Antibody Responses after a Third Dose of COVID-19 Vaccine in Kidney Transplant Recipients and Patients Treated for Chronic Lymphocytic Leukemia
Abstract
:1. Introduction
2. Materials and Methods
3. Results
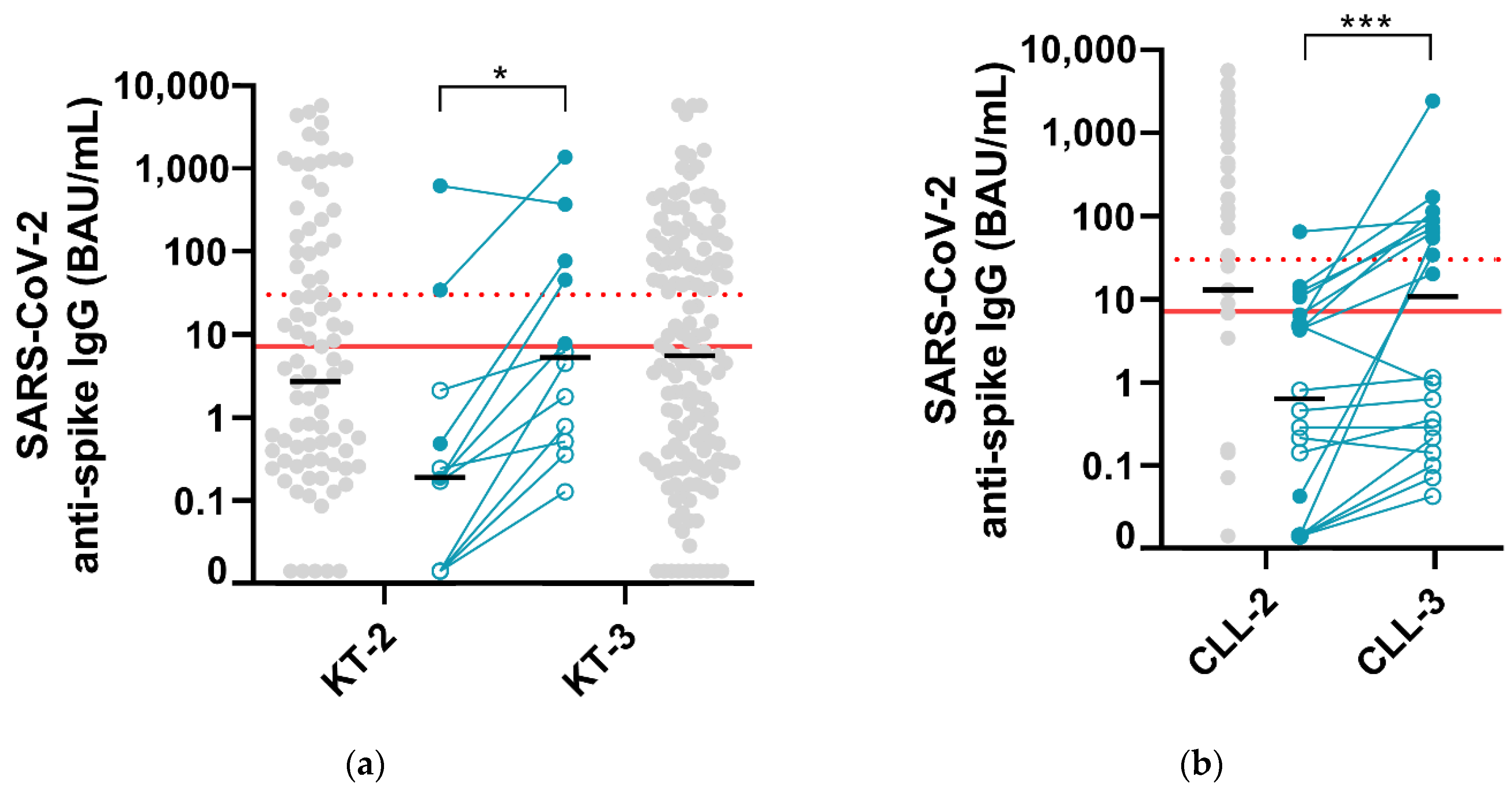
3.1. Kidney Transplant Recipients
3.2. Patients Treated for Chronic Lymphocytic Leukemia
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and Effectiveness of MRNA BNT162b2 Vaccine against SARS-CoV-2 Infections and COVID-19 Cases, Hospitalisations, and Deaths Following a Nationwide Vaccination Campaign in Israel: An Observational Study Using National Surveillance Data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Olson, S.M.; Self, W.H.; Talbot, H.K.; Lindsell, C.J.; Steingrub, J.S.; Shapiro, N.I.; Ginde, A.A.; Douin, D.J.; Prekker, M.E.; et al. Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Among Hospitalized Adults Aged ≥65 Years-United States, January-March 2021. MMWR Morb. Mortal Wkly. Rep. 2021, 70, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; Tessier, E.; Simmons, R.; Cottrell, S.; Roberts, R.; O’Doherty, M.; et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca Vaccines on Covid-19 Related Symptoms, Hospital Admissions, and Mortality in Older Adults in England: Test Negative Case-Control Study. BMJ 2021, 373, n1088. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.X.; Moore, L.W.; Anjan, S.; Rahamimov, R.; Sifri, C.D.; Ali, N.M.; Morales, M.K.; Tsapepas, D.S.; Basic-Jukic, N.; Miller, R.A.; et al. Risk of Breakthrough SARS-CoV-2 Infections in Adult Transplant Recipients. Transplantation 2021. [Google Scholar] [CrossRef]
- Stumpf, J.; Siepmann, T.; Lindner, T.; Karger, C.; Schwöbel, J.; Anders, L.; Faulhaber-Walter, R.; Schewe, J.; Martin, H.; Schirutschke, H.; et al. Humoral and Cellular Immunity to SARS-CoV-2 Vaccination in Renal Transplant versus Dialysis Patients: A Prospective, Multicenter Observational Study Using MRNA-1273 or BNT162b2 MRNA Vaccine. Lancet Reg. Health Eur. 2021, 100178. [Google Scholar] [CrossRef] [PubMed]
- Grupper, A.; Rabinowich, L.; Schwartz, D.; Schwartz, I.F.; Ben-Yehoyada, M.; Shashar, M.; Katchman, E.; Halperin, T.; Turner, D.; Goykhman, Y.; et al. Reduced Humoral Response to MRNA SARS-Cov-2 BNT162b2 Vaccine in Kidney Transplant Recipients without Prior Exposure to the Virus. Am. J. Transplant. 2021. [Google Scholar] [CrossRef]
- Roeker, L.E.; Knorr, D.A.; Thompson, M.C.; Nivar, M.; Lebowitz, S.; Peters, N.; Deonarine, I.; Momotaj, S.; Sharan, S.; Chanlatte, V.; et al. COVID-19 Vaccine Efficacy in Patients with Chronic Lymphocytic Leukemia. Leukemia 2021. [Google Scholar] [CrossRef] [PubMed]
- Herishanu, Y.; Avivi, I.; Aharon, A.; Shefer, G.; Levi, S.; Bronstein, Y.; Morales, M.; Ziv, T.; Shorer Arbel, Y.; Scarfò, L.; et al. Efficacy of the BNT162b2 MRNA COVID-19 Vaccine in Patients with Chronic Lymphocytic Leukemia. Blood 2021, 137, 3165–3173. [Google Scholar] [CrossRef] [PubMed]
- Agha, M.; Blake, M.; Chilleo, C.; Wells, A.; Haidar, G. Suboptimal Response to COVID-19 MRNA Vaccines in Hematologic Malignancies Patients. medRxiv 2021. [Google Scholar] [CrossRef]
- Benotmane, I.; Gautier, G.; Perrin, P.; Olagne, J.; Cognard, N.; Fafi-Kremer, S.; Caillard, S. Antibody Response After a Third Dose of the MRNA-1273 SARS-CoV-2 Vaccine in Kidney Transplant Recipients With Minimal Serologic Response to 2 Doses. JAMA 2021. [Google Scholar] [CrossRef] [PubMed]
- Werbel, W.A.; Boyarsky, B.J.; Ou, M.T.; Massie, A.B.; Tobian, A.A.R.; Garonzik-Wang, J.M.; Segev, D.L. Safety and Immunogenicity of a Third Dose of SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients: A Case Series. Ann. Intern. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Abravanel, F.; Marion, O.; Couat, C.; Izopet, J.; Del Bello, A. Three Doses of an MRNA Covid-19 Vaccine in Solid-Organ Transplant Recipients. N. Engl. J. Med. 2021, 385, 661–662. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, P.A.; Page, M.; Bernasconi, V.; Mattiuzzo, G.; Dull, P.; Makar, K.; Plotkin, S.; Knezevic, I. WHO International Standard for Anti-SARS-CoV-2 Immunoglobulin. Lancet 2021, 397, 1347–1348. [Google Scholar] [CrossRef]
- Ebinger, J.E.; Fert-Bober, J.; Printsev, I.; Wu, M.; Sun, N.; Figueiredo, J.C.; Eyk, J.E.V.; Braun, J.G.; Cheng, S.; Sobhani, K. Prior COVID-19 Infection and Antibody Response to Single Versus Double Dose MRNA SARS-CoV-2 Vaccination. medRxiv 2021. [Google Scholar] [CrossRef]
- Narasimhan, M.; Mahimainathan, L.; Araj, E.; Clark, A.E.; Markantonis, J.; Green, A.; Xu, J.; SoRelle, J.A.; Alexis, C.; Fankhauser, K.; et al. Clinical Evaluation of the Abbott Alinity SARS-CoV-2 Spike-Specific Quantitative IgG and IgM Assays in Infected, Recovered, and Vaccinated Groups. medRxiv 2021. [Google Scholar] [CrossRef]
- Prendecki, M.; Clarke, C.; Brown, J.; Cox, A.; Gleeson, S.; Guckian, M.; Randell, P.; Pria, A.D.; Lightstone, L.; Xu, X.-N.; et al. Effect of Previous SARS-CoV-2 Infection on Humoral and T-Cell Responses to Single-Dose BNT162b2 Vaccine. Lancet 2021, 397, 1178–1181. [Google Scholar] [CrossRef]
- Gobbi, F.; Buonfrate, D.; Moro, L.; Rodari, P.; Piubelli, C.; Caldrer, S.; Riccetti, S.; Sinigaglia, A.; Barzon, L. Antibody Response to the BNT162b2 MRNA COVID-19 Vaccine in Subjects with Prior SARS-CoV-2 Infection. Viruses 2021, 13, 422. [Google Scholar] [CrossRef] [PubMed]
- Perkmann, T.; Perkmann-Nagele, N.; Koller, T.; Mucher, P.; Radakovics, A.; Marculescu, R.; Wolzt, M.; Wagner, O.F.; Binder, C.J.; Haslacher, H. Anti-Spike Protein Assays to Determine SARS-CoV-2 Antibody Levels: A Head-to-Head Comparison of Five Quantitative Assays. Microbiol. Spectr. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of Protection against Symptomatic and Asymptomatic SARS-CoV-2 Infection. medRxiv 2021. [Google Scholar] [CrossRef]
- Danthu, C.; Hantz, S.; Dahlem, A.; Duval, M.; Ba, B.; Guibbert, M.; El Ouafi, Z.; Ponsard, S.; Berrahal, I.; Achard, J.M.; et al. Humoral Response after SARS-Cov-2 MRNA Vaccine in a Cohort of Hemodialysis Patients and Kidney Transplant Recipients. JASN 2021. [Google Scholar] [CrossRef] [PubMed]
| Kidney Transplant | Chronic Lymphocytic Leukemia | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| After 2nd Dose (n = 97) | After 3rd Dose (n = 160) | After 2nd Dose (n = 51) | After 3rd Dose (n = 20) | |||||||||
| Response to COVID vaccine | No (n = 55) | Yes (n = 42) | p | No (n = 85) | Yes (n = 75) | p | No (n = 22) | Yes (n = 29) | p | No (n = 10) | Yes (n = 10) | p |
| Age > 65 year | 31 (56) | 13 (31) | 0.02 | 41 (48) | 26 (35) | 0.08 | 17 (77) | 20 (69) | 0.55 | 8 (80) | 6 (60) | 0.63 |
| Age < 50 year | 5 (9) | 8 (19) | 0.23 | 15 (18) | 24 (32) | 0.06 | 0 | 1 (4) | 1.0 | 0 | 0 | - |
| Sex (female) | 18 (33) | 21 (5) | 0.10 | 36 (42) | 21 (28) | 0.07 | 7 (32) | 11 (38) | 0.77 | 2 (20) | 4 (40) | 0.64 |
| History of positive SARS-CoV-2 RT-PCR | 1 (1.8) | 6 (14) | 0.04 | 0 | 2 (2.7) | 0.22 | - | - | - | - | - | - |
| COVID-19 vaccine | ||||||||||||
| Pfizer (BNT162b2) | 43 (78) | 32 (76) | 0.81 | 53 (62) | 45 (60) | 0.88 | - | - | - | - | - | - |
| Moderna (mRNA-1273) | 3 (5.5) | 4 (9.5) | 0.46 | 11 (13) | 16 (21) | 0.20 | - | - | - | - | - | - |
| Tyrosine kinase inhibitors or venetoclax | - | - | - | - | - | - | 19 (86) | 14 (48) | 0.007 | 9 (90) | 9 (90) | 1.0 |
| Prior-CLL directed therapy | - | - | - | - | - | - | 15 (68) | 12 (41) | 0.54 | 9 (90) | 7 (70) | 0.58 |
| Anti-CD20 monoclonal antibody within 2 years | - | - | - | - | - | - | 2 (9) | 0 | 0.18 | 1 (10) | 0 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marlet, J.; Gatault, P.; Maakaroun, Z.; Longuet, H.; Stefic, K.; Handala, L.; Eymieux, S.; Gyan, E.; Dartigeas, C.; Gaudy-Graffin, C. Antibody Responses after a Third Dose of COVID-19 Vaccine in Kidney Transplant Recipients and Patients Treated for Chronic Lymphocytic Leukemia. Vaccines 2021, 9, 1055. https://doi.org/10.3390/vaccines9101055
Marlet J, Gatault P, Maakaroun Z, Longuet H, Stefic K, Handala L, Eymieux S, Gyan E, Dartigeas C, Gaudy-Graffin C. Antibody Responses after a Third Dose of COVID-19 Vaccine in Kidney Transplant Recipients and Patients Treated for Chronic Lymphocytic Leukemia. Vaccines. 2021; 9(10):1055. https://doi.org/10.3390/vaccines9101055
Chicago/Turabian StyleMarlet, Julien, Philippe Gatault, Zoha Maakaroun, Hélène Longuet, Karl Stefic, Lynda Handala, Sébastien Eymieux, Emmanuel Gyan, Caroline Dartigeas, and Catherine Gaudy-Graffin. 2021. "Antibody Responses after a Third Dose of COVID-19 Vaccine in Kidney Transplant Recipients and Patients Treated for Chronic Lymphocytic Leukemia" Vaccines 9, no. 10: 1055. https://doi.org/10.3390/vaccines9101055
APA StyleMarlet, J., Gatault, P., Maakaroun, Z., Longuet, H., Stefic, K., Handala, L., Eymieux, S., Gyan, E., Dartigeas, C., & Gaudy-Graffin, C. (2021). Antibody Responses after a Third Dose of COVID-19 Vaccine in Kidney Transplant Recipients and Patients Treated for Chronic Lymphocytic Leukemia. Vaccines, 9(10), 1055. https://doi.org/10.3390/vaccines9101055






