeric antigen receptor T cell (CAR-T) immunotherapy for solid tumors: Lessons learned and strategies for moving forward. J. Hematol. Oncol. 2018, 11, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gargett, T.; Brown, M.P. The inducible caspase-9 suicide gene system as a “safety switch” to limit on-target, off-tumor toxicities of chimeric antigen receptor T cells. Front. Pharmacol. 2014, 5, 235. [Google Scholar] [CrossRef] [PubMed]
- Iuliucci, J.D.; Oliver, S.D.; Morley, S.; Ward, C.; Ward, J.; Dalgarno, D.; Clackson, T.; Berger, H.J. Intravenous safety and pharmacokinetics of a novel dimerizer drug, AP1903, in healthy volunteers. J. Clin. Pharmacol. 2001, 41, 870–879. [Google Scholar] [CrossRef]
- Park, J.R.; Digiusto, D.L.; Slovak, M.; Wright, C.; Naranjo, A.; Wagner, J.; Meechoovet, H.B.; Bautista, C.; Chang, W.C.; Ostberg, J.R.; et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol. Ther. 2007, 15, 825–833. [Google Scholar] [CrossRef]
- Pule, M.A.; Savoldo, B.; Myers, G.D.; Rossig, C.; Russell, H.V.; Dotti, G.; Huls, M.H.; Liu, E.; Gee, A.P.; Mei, Z.; et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: Persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 2008, 14, 1264–1270. [Google Scholar] [CrossRef]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef] [Green Version]
- Heczey, A.; Louis, C.U.; Savoldo, B.; Dakhova, O.; Durett, A.; Grilley, B.; Liu, H.; Wu, M.F.; Mei, Z.; Gee, A.; et al. CAR T Cells Administered in Combination with Lymphodepletion and PD-1 Inhibition to Patients with Neuroblastoma. Mol. Ther. 2017, 25, 2214–2224. [Google Scholar] [CrossRef] [Green Version]
- Louis, C.U.; Savoldo, B.; Dotti, G.; Pule, M.; Yvon, E.; Myers, G.D.; Rossig, C.; Russell, H.V.; Diouf, O.; Liu, E.; et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 2011, 118, 6050–6056. [Google Scholar] [CrossRef]
- Quintarelli, C.; Orlando, D.; Boffa, I.; Guercio, M.; Polito, V.A.; Petretto, A.; Lavarello, C.; Sinibaldi, M.; Weber, G.; Del Bufalo, F.; et al. Choice of costimulatory domains and of cytokines determines CAR T-cell activity in neuroblastoma. Oncoimmunology 2018, 7, e1433518. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, C.; Landoni, E.; Metelitsa, L.; Dotti, G.; Savoldo, B. Eradication of Neuroblastoma by T Cells Redirected with an Optimized GD2-Specific Chimeric Antigen Receptor and Interleukin-15. Clin. Cancer Res. 2019, 25, 2915–2924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Künkele, A.; Taraseviciute, A.; Finn, L.S.; Johnson, A.J.; Berger, C.; Finney, O.; Chang, C.A.; Rolczynski, L.S.; Brown, C.; Mgebroff, S.; et al. Preclinical Assessment of CD171-Directed CAR T-cell Adoptive Therapy for Childhood Neuroblastoma: CE7 Epitope Target Safety and Product Manufacturing Feasibility. Clin. Cancer Res. 2017, 23, 466–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawaisorn, P.; Atjanasuppat, K.; Anurathapan, U.; Chutipongtanate, S.; Hongeng, S. Strategies to Improve Chimeric Antigen Receptor Therapies for Neuroblastoma. Vaccines 2020, 8, 753. [Google Scholar] [CrossRef] [PubMed]
- Vermijlen, D.; Gatti, D.; Kouzeli, A.; Rus, T.; Eberl, M. γδ T cell responses: How many ligands will it take till we know? Semin. Cell Dev. Biol. 2018, 84, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Minculescu, L.; Sengeløv, H. The role of gamma delta T cells in haematopoietic stem cell transplantation. Scand. J. Immunol. 2015, 81, 459–468. [Google Scholar] [CrossRef]
- Davey, M.S.; Willcox, C.R.; Joyce, S.P.; Ladell, K.; Kasatskaya, S.A.; McLaren, J.E.; Hunter, S.; Salim, M.; Mohammed, F.; Price, D.A.; et al. Clonal selection in the human Vδ1 T cell repertoire indicates γδ TCR-dependent adaptive immune surveillance. Nat. Commun. 2017, 8, 14760. [Google Scholar] [CrossRef]
- Nussbaumer, O.; Koslowski, M. The emerging role of γδ T cells in cancer immunotherapy. Immuno-Oncol. Technol. 2019, 1, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Brandes, M.; Willimann, K.; Moser, B. Professional antigen-presentation function by human gammadelta T Cells. Science 2005, 309, 264–268. [Google Scholar] [CrossRef]
- Capsomidis, A.; Benthall, G.; Van Acker, H.H.; Fisher, J.; Kramer, A.M.; Abeln, Z.; Majani, Y.; Gileadi, T.; Wallace, R.; Gustafsson, K.; et al. Chimeric Antigen Receptor-Engineered Human Gamma Delta T Cells: Enhanced Cytotoxicity with Retention of Cross Presentation. Mol. Ther. 2018, 26, 354–365. [Google Scholar] [CrossRef] [Green Version]
- Silva-Santos, B.; Serre, K.; Norell, H. γδ T cells in cancer. Nat. Rev. Immunol. 2015, 15, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Morandi, F.; Yazdanifar, M. Engineering the Bridge between Innate and Adaptive Immunity for Cancer Immunotherapy: Focus on γδ T and NK Cells. Cells 2020, 9, 1757. [Google Scholar] [CrossRef] [PubMed]
- Lo Presti, E.; Pizzolato, G.; Corsale, A.M.; Caccamo, N.; Sireci, G.; Dieli, F.; Meraviglia, S. γδ T Cells and Tumor Microenvironment: From Immunosurveillance to Tumor Evasion. Front. Immunol. 2018, 9, 1395. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.R.; Mirzaei, H.; Lee, S.Y.; Hadjati, J.; Till, B.G. Prospects for chimeric antigen receptor (CAR) γδ T cells: A potential game changer for adoptive T cell cancer immunotherapy. Cancer Lett. 2016, 380, 413–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polito, V.A.; Cristantielli, R.; Weber, G.; Del Bufalo, F.; Belardinilli, T.; Arnone, C.M.; Petretto, A.; Antonucci, L.; Giorda, E.; Tumino, N.; et al. Universal Ready-to-Use Immunotherapeutic Approach for the Treatment of Cancer: Expanded and Activated Polyclonal γδ Memory T Cells. Front. Immunol. 2019, 10, 2717. [Google Scholar] [CrossRef] [Green Version]
- Buccheri, S.; Guggino, G.; Caccamo, N.; Li Donni, P.; Dieli, F. Efficacy and safety of γδT cell-based tumor immunotherapy: A meta-analysis. J. Biol. Regul. Homeost. Agents 2014, 28, 81–90. [Google Scholar]
- Rezvani, K.; Rouce, R.; Liu, E.; Shpall, E. Engineering Natural Killer Cells for Cancer Immunotherapy. Mol. Ther. 2017, 25, 1769–1781. [Google Scholar] [CrossRef]
- Boissel, L.; Betancur-Boissel, M.; Lu, W.; Krause, D.S.; Van Etten, R.A.; Wels, W.S.; Klingemann, H. Retargeting NK-92 cells by means of CD19- and CD20-specific chimeric antigen receptors compares favorably with antibody-dependent cellular cytotoxicity. Oncoimmunology 2013, 2, e26527. [Google Scholar] [CrossRef] [Green Version]
- Herrera, L.; Santos, S.; Vesga, M.A.; Anguita, J. Adult peripheral blood and umbilical cord blood NK cells are good sources for effective CAR therapy against CD19 positive leukemic cells. Sci. Rep. 2019, 9, 18729. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Yang, L.; Li, Z.; Nalin, A.P.; Dai, H.; Xu, T.; Yin, J.; You, F.; Zhu, M.; Shen, W.; et al. First-in-man clinical trial of CAR NK-92 cells: Safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am. J. Cancer Res. 2018, 8, 1083–1089. [Google Scholar]
- You, F.; Wang, Y.; Jiang, L.; Zhu, X.; Chen, D.; Yuan, L.; An, G.; Meng, H.; Yang, L. A novel CD7 chimeric antigen receptor-modified NK-92MI cell line targeting T-cell acute lymphoblastic leukemia. Am. J. Cancer Res. 2019, 9, 64–78. [Google Scholar] [PubMed]
- Colamartino, A.B.L.; Lemieux, W.; Bifsha, P.; Nicoletti, S.; Chakravarti, N.; Sanz, J.; Roméro, H.; Selleri, S.; Béland, K.; Guiot, M.; et al. Efficient and Robust NK-Cell Transduction With Baboon Envelope Pseudotyped Lentivector. Front. Immunol. 2019, 10, 2873. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Burger, M.C.; Jennewein, L.; Genßler, S.; Schönfeld, K.; Zeiner, P.; Hattingen, E.; Harter, P.N.; Mittelbronn, M.; Tonn, T.; et al. ErbB2/HER2-Specific NK Cells for Targeted Therapy of Glioblastoma. J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Han, J.; Chu, J.; Zhang, L.; Zhang, J.; Chen, C.; Chen, L.; Wang, Y.; Wang, H.; Yi, L.; et al. A combinational therapy of EGFR-CAR NK cells and oncolytic herpes simplex virus 1 for breast cancer brain metastases. Oncotarget 2016, 7, 27764–27777. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Chu, J.; Keung Chan, W.; Zhang, J.; Wang, Y.; Cohen, J.B.; Victor, A.; Meisen, W.H.; Kim, S.H.; Grandi, P.; et al. CAR-Engineered NK Cells Targeting Wild-Type EGFR and EGFRvIII Enhance Killing of Glioblastoma and Patient-Derived Glioblastoma Stem Cells. Sci. Rep. 2015, 5, 11483. [Google Scholar] [CrossRef]
- Pinz, K.G.; Yakaboski, E.; Jares, A.; Liu, H.; Firor, A.E.; Chen, K.H.; Wada, M.; Salman, H.; Tse, W.; Hagag, N.; et al. Targeting T-cell malignancies using anti-CD4 CAR NK-92 cells. Oncotarget 2017, 8, 112783–112796. [Google Scholar] [CrossRef] [Green Version]
- Zhang, E.; Yang, P.; Gu, J.; Wu, H.; Chi, X.; Liu, C.; Wang, Y.; Xue, J.; Qi, W.; Sun, Q.; et al. Recombination of a dual-CAR-modified T lymphocyte to accurately eliminate pancreatic malignancy. J. Hematol. Oncol. 2018, 11, 102. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Luo, H.; Fan, M.; Wu, X.; Shi, B.; Di, S.; Liu, Y.; Pan, Z.; Jiang, H.; Li, Z. Development of GPC3-Specific Chimeric Antigen Receptor-Engineered Natural Killer Cells for the Treatment of Hepatocellular Carcinoma. Mol. Ther. 2018, 26, 366–378. [Google Scholar] [CrossRef] [Green Version]
- Chu, J.; Deng, Y.; Benson, D.M.; He, S.; Hughes, T.; Zhang, J.; Peng, Y.; Mao, H.; Yi, L.; Ghoshal, K.; et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia 2014, 28, 917–927. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Zhang, H.; Ding, J.; Liu, H.; Li, H. Combination Therapy with EpCAM-CAR-NK-92 Cells and Regorafenib against Human Colorectal Cancer Models. J. Immunol. Res. 2018, 2018, 4263520. [Google Scholar] [CrossRef] [Green Version]
- Esser, R.; Müller, T.; Stefes, D.; Kloess, S.; Seidel, D.; Gillies, S.D.; Aperlo-Iffland, C.; Huston, J.S.; Uherek, C.; Schönfeld, K.; et al. NK cells engineered to express a GD2-specific antigen receptor display built-in ADCC-like activity against tumour cells of neuroectodermal origin. J. Cell. Mol. Med. 2012, 16, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Romanski, A.; Uherek, C.; Bug, G.; Seifried, E.; Klingemann, H.; Wels, W.S.; Ottmann, O.G.; Tonn, T. CD19-CAR engineered NK-92 cells are sufficient to overcome NK cell resistance in B-cell malignancies. J. Cell. Mol. Med. 2016, 20, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Kailayangiri, S.; Altvater, B.; Spurny, C.; Jamitzky, S.; Schelhaas, S.; Jacobs, A.H.; Wiek, C.; Roellecke, K.; Hanenberg, H.; Hartmann, W.; et al. Targeting Ewing sarcoma with activated and GD2-specific chimeric antigen receptor-engineered human NK cells induces upregulation of immune-inhibitory HLA-G. Oncoimmunology 2017, 6, e1250050. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| NRG Stage | Age (Months) | Histologic Category | Grade of Tumor Differentiation | MYCN | 11q Aberration | Ploidy | Pretreatment Risk Group |
|---|---|---|---|---|---|---|---|
| L1/L2 | GN maturing, GNB intermixed | A (very low) | |||||
| L1 | Any, except GN maturing or GNB intermixed | NA | B (very low) | ||||
| Amplified | K (high) | ||||||
| L2 | <18 | Any, except GN maturing or GNB intermixed | NA | No | D (low) | ||
| Yes | G (intermediate) | ||||||
| ≥18 | GNB nodular, neuroblastoma | Differentiating | NA | No | E (low) | ||
| Yes | H (intermediate) | ||||||
| Poorly differentiated or undifferentiated | NA | H (intermediate) | |||||
| Amplified | N (high) | ||||||
| M | <18 | NA | Hyperdiploid | F (low) | |||
| <12 | NA | Diploid | I (intermediate) | ||||
| 12 < 18 | NA | Diploid | J (intermediate) | ||||
| <18 | Amplified | O (high) | |||||
| ≥18 | P (high) | ||||||
| MS | <18 | NA | No | C (very low) | |||
| Yes | Q (high) | ||||||
| Amplified | R (high) |
| Identifier | Study Title | Phase | Start Date | Patients enrolled | Status | Primary Aims and References |
|---|---|---|---|---|---|---|
| NCT01183897 | 3F8/GM-CSF immunotherapy plus 13-cis-retinoic acid for primary refractory Neuroblastoma in bone marrow | II | 2010 | 31 | Completed | To find out what effects, good and/or bad, the combination of 3F8 and GM-CSF had on the patient and the cancer Another purpose was to see if high-dose 3F8 combined with GM-CSF is better than standard dose 3F8 in treating neuroblastoma Kushner BH et al. Cancer. 2013. |
| NCT01183429 | 3F8/GM-CSF immunotherapy plus 13-cis-retinoic acid for consolidation of first remission after non-myeloablative therapy in patients with high-risk neuroblastoma | II | 2010 | 39 | Completed | |
| NCT01183416 | High-Dose 3F8/GM-CSF immunotherapy plus 13-Cis-Retinoic acid for consolidation of first remission after myeloablative therapy and autologous stem-cell transplantation | II | 2010 | 4 | Completed | |
| NCT01041638 | Monoclonal antibody Ch14.18, sargramostim, aldesleukin and isotretinoin after autologous stem cell transplant in treating patients with neuroblastoma | III | 2010 | 105 | Active, not recruiting | To study the side effects of giving monoclonal antibody Ch14.18 together with sargramostim, aldesleukin and isotretinoin after autologous stem cell transplant in treating patients with neuroblastoma |
| NCT01334515 | Biological therapy, sargramostim and isotretinoinin treating patients with relapsed or refractory neuroblastoma | II | 2011 | 52 | Completed | To analyse how well hu14.18-interleukin-2 (IL2) fusion protein works when given together with sargramostim and isotretinoin in treating patients with relapsed or refractory neuroblastoma (Shusteman S et al. Clin Cancer Res. 2019) |
| NCT01757626 | Combination therapy of antibody Hu3F8 with GM-CSF in patients with relapsed/refractory high-risk neuroblastoma | I/II | 2012 | 224 | Recruiting | To find out if an antibody called Humanized 3F8 (Hu3F8) combined with granulocyte-macrophage colony stimulating factor is safe for treating neuroblastoma (Kushner BH et al. JAMA Oncol. 2018). |
| NCT01592045 | ch14.18 Pharmacokinetic study in high-risk neuroblastoma | I/II | 2012 | 28 | Completed | To compare the pharmacokinetics (blood levels) and safety of chimeric 14.18 manufactured by two independent drug makers |
| NCT01767194 | Irinotecan hydrochloride and temozolomide with temsirolimus or dinutuximab in treating younger patients with refractory or relapsed neuroblastoma | II | 2013 | 73 | Completed | To investigate how well irinotecan hydrochloride and temozolomide with temsirolimus or dinutuximab work in treating younger patients with neuroblastoma that has returned or does not respond to treatment. Mody R et al. Lancet Oncol. 2017. |
| NCT02100930 | Anti-GD2 3F8 monoclonal antibody and GM-CSF for high-risk neuroblastoma | NA | 2014 | 69 | Completed | To supply an experimental combination of drugs called 3F8 and sargramostim to patients with high-risk neuroblastoma who may benefit from treatment. |
| NCT02173093 | Activated T cells armed with GD2 bispecific antibody in children and young adults with neuroblastoma and osteosarcoma | I/II | 2014 | 40 | Recruiting | To study the side effects and best dose of activated T cells armed with GD2 bispecific antibody and how well they work in treating patients with neuroblastoma, osteosarcoma and other GD2-positive solid tumors. |
| NCT02130869 | A pilot study of immunotherapy including haploidentical NK cell infusion following CD133+ positively-selected autologous hematopoietic stem cells in children with high risk solid tumors or lymphomas | I | 2014 | 8 | Completed | In NB, to establish NK cell engraftment in patients receiving high-dose chemotherapy, stem cell infusion, hu14.18K322A, IL-2, haploidentical natural killer cell infusion, G-CSF and GM-CSF. |
| NCT02786719 | High-risk neuroblastoma chemotherapy without G-CSF | NA | 2016 | 13 | Completed | Whittle SB et al. Blood Cancer. 2020 |
| NCT03363373 | Naxitamab for high-risk neuroblastoma patients with primary refractory disease or incomplete response to salvage treatment in bone and/or bone marrow | II | 2017 | 95 | Recruiting | To define the effects of naxitamab and GM-CSF in children diagnosed with high-risk neuroblastoma with primary refractory disease or incomplete response to salvage treatment |
| NCT03033303 | A study of the effect of Hu3F8/GM-CSF immunotherapy plus isotretinoin in patients in first remission of high-risk neuroblastoma | II | 2017 | 59 | Recruiting | To test the combined effects of Humanized 3F8 in combination with granulocyte-macrophage colony stimulating factor |
| NCT03189706 | Study of chemoimmunotherapy for high-risk neuroblastoma | I | 2017 | 62 | Recruiting | To test the efficacy and safety of Hu3F8 combined with the chemotherapy drugs irinotecan and temozolomide or GM-CSF |
| NCT04211675 | NK cells infusions with irinotecan, temozolomide, and dinutuximab | I/II | 2019 | 31 | Not yet recruiting | Phase 1: to assess the safety and tolerability of autologous expanded NK cells in combination with irinotecan, temozolomide and dinituximab. Phase 2: to estimate the response to treatment |
| NCT03794349 | Irinotecan hydrochloride, temozolomide, and dinutuximab with or without eflornithine in treating patients with relapsed or refractory neuroblastoma | II | 2019 | 95 | Recruiting | To study how well irinotecan hydrochloride, temozolomide and dinutuximab work with or without eflornithine in patients with relapsed or refractory neuroblastoma |
| NCT04560166 | IT with or without naxitamab and GM CSF in patients with high-risk neuroblastoma | III | 2020 | 117 | Not yet recruiting | This is an open label, randomized, controlled, multicenter phase 3 trial, in patients ≥ 12 months of age with high-risk NB with primary refractory disease or in first relapse |
| NCT04385277 | Treatment with dinutuximab, sargramostim (GM-CSF), and isotretinoin in combination with irinotecan and temozolomide after intensive therapy for people with high-risk neuroblastoma (NBL) | II | 2020 | 45 | Not yet recruiting | Safety of dinutuximab, GM-CSF and isotretinoin in combination with irinotecan and temozolomide, in high-risk neuroblastoma patients after consolidation therapy |
| Identifier | Study Title | Phase | Start Date | Patients Enrolled | Status | Primary Aims and References |
|---|---|---|---|---|---|---|
| NCT01386619 | NK DLI in patients after human leukocyte antigen (HLA)-haploidentical hematopoietic stem cell transplantation (HSCT) | I/II | 2004 | 15 | Completed | To analyse the feasibility of expanded NK-cell DLI production and their safety, by evaluating regards transfusion associated adverse events and the absence of acute graft-versus-host disease 30 days after the last NK DLI infusion. The efficacy of NK DLI infusions has been also assessed by evaluating the rates of overall and disease free survival and the rate of disease relapse compared to patients treated with haploidentical HSCT without NK DLI infusions. |
| NCT00569283 | Donor natural killer cell infusion in preventing relapse or graft failure in patients who have undergone donor bone marrow transplant | I | 2007 | 18 | Completed | To evaluate the safety of donor natural killer (NK) cells, given as a single intravenous infusion in patients that underwent HLA-haploidentical familial donor bone marrow transplantation and to determine the maximum tolerated dose of donor NK cells infusion. The effectiveness of donor NK cell infusion was evaluated as ability to prevent tumor relapse and graft failure. |
| NCT00877110 | Anti-GD2 3F8 antibody and allogeneic natural killer cells for high-risk neuroblastoma | I | 2009 | 71 | Completed | To assess the feasibility and safety of administering allogeneic haploidentical NK infusions with mAb 3F8 in patients with high-risk NB. Moreover, the efficacy of allogeneic NK infusions plus 3F8 was evaluated as anti-tumor activity. The impact of KIR/HLA immunogenetics and CD16 polymorphism on disease response to NK/3F8 was also evaluated. |
| NCT01462396 | Allogeneic stem cell transplantation for advanced neuroblastoma using MHC mismatched related donors | I | 2011 | 4 | Completed | To evaluate the safety of a fludarabine based reduced intensity conditioning regimen and CD34+ stem cell selected mis-matched, related, allogeneic transplant in patients with relapsed/refractory neuroblastoma, by monitoring mortality, toxicity, acute and chronic graft versus host disease and engraftment rate. Anti-tumor effect has been also evaluated. |
| NCT01287104 | A phase I study of NK cell infusion following allogeneic peripheral blood stem cell transplantation from related or matched unrelated donors in pediatric patients with solid tumors and leukemias | I | 2011 | 34 | Completed | To assess the feasibility and toxicity of infusing escalating doses of donor-derived activated NK cell donor lymphocyte infusions (NK-DLI) following human leukocyte antigen (HLA)-matched T cell depleted (TCD) peripheral blood stem cell transplant (PBSCT) in patients with metastatic or recurrent pediatric solid tumors and high risk leukemias who have unrelated donors or related donors. In addition, donor engraftment and acute graft versus host disease have been evaluated. Shah NN et al. Blood. 2015. |
| NCT01386619 | NK DLI in patients after human leukocyte antigen (HLA)-haploidentical hematopoietic stem cell transplantation (HSCT) | I/II | 2011 | 15 | Completed | To evaluate the feasibility of NK-DLI production. The safety of NK DLI Infusion has been evaluated as transfusion associated adverse events (fever, fall in blood pressure, transfusion site reactions, etc) at the time of NK DLI infusion. The primary long-term safety measure is the absence of acute graft-versus-host disease 30 days after the last NK DLI infusion. The efficacy of NK DLI infusions has been assessed as rate of overall and disease free survival and disease relapse. |
| NCT01576692 | Combination chemotherapy, monoclonal antibody, and natural killer cells in treating young patients with recurrent or refractory neuroblastoma | I | 2012 | 34 | Completed | To evaluate the toxicity associated with humanized anti-GD2 antibody/chemotherapy associated or not with NK cells infusion. Clinical outcome was measured as response to therapy using response evaluation criteria in solid tumors, clearing of bone marrow and improvement in MIBG scans. Event-free and overall survival have been also analyzed. |
| NCT01701479 | Long term continuous infusion ch14.18/CHO plus s.c. aldesleukin (IL-2) | I/II | 2012 | 288 | Active, not recruiting | To find a way of giving ch14.18/CHO, in combination with subcutaneous aldesleukin (IL-2) and oral isotretinoin (13-cis-RA), to children and young people with primary refractory or relapsed neuroblastoma without intravenous morphine. |
| NCT01875601 | NK white blood cells and interleukin in children and young adults with advanced solid tumors | I | 2013 | 16 | Completed | To evaluate the feasibility of harvesting and expanding activated NK cells and to assess the toxicity of infusing escalating doses of activated NK cells following lymphodepleting chemotherapy with or without escalating doses of rhIL15 in pediatric patients with refractory malignant solid tumors. Kontny HU et al. Cell Death Differ. 2001. Yu AL et al. N Engl J Med. 2010. Dudley ME et al. Nat Rev Cancer. 2003. |
| NCT01857934 | Therapy for children with advanced stage neuroblastoma | II | 2013 | 153 | Active, not recruiting | To analyze event-free survival of patients with newly diagnosed high-risk NB treated with hu14.18K322A in addition to standard treatment. In addition, the tolerability of hu14.18K322A with allogeneic natural killer (NK) cells from an acceptable parent, in the immediate post-transplant period will be evaluated. Tolerability of hu14.18K322A with interleukin-2 and GM-CSF as treatment for minimal residual disease (MRD) will be assessed. Nguyen R et al. J Immunother Cancer. 2020. |
| NCT01807468 | Haploidentical stem cell transplantation and NK cell therapy in patients with high-risk solid tumors | II | 2013 | 12 | Active, not recruiting | To evaluate feasibility and efficacy of haploidentical stem cell transplantation followed (or not) by NK cell infusion in patients with high-risk solid tumors who failed after tandem high-dose chemotherapy and autologous stem cell transplantation. |
| NCT02130869 | A pilot study of immunotherapy including haploidentical NK cell infusion following CD133+positively-selected autologous hematopoietic stem cells in children with high risk solid tumors or lymphomas | I | 2014 | 8 | Completed | To investigate the addition of haploidentical natural killer (NK) cell infusion to high dose chemotherapy and autologous stem cell transplantation in children with high-risk solid tumors. In patients with neuroblastoma, the anti-GD2 antibody hu14.18K322A has been also given. Survival of children treated with this approach has been analyzed. |
| NCT02100891 | Phase 2 STIR trial: Haploidentical transplant and donor natural killer cells for solid tumors | II | 2014 | 15 | Active, not recruiting | In this study, patients with high-risk solid tumors (Ewings sarcoma, neuroblastoma and rhabdomyosarcoma) underwent haploidentical hematopoietic cell transplantation (HCT) followed by an early, post-transplant infusion of donor natural killer (NK) cells. Safety and efficacy will be analyzed. Efficacy will be evaluated as complete (CR) and partial (PR) response and stable disease (SD). Hattinger CM et al. Expert Opin Emerg Drugs. 2019. |
| NCT02573896 | Immunotherapy of relapsed refractory neuroblastoma with expanded NK cells | I | 2015 | 24 | Recruiting | To determine the maximum tolerated dose of autologous expanded natural killer (NK) cells when combined with standard dosing of ch14.18 and will assess the feasibility of adding lenalidomide at the recommended Phase II dose of the expanded NK cells with ch14.18, for treatment of children with refractory or recurrent neuroblastoma. |
| NCT02650648 | Humanized anti-GD2 antibody Hu3F8 and allogeneic natural killer cells for high-risk neuroblastoma | I | 2016 | 85 | Active, not recruiting | To see if it is safe and feasible to give the participant cyclophosphamide, natural killer (NK) cells and Hu3F8 antibody as a treatment for neuroblastoma. |
| NCT02508038 | Alpha/Beta CD19+Depleted haploidentical transplantation+zometa for pediatric hematologic malignancies and solid tumors | I | 2016 | 22 | Recruiting | To study safety of transplantation with a haploidentical donor peripheral blood stem cell graft depleted of TCRαβ+ and CD19+ cells in conjunction with the immunomodulating drug Zoledronate, given in the post-transplant period to treat pediatric patients with relapsed or refractory hematologic malignancies or high risk solid tumors. |
| NCT03242603 | Immunotherapy of Neuroblastoma Patients Using a Combination of Anti-GD2 and NK Cells | I/II | 2017 | 5 | Unknown | To measure tumor response after infusion of expanded activated haploidentical NK cells with anti-GD2. Disease response will be defined as complete response/remission (CR), partial response (PR), minor response, stable disease (SD), or progressive disease (PD). |
| NCT03209869 | Treatment of relapsed or refractory neuroblastoma with expanded haploidentical NK cells and Hu14.18-IL2 | I | 2018 | 6 | Suspended | Patients with relapsed or refractory neuroblastoma received ex vivo expanded and activated natural killer (NK) cells from haploidentical donor with the immunocytokine, hu14.18-IL2. Safety has been evaluated as adverse event and GvHD rate and efficacy was evaluated as progression-free and overall survival and/or objective tumor response. |
| NCT04211675 | NK cells infusions with irinotecan, temozolomide, and dinutuximab | I/II | 2019 | 31 | Not recruiting | Phase 1: to assess the safety and tolerability of autologous expanded NK cells in combination with irinotecan, temozolomide and dinituximab. Phase 2: to estimate the response to treatment. |
| Identifier | Study Title | Phase | Start Date | Patients Enrolled | Status | Primary Aims |
|---|---|---|---|---|---|---|
| NCT00085930 | Blood T cells and EBV specific CTLs expressing GD2 specific chimeric T cell receptors to neuroblastoma patients | I | 2004 | 19 | Active, not recruiting | To evaluate the safety of escalating doses of 14g2a.zeta chimeric receptor transduced autologous EBV specific cytotoxic T-lymphocytes and 14g2a.zeta transduced autologous peripheral blood T cells |
| NCT01822652 | 3rd generation GD-2 chimeric antigen receptor and iCaspase suicide safety switch, neuroblastoma, GRAIN | I | 2013 | 11 | Active, not recruiting | To define the dose limiting toxicities at six weeks post T cell infusion |
| NCT01953900 | iC9-GD2-CAR-VZV-CTLs/refractory or metastatic GD2-positive sarcoma and neuroblastoma | I | 2013 | 26 | Active, not recruiting | To evaluate the safety and feasibility of intravenous injections of autologous iC9-GD2-CAR-VZV-CTLs in combination with VZV vaccination in patients with advanced GD2-positive sarcomas or neuroblastoma |
| NCT02311621 | Engineered neuroblastoma cellular immunotherapy (ENCIT)-01 | I | 2014 | 40 | Recruiting | Patients will be evaluated through day 28 for occurrence of dose limiting toxicity |
| NCT02761915 | A cancer research UK trial of anti-GD2 T-cells (1RG-CART) | I | 2016 | 27 | Recruiting | To evaluate (i) the feasibility of 1RG-CART therapy in patients with relapsed or refractory neuroblastoma, (ii) safety and tolerability of 1RG-CART therapy and (iii) to assess the incidence, severity and causality of adverse events to 1RG-CART and/or the lymphodepleting regimen |
| NCT02919046 | Study evaluating the efficacy and safety with CAR-T for relapsed or refractory neuroblastoma in children | NA | 2016 | 22 | Unknown | To establish the overall efficiency of patients with neuroblastoma after autologous CAR-T cell therapy |
| NCT02765243 | Anti-GD2 4th generation CART cells targeting refractory and/or recurrent neuroblastoma | I | 2016 | 20 | Recruiting | To determine the toxicity profile of the 4SCAR-GD2-modified T cells with common toxicity criteria for adverse effects |
| NCT03373097 | Anti-GD2 CAR T cells in pediatric patients affected by high risk and/or relapsed/refractory neuroblastoma or other GD2-positive solid tumors | I/II | 2017 | 42 | Recruiting | Phase I—Identification of the dose limiting toxicity Phase II—Assessment of Antitumor effect and best overall response |
| NCT03294954 | GD2 specific CAR and interleukin-15 expressing autologous NKT cells to treat children with neuroblastoma | I | 2017 | 24 | Recruiting | To define the maximum tolerated dose of autologous NKTs expressing a second generation GD2-specific chimeric antigen receptor administered to patients with relapsed or refractory neuroblastoma. |
| NCT03721068 | Study of CAR T-Cells targeting the GD2 with IL-15+iCaspase9 for relapsed/refractory neuroblastoma | I | 2018 | 18 | Recruiting | To establish the number of participants with adverse events as a measure of safety and tolerability of iC9.GD2.CAR.IL-15 T cells administered to pediatric subjects with relapsed or refractory neuroblastoma |
| NCT03635632 | C7R-GD2.CART cells for patients with relapsed or refractory Neuroblastoma and other GD2 positive cancers (GAIL-N) | I | 2018 | 94 | Recruiting | To determine maximum tolerated dose of C7R-GD2.CART Cells and toxicity. |
| NCT03618381 | EGFR806 CAR T cell immunotherapy for recurrent/refractory solid tumors in children and young adults | I | 2018 | 36 | Recruiting |
|
| NCT04483778 | B7H3 CAR T cell immunotherapy for recurrent/refractory solid tumors in children and young adults | I | 2020 | 68 | Recruiting |
|
| NCT04637503 | 4SCAR-T therapy targeting GD2, PSMA and CD276 for treating neuroblastoma | I/II | 2020 | 100 | Recruiting | To determine the number of patients with adverse events and the toxicity profile |
| NCT04539366 | Testing a new immune cell therapy, GD2-targeted modified T-cells (GD2CART), in children, adolescents, and young adults with relapsed/refractory osteosarcoma and neuroblastoma, the GD2-CAR PERSIST trial | I | 2020 | 67 | Not yet recruiting | To define (i) feasibility of producing GD2-CAR-expressing autologous T-lymphocytes (GD2CART), (ii) incidence of adverse events and (iii) maximum tolerated dose and best response to GD2CART cells |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morandi, F.; Sabatini, F.; Podestà, M.; Airoldi, I. Immunotherapeutic Strategies for Neuroblastoma: Present, Past and Future. Vaccines 2021, 9, 43. https://doi.org/10.3390/vaccines9010043
Morandi F, Sabatini F, Podestà M, Airoldi I. Immunotherapeutic Strategies for Neuroblastoma: Present, Past and Future. Vaccines. 2021; 9(1):43. https://doi.org/10.3390/vaccines9010043
Chicago/Turabian StyleMorandi, Fabio, Federica Sabatini, Marina Podestà, and Irma Airoldi. 2021. "Immunotherapeutic Strategies for Neuroblastoma: Present, Past and Future" Vaccines 9, no. 1: 43. https://doi.org/10.3390/vaccines9010043
APA StyleMorandi, F., Sabatini, F., Podestà, M., & Airoldi, I. (2021). Immunotherapeutic Strategies for Neuroblastoma: Present, Past and Future. Vaccines, 9(1), 43. https://doi.org/10.3390/vaccines9010043