Advances in Understanding of the Immune Response to Mycobacterial Pathogens and Vaccines through Use of Cattle and Mycobacterium avium subsp. paratuberculosis as a Prototypic Mycobacterial Pathogen
Abstract
:1. Introduction
2. Mycobacterium avium subsp. paratuberculosis, a Prototypic Mycobacterial Pathogen
3. Immune Response to Map
4. Early Events of Infection through Direct Infection of the Ileum
5. Original Isolates of Map from Humans with CD Elicited an Immune Response Similar to the Response Elicited by Isolates from Cattle
6. Site-Directed Mutagenesis
7. Analysis of Immune Response Elicited by Map/relA
8. Identification of a Target of the Immune Response Elicited by Map/relA
9. Analysis of the Effector Activity of CD4 and CD8 T Cells Proliferating in Response to Stimulation with APC Pulsed with Map/relA and MMP
10. Simultaneous Recognition of Antigens Processed and Presented by APC Is Essential for Development of CD8 CTL
11. Candidate Vaccines for Map and Mbv
12. relA Deletion Mutants
13. Peptide-Based Vaccines
14. Method of Delivery of Peptide-Based Vaccines
15. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reva, O.; Korotetskiy, I.; Ilin, A. Role of the horizontal gene exchange in evolution of pathogenic Mycobacteria. BMC Evol. Biol. 2015, 15, S2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachmann, N.L.; Salamzade, R.; Manson, A.L.; Whittington, R.; Sintchenko, V.; Earl, A.M.; Marais, B.J. Key transitions in the evolution of rapid and slow growing Mycobacteria identified by comparative genomics. Front. Microbiol. 2019, 10, 3019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bollongino, R.; Burger, J.; Powell, A.; Mashkour, M.; Vigne, J.D.; Thomas, M.G. Modern taurine cattle descended from small number of near-eastern founders. Mol. Biol. Evol. 2012, 29, 2101–2104. [Google Scholar] [CrossRef] [PubMed]
- Zeder, M.A. Domestication and early agriculture in the Mediterranean Basin: Origins, diffusion, and impact. Proc. Natl. Acad. Sci. USA 2008, 105, 11597–11604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeder, M.A.; Hesse, B. The initial domestication of goats (Capra hircus) in the Zagros mountains 10,000 years ago. Science 2000, 287, 2254–2257. [Google Scholar] [CrossRef]
- Frothingham, R. Evolutionary bottlenecks in the agents of tuberculosis, leprosy, and paratuberculosis. Med. Hypotheses 1999, 52, 95–99. [Google Scholar] [CrossRef]
- Johne, H.A.; Frothingham, L. Ein eigenthumlicher fall von tuberculosis beim rind [A peculiar case of tuberculosis in a cow]. Deutsche. Zeitschr. Tierm. Path 1895, 21, 438–454. [Google Scholar]
- Koch, R. Die Aetiologie der tuberkulose. Mitth. Ausdem Kais. Gesundh. 1884, 2, 1–88. [Google Scholar] [CrossRef]
- Chung, J.; Ince, D.; Ford, B.A.; Wanat, K.A. Cutaneous infections due to nontuberculosis Mycobacterium: Recognition and management. Am. J. Clin. Dermatol. 2018, 19, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Ratnatunga, C.N.; Lutzky, V.P.; Kupz, A.; Doolan, D.L.; Reid, D.W.; Field, M.; Bell, S.C.; Thomson, R.M.; Miles, J.J. The rise of non-tuberculosis Mycobacterial Lung disease. Front. Immunol. 2020, 11, 303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gcebe, N.; Hlokwe, T.M. Non-tuberculous Mycobacteria in South African wildlife: Neglected pathogens and potential impediments for bovine Tuberculosis diagnosis. Front. Cell Infect. Microbiol. 2017, 7, 15. [Google Scholar] [CrossRef] [Green Version]
- Prasanna, A.N.; Mehra, S. Comparative phylogenomics of pathogenic and non-pathogenic mycobacterium. PLoS ONE 2013, 8, e71248. [Google Scholar] [CrossRef]
- Tortoli, E. The new mycobacteria: An update. FEMS Immunol. Med. Microbiol. 2006, 48, 159–178. [Google Scholar] [CrossRef] [Green Version]
- Davis, W.C.; Marusic, S.; Lewin, H.A.; Splitter, G.A.; Perryman, L.E.; McGuire, T.C.; Gorham, J.R. The development and analysis of species specific and cross reactive monoclonal antibodies to leukocyte differentiation antigens and antigens of the major histocompatibility complex for use in the study of the immune system in cattle and other species. Vet. Immunol. Immunopathol. 1987, 15, 337–376. [Google Scholar] [CrossRef]
- Davis, W.C.; Perryman, L.E.; McGuire, T.C. The identification and analysis of major functional populations of differentiated cells. In Hydridoma Technology in Agriculture and Veterinary Research; Stern, N.J., Gamble, H.R., Eds.; Rowan and Allanheld Publishers: Totowa, NJ, USA, 1984; pp. 121–150. [Google Scholar]
- Morrison, W.I.; Davis, W.C. Individual antigens of cattle. Differentiation antigens expressed predominantly on CD4-CD8- T lymphocytes (WC1, WC2). Vet. Immunol. Immunopathol. 1991, 27, 71–76. [Google Scholar] [CrossRef]
- Naessens, J.; Hopkins, J. Introduction and summary of workshop findings. Vet. Immunol. Immunopathol. 1996, 52, 213–235. [Google Scholar] [CrossRef]
- Wijngaard, P.L.; MacHugh, N.D.; Metzelaar, M.J.; Romberg, S.; Bensaid, A.; Pepin, L.; Davis, W.C.; Clevers, H.C. Members of the novel WC1 gene family are differentially expressed on subsets of bovine CD4-CD8- gamma delta T lymphocytes. J. Immunol. 1994, 152, 3476–3482. [Google Scholar] [PubMed]
- Flynn, J.L.; Chan, J. Immunology of tuberculosis. Annu. Rev. Immunol. 2001, 19, 93–129. [Google Scholar] [CrossRef] [PubMed]
- Rideout, B.A.; Brown, S.T.; Davis, W.C.; Gay, J.M.; Giannella, R.A.; Hines, M.E.; Hueston, W.D.; Hutchinson, L.J. Diagnosis and Control of Johne’s Disease; Kelly, K., Ed.; The National Academy Press: Washington, DC, USA, 2003. [Google Scholar]
- Chiodini, R.J.; Van Kruiningen, H.J.; Merkal, R.S.; Thayer, W.R., Jr.; Coutu, J.A. Characteristics of an unclassified Mycobacterium species isolated from patients with Crohn’s disease. J. Clin. Microbiol. 1984, 20, 966–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiodini, R.J.; Davis, W.C. The cellular immunology of bovine paratuberculosis: The predominant response is mediated by cytotoxic gamma/delta T lymphocytes which prevent CD4+ activity. Microb. Pathog. 1992, 13, 447–463. [Google Scholar] [CrossRef]
- Waters, W.R.; Miller, J.M.; Palmer, M.V.; Stabel, J.R.; Jones, D.E.; Koistinen, K.A.; Steadham, E.M.; Hamilton, M.J.; Davis, W.C.; Bannantine, J.P. Early induction of humoral and cellular immune responses during experimental Mycobacterium avium subsp. paratuberculosis infection of calves. Infect. Immun. 2003, 71, 5130–5138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, H.C.; Park, Y.H.; Hamilton, M.J.; Barrington, G.M.; Davies, C.J.; Kim, J.B.; Dahl, J.L.; Waters, W.R.; Davis, W.C. Analysis of the immune response to Mycobacterium avium subsp. paratuberculosis in experimentally infected calves. Infect. Immun. 2004, 72, 6870–6883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.W.; Livesey, M.; Schmoller, S.K.; Manning, E.J.; Steinberg, H.; Davis, W.C.; Hamilton, M.J.; Talaat, A.M. Invasion and persistence of Mycobacterium avium subsp. paratuberculosis during early stages of Johne’s disease in calves. Infect. Immun. 2007, 75, 2110–2119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
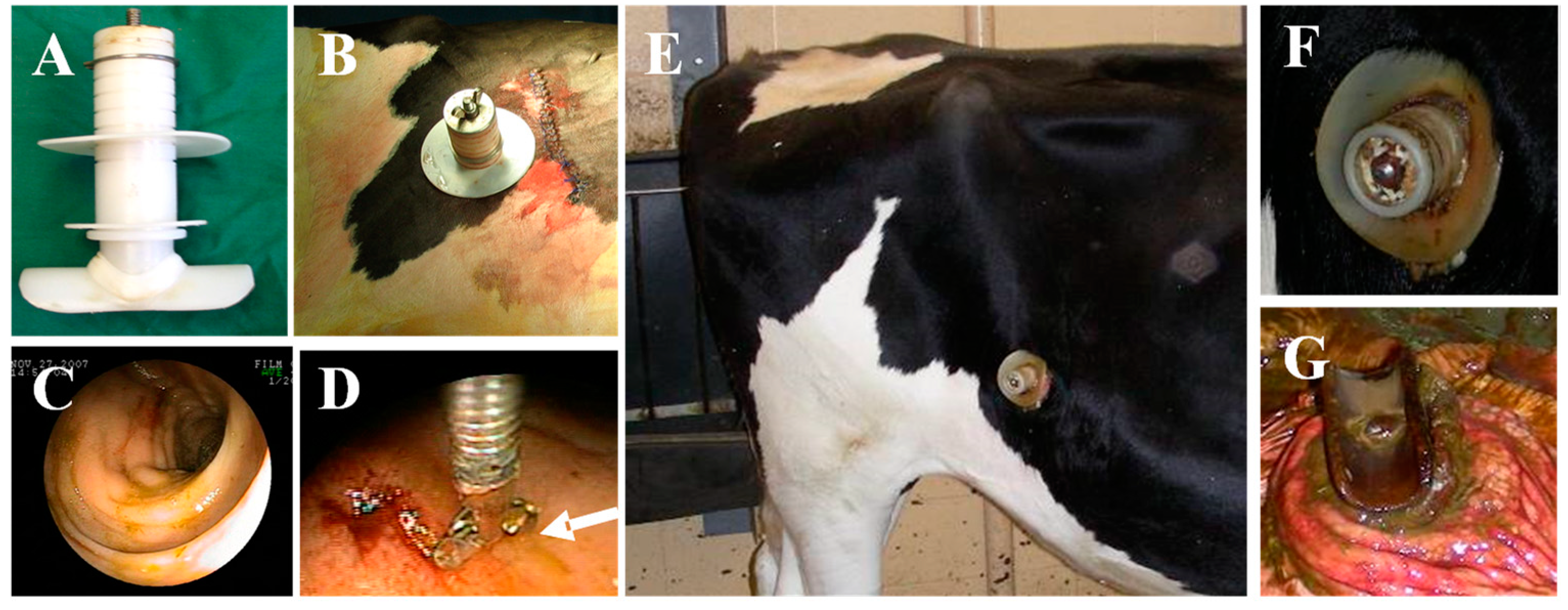
- Allen, A.J.; Park, K.T.; Barrington, G.M.; Hamilton, M.J.; Davis, W.C. Development of a bovine ileal cannulation model to study the immune response and mechanisms of pathogenesis paratuberculosis. Clin. Vaccine Immunol. 2009, 16, 453–463. [Google Scholar] [CrossRef] [Green Version]
- Allen, A.J.; Park, K.T.; Barrington, G.M.; Lahmers, K.K.; Abdellrazeq, G.S.; Rihan, H.M.; Sreevatsan, S.; Davies, C.; Hamilton, M.J.; Davis, W.C. Experimental infection of a bovine model with human isolates of Mycobacterium avium subsp. paratuberculosis. Vet. Immunol. Immunpathol. 2011, 141, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Kuenstner, J.T.; Potula, R.; Bull, T.J.; Grant, I.R.; Foddai, A.; Naser, S.A.; Bach, H.; Zhang, P.; Yu, D.; Lu, X.; et al. Presence of infection by Mycobacterium avium subsp. paratuberculosis in the blood of patients with Crohn’s disease and control subjects shown by multiple laboratory culture and antibody methods. Microorganisms 2020, 8, 2054. [Google Scholar] [CrossRef]
- Singh, S.V.; Kuenstner, J.T.; Davis, W.C.; Agarwal, P.; Kumar, N.; Singh, D.; Gupta, S.; Chaubey, K.K.; Kumar, A.; Misri, J.; et al. Concurrent resolution of chronic diarrhea likely due to Crohn’s disease and infection with Mycobacterium avium paratuberculosis. Front. Med. 2016, 3, 49. [Google Scholar] [CrossRef]
- Davis, W.C. Why is the obvious not obvious, it is Johne’s disease (Paratuberculosis) not Crohn’s disease. EC Gastroenterol. Dig. Syst. 2018, 5, 752–758. [Google Scholar]
- Harris, N.B.; Feng, Z.; Liu, X.; Cirillo, S.L.G.; Cirillop, J.D.; Barletta, R.G. Development of a transposon mutagenesis system for Mycobacterium avium subsp. paratuberculosis. FEMS Microbiol. Lett. 1999, 175, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.J.; Wu, C.; Steinberg, H.; Talaat, A.M. Identification of novel virulence determinants in Mycobacterium paratuberculosis by screening a library of insertional mutants. Infect. Immun. 2006, 74, 3825–3833. [Google Scholar] [CrossRef] [Green Version]
- Cavaignac, S.M.; White, S.J.; de Lisle, G.W.; Collins, D.M. Construction and screening of Mycobacterium paratuberculosis insertional mutant libraries. Arch. Microbiol. 2000, 173, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Bardarov, S.; Bardarov, S.J.; Pavelka, M.S.J.; Sambandamurthy, V.; Larsen, M.; Tufariello, J.; Chan, J.; Hatfull, G.; Jacobs, W.R.J. Specialized transduction: An efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology 2002, 148, 3007–3017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.T.; Dahl, J.L.; Bannantine, J.P.; Barletta, R.G.; Ahn, J.; Allen, A.J.; Hamilton, M.J.; Davis, W.C. Demonstration of allelic exchange in the slow-growing bacterium Mycobacterium avium subsp. paratuberculosis, and generation of mutants with deletions at the pknG, relA, and lsr2 loci. Appl. Environ. Microbiol. 2008, 74, 1687–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalebroux, Z.D.; Svensson, S.L.; Gaynor, E.C.; Swanson, M.S. ppGpp conjures bacterial virulence. Microbiol. Mol. Biol. Rev. 2010, 74, 171–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bannantine, J.P.; Hines, M.E., 2nd; Bermudez, L.E.; Talaat, A.M.; Sreevatsan, S.; Stabel, J.R.; Chang, Y.F.; Coussens, P.M.; Barletta, R.G.; Davis, W.C.; et al. A rational framework for evaluating the next generation of vaccines against Mycobacterium avium subspecies paratuberculosis. Front. Cell. Infect. Microbiol. 2014, 4, 126. [Google Scholar] [CrossRef]
- Walburger, A.; Koul, A.; Ferrari, G.; Nguyen, L.; Prescianotto-Baschong, C.; Huygen, K.; Klebl, B.; Thompson, C.; Bacher, G.; Pieters, J. Protein kinase G from pathogenic mycobacteria promotes survival within macrophages. Science 2004, 304, 1800–1804. [Google Scholar] [CrossRef] [Green Version]
- Dahl, J.L.; Kraus, C.N.; Boshoff, H.I.M.; Doan, B.; Foley, K.; Avarbock, D.; Kaplan, G.; Mizrahi, V.; Rubin, H.; Barry, C.E.I. The role of RelMtb-mediated adaptation to stationary phase in long-term persistence of Mycobacterium tuberculosis in mice. Proc. Natl. Acad. Sci. USA 2003, 100, 10026–10031. [Google Scholar] [CrossRef] [Green Version]
- Park, K.T.; Allen, A.J.; Bannantine, J.P.; Seo, K.S.; Hamilton, M.J.; Abdellrazeq, G.S.; Rihan, H.M.; Grimm, A.; Davis, W.C. Evaluation of two mutants of Mycobacterium avium subsp. paratuberculosis as candidates for a live attenuated vaccine for Johne’s disease. Vaccine 2011, 29, 4709–4719. [Google Scholar] [CrossRef] [Green Version]
- Hauryliuk, V.; Atkinson, G.C.; Murakami, K.S.; Tenson, T.; Gerdes, K. Recent functional insights into the role of (p) ppGpp in bacterial physiology. Nat. Rev. Microbiol. 2015, 13, 298–309. [Google Scholar] [CrossRef] [Green Version]
- Geijtenbeek, T.B.; Torensma, R.; van Vliet, S.J.; van Duijnhoven, G.C.; Adema, G.J.; van Kooyk, Y.; Figdor, C.G. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 2000, 100, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Park, K.T.; ElNaggar, M.M.; Abdellrazeq, G.S.; Bannantine, J.P.; Mack, V.; Fry, L.M.; Davis, W.C. Phenotype and function of CD209 + bovine blood dendritic cells, monocyte-derived-dendritic cells and monocyte-derived macrophages. PLoS ONE 2016, 11, e0165247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bannantine, J.P.; Paustian, M.L.; Waters, W.R.; Stabel, J.R.; Palmer, M.V.; Li, L.; Kapur, V. Profiling bovine antibody responses to Mycobacterium avium subsp. paratuberculosis infection by using protein arrays. Infect. Immun. 2008, 76, 739–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bannantine, J.P.; Huntley, J.F.J.; Miltner, E.; Stabel, J.R.; Bermudez, L.E. The Mycobacterium avium subsp. paratuberculosis 35 kDa protein plays a role in invasion of bovine epithelial cells. Microbiology 2003, 149, 2061–2069. [Google Scholar] [CrossRef] [PubMed]
- Abdellrazeq, G.S.; Elnaggar, M.M.; Bannantine, J.P.; Park, K.T.; Souza, C.D.; Backer, B.; Hulubei, V.; Fry, L.M.; Khaliel, S.A.; Torky, H.A.; et al. A Mycobacterium avium subsp. paratuberculosis relA deletion mutant and a 35 kDa major membrane protein elicit development of cytotoxic T lymphocytes with ability to kill intracellular bacteria. Vet. Res. 2018, 49, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
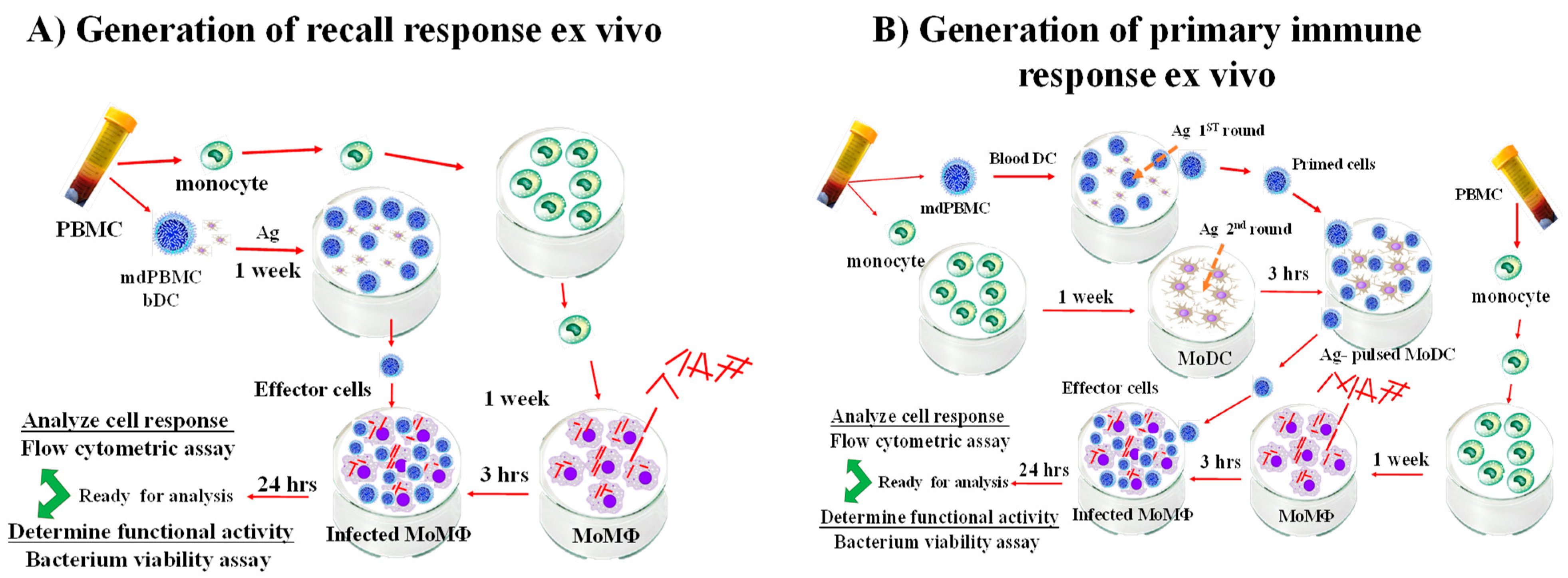
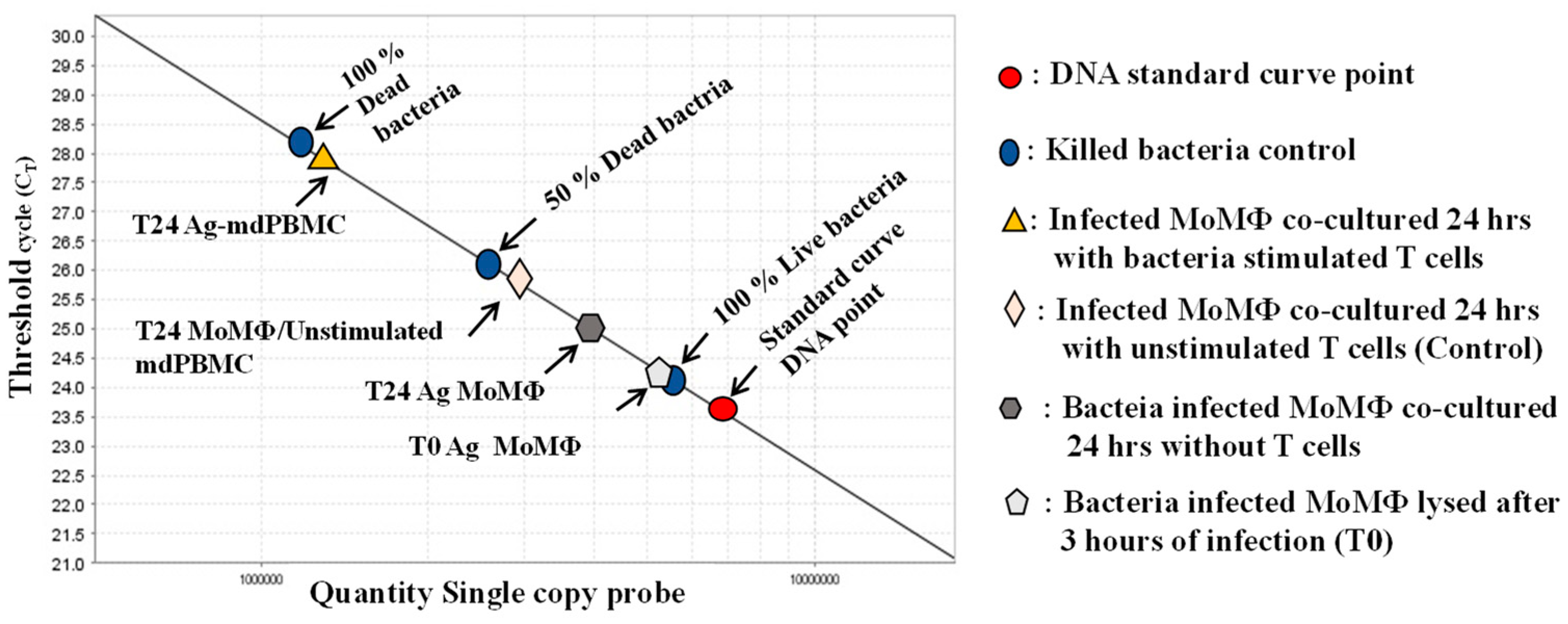
- Worku, S.; Hoft, D.F. Differential effects of control and antigen-specific T cells on intracellular mycobacterial growth. Infect. Immun. 2003, 71, 1763–1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nocker, A.; Sossa-Fernandez, P.; Burr, M.D.; Camper, A.K. Use of propidium monoazide for live/dead distinction in microbial ecology. Appl. Environ. Microbiol. 2007, 73, 5111–5117. [Google Scholar] [CrossRef] [Green Version]
- Nocker, A.; Cheung, C.-Y.; Camper, A.K. Comparison of propidium monoazide with ethidium monoazide for differentiation of live vs. dead bacteria by selective removal of DNA from dead cells. J. Microbiol. Methods 2006, 67, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Kralik, P.; Nocker, A.; Pavlik, I. Mycobacterium avium subsp. paratuberculosis viability determination using F57 quantitative PCR in combination with propidium monoazide treatment. Int. J. Food Microbiol. 2010, 141, S80–S86. [Google Scholar] [CrossRef]
- Abdellrazeq, G.S.; Mahmoud, A.H.; Park, K.T.; Fry, L.M.; Elnaggar, M.M.; Schneider, D.A.; Hulubei, V.; Davis, W.C. relA is Achilles’ heel for mycobacterial pathogens as demonstrated with deletion mutants in Mycobacterium avium subsp. paratuberculosis and mycobacterium bovis bacillus Calmette-Guerin (BCG). Tuberculosis 2020, 120, 101904. [Google Scholar] [CrossRef]
- Corripio-Miyar, Y.; Hope, J.; McInnes, C.J.; Wattegedera, S.R.; Jensen, K.; Pang, Y.; Entrican, G.; Glass, E.J. Phenotypic and functional analysis of monocyte populations in cattle peripheral blood identifies a subset with high endocytic and allogeneic T-cell stimulatory capacity. Vet. Res. 2015, 46, 112. [Google Scholar] [CrossRef] [Green Version]
- Ababou, A.; Goyeneche, J.; Davis, W.C.; Levy, D. Evidence for the expression of three different BoLa-class II molecules on the bovine BL-3 cell line: Determination of a non-DR non-DQ gene product. J. Leukoc. Biol. 1994, 56, 182–186. [Google Scholar] [CrossRef]
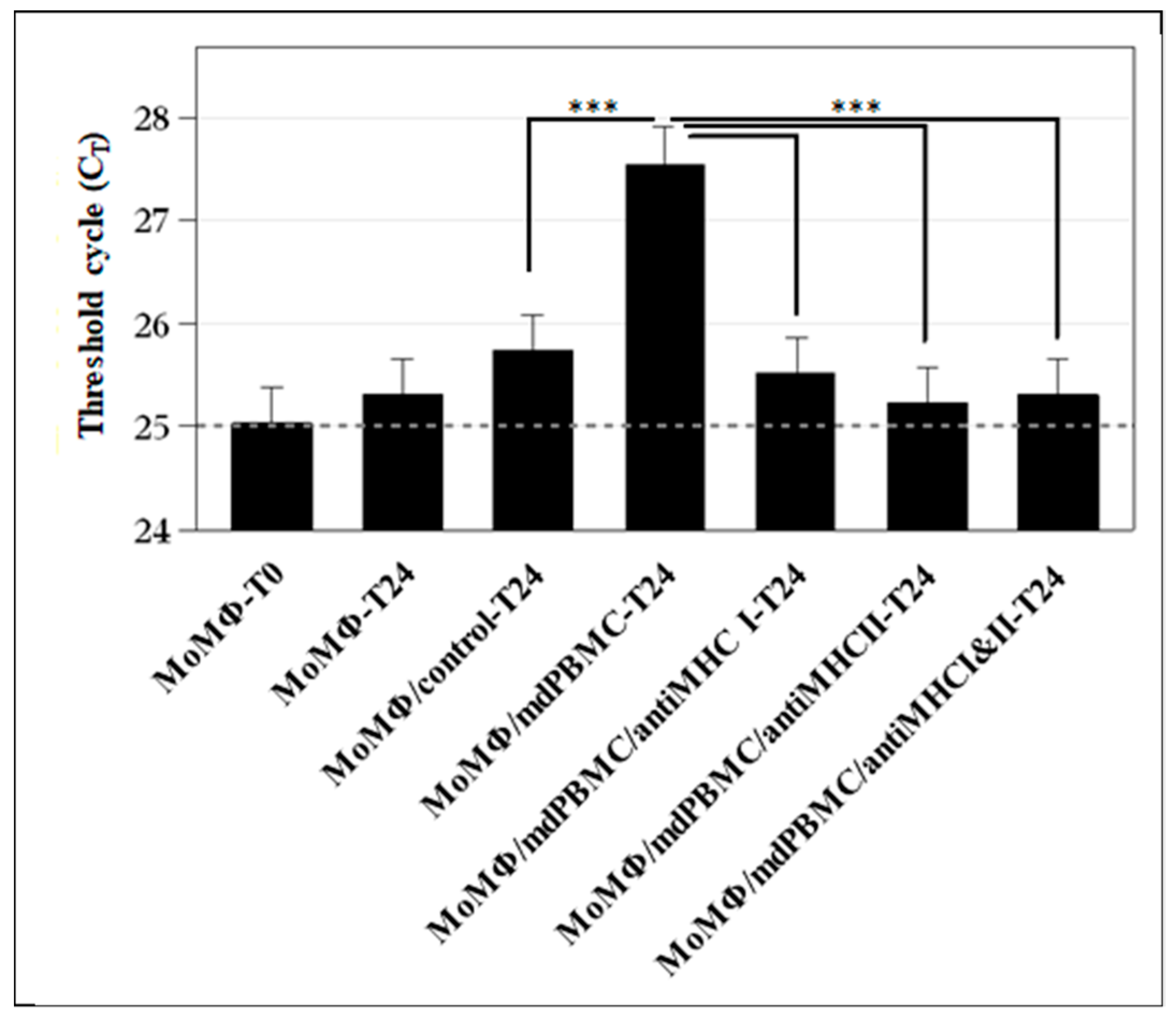
- Abdellrazeq, G.S.; Fry, L.M.; Elnaggar, M.M.; Bannantine, J.P.; Schneider, D.A.; Chamberlin, W.M.; Mahmoud, A.H.A.; Park, K.T.; Hulubei, V.; Davis, W.C. Simultaneous cognate epitope recognition by bovine CD4 and CD8 T cells is essential for primary expansion of antigen-specific cytotoxic T-cells following ex vivo stimulation with a candidate Mycobacterium avium subsp. paratuberculosis peptide vaccine. Vaccine 2020, 38, 2016–2025. [Google Scholar] [CrossRef]
- Dockrell, H.M.; Smith, S.G. What have we learnt about BCG vaccination in the last 20 years? Front. Immunol. 2017, 8, 1134. [Google Scholar] [CrossRef]
- Lurie, M.B. The Fate of Bcg and associated changes in the organs of rabbits. J. Exp. Med. 1934, 60, 163–178. [Google Scholar] [CrossRef]
- Black, M.; Trent, A.; Tirrell, M.; Olive, C. Advances in the design and delivery of peptide subunit vaccines with a focus on toll-like receptor agonists. Expert Rev. Vaccines 2010, 9, 157–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdellrazeq, G.S.; Elnaggar, M.M.; Bannantine, J.P.; Schneider, D.A.; Souza, C.D.; Hwang, J.; Mahmoud, A.H.A.; Hulubei, V.; Fry, L.M.; Park, K.T.; et al. A peptide-based vaccine for Mycobacterium avium subspecies paratuberculosis. Vaccine 2019, 37, 2783–2790. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, V.; Mahmoud, A.H.; Abdellrazeq, G.S.; Tebaldi, G.; Macchi, F.; Russo, L.; Fry, L.M.; Elnaggar, M.M.; Bannantine, J.P.; Park, K.T.; et al. Capacity to Elicit Cytotoxic CD8 T Cell Activity Against Mycobacterium avium subsp. paratuberculosis Is Retained in a Vaccine candidate 35 kDa peptide modified for expression in mammalian cells. Front. Immunol. 2019, 10, 2859. [Google Scholar] [CrossRef] [PubMed]
- Capocefalo, A.; Mangia, C.; Franceschi, V.; Jacca, S.; van Santen, V.L.; Donofrio, G. Efficient heterologous antigen gene delivery and expression by a replication-attenuated BoHV-4-based vaccine vector. Vaccine 2013, 31, 3906–3914. [Google Scholar] [CrossRef]
- Kundra, S.; Colomer-Winter, C.; Lemos, J.A. Survival of the fittest: The relationship of (p) ppGpp with bacterial virulence. Front. Microbiol. 2020, 11, 3124. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, W.C.; Abdellrazeq, G.S.; Mahmoud, A.H.; Park, K.-T.; Elnaggar, M.M.; Donofrio, G.; Hulubei, V.; Fry, L.M. Advances in Understanding of the Immune Response to Mycobacterial Pathogens and Vaccines through Use of Cattle and Mycobacterium avium subsp. paratuberculosis as a Prototypic Mycobacterial Pathogen. Vaccines 2021, 9, 1085. https://doi.org/10.3390/vaccines9101085
Davis WC, Abdellrazeq GS, Mahmoud AH, Park K-T, Elnaggar MM, Donofrio G, Hulubei V, Fry LM. Advances in Understanding of the Immune Response to Mycobacterial Pathogens and Vaccines through Use of Cattle and Mycobacterium avium subsp. paratuberculosis as a Prototypic Mycobacterial Pathogen. Vaccines. 2021; 9(10):1085. https://doi.org/10.3390/vaccines9101085
Chicago/Turabian StyleDavis, William C., Gaber S. Abdellrazeq, Asmaa H. Mahmoud, Kun-Taek Park, Mahmoud M. Elnaggar, Gaetano Donofrio, Victoria Hulubei, and Lindsay M. Fry. 2021. "Advances in Understanding of the Immune Response to Mycobacterial Pathogens and Vaccines through Use of Cattle and Mycobacterium avium subsp. paratuberculosis as a Prototypic Mycobacterial Pathogen" Vaccines 9, no. 10: 1085. https://doi.org/10.3390/vaccines9101085
APA StyleDavis, W. C., Abdellrazeq, G. S., Mahmoud, A. H., Park, K.-T., Elnaggar, M. M., Donofrio, G., Hulubei, V., & Fry, L. M. (2021). Advances in Understanding of the Immune Response to Mycobacterial Pathogens and Vaccines through Use of Cattle and Mycobacterium avium subsp. paratuberculosis as a Prototypic Mycobacterial Pathogen. Vaccines, 9(10), 1085. https://doi.org/10.3390/vaccines9101085





