The Epidemiological and Economic Impact of a Cell-Based Quadrivalent Influenza Vaccine in Adults in the US: A Dynamic Modeling Approach
Abstract
:1. Introduction
2. Materials
2.1. Epidemiology and Vaccine Effectiveness
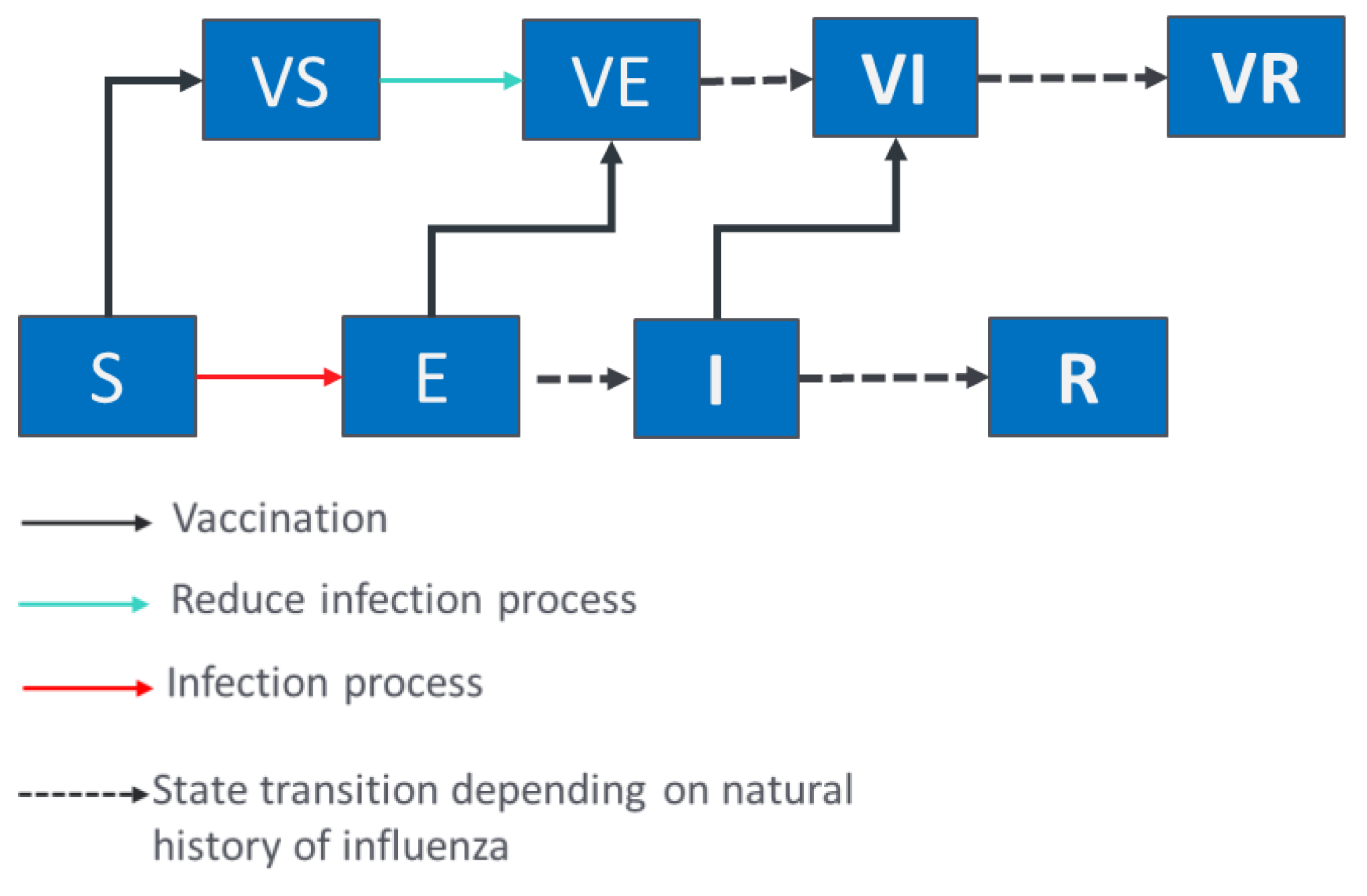
2.2. Epidemiological and Economic Model
2.3. Economic Data
2.4. Scenarios
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- Centers for Disease Control & Prevention. Estimated Influenza Illnesses, Medical Visits, Hospitalizations, and Deaths in the United States—2018–2019 Influenza Season. Available online: https://www.cdc.gov/flu/about/burden/2018-2019.html (accessed on 11 August 2021).
- Belongia, E.A.; Simpson, M.D.; King, J.P.; Sundaram, M.E.; Kelley, N.S.; Osterholm, M.T.; McLean, H.Q. Variable influenza vaccine effectiveness by subtype: A systematic review and meta-analysis of test-negative design studies. Lancet Infect. Dis. 2016, 16, 942–951. [Google Scholar] [CrossRef]
- Wu, N.C.; Zost, S.J.; Thompson, A.J.; Oyen, D.; Nycholat, C.M.; McBride, R.; Paulson, J.C.; Hensley, S.E.; Wilson, I.A. A structural explanation for the low effectiveness of the seasonal influenza H3N2 vaccine. PLoS Pathog. 2017, 13, e1006682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zost, S.J.; Parkhouse, K.; Gumina, M.E.; Kim, K.; Diaz, P.S.; Wilson, P.C.; Treanor, J.J.; Sant, A.J.; Cobey, S.; Hensley, S.E. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc. Natl. Acad. Sci. USA 2017, 114, 12578–12583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeMarcus, L.; Shoubaki, L.; Federinko, S. Comparing influenza vaccine effectiveness between cell-derived and egg-derived vaccines, 2017–2018 influenza season. Vaccine 2019, 37, 4015–4021. [Google Scholar] [CrossRef]
- Izurieta, H.S.; Chillarige, Y.; Kelman, J.; Wei, Y.; Lu, Y.; Xu, W.; Lu, M.; Pratt, D.; Chu, S.; Wernecke, M.; et al. Relative Effectiveness of Cell-Cultured and Egg-Based Influenza Vaccines Among Elderly Persons in the United States, 2017–2018. J. Infect. Dis. 2019, 220, 1255–1264. [Google Scholar] [CrossRef]
- World Health Organization. Guidance on the Economic Evaluation of Influenza Vaccination; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- de Boer, P.T.; van Maanen, B.M.; Damm, O.; Ultsch, B.; Dolk, F.C.K.; Crepey, P.; Pitman, R.; Wilschut, J.C.; Postma, M.J. A systematic review of the health economic consequences of quadrivalent influenza vaccination. Expert Rev. Pharm. Outcomes Res. 2017, 17, 249–265. [Google Scholar] [CrossRef]
- Fleming, D.M.; Elliot, A.J. Health benefits, risks, and cost-effectiveness of influenza vaccination in children. Pediatr. Infect. Dis. J. 2008, 27, S154–S158. [Google Scholar] [CrossRef]
- Centers for Disease Control & Prevention. Weekly U.S. Influenza Surveillance Report: 2013–2018 Influenza Seasons. Available online: https://www.cdc.gov/flu/weekly/pastreports.htm (accessed on 11 August 2021).
- World Health Organization. FluNet, Global Influenza Surveillance and Response System (GISRS). Available online: https://www.who.int/tools/flunet (accessed on 2 June 2019).
- Carrat, F.; Vergu, E.; Ferguson, N.M.; Lemaitre, M.; Cauchemez, S.; Leach, S.; Valleron, A.J. Time lines of infection and disease in human influenza: A review of volunteer challenge studies. Am. J. Epidemiol. 2008, 167, 775–785. [Google Scholar] [CrossRef] [Green Version]
- Baguelin, M.; Flasche, S.; Camacho, A.; Demiris, N.; Miller, E.; Edmunds, W.J. Assessing optimal target populations for influenza vaccination programmes: An evidence synthesis and modelling study. PLoS Med. 2013, 10, e1001527. [Google Scholar] [CrossRef]
- Ferguson, N.M.; Cummings, D.A.; Cauchemez, S.; Fraser, C.; Riley, S.; Meeyai, A.; Iamsirithaworn, S.; Burke, D.S. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature 2005, 437, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Rolfes, M.A.; Flannery, B.; Chung, J.R.; O’Halloran, A.; Garg, S.; Belongia, E.A.; Gaglani, M.; Zimmerman, R.K.; Jackson, M.L.; Monto, A.S.; et al. Effects of Influenza Vaccination in the United States during the 2017–2018 Influenza Season. Clin. Infect. Dis. 2019, 69, 1845–1853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boikos, C.; Sylvester, G.C.; Sampalis, J.S.; Mansi, J.A. Relative Effectiveness of the Cell-Cultured Quadrivalent Influenza Vaccine Compared to Standard, Egg-derived Quadrivalent Influenza Vaccines in Preventing Influenza-like Illness in 2017–2018. Clin. Infect. Dis. 2020, 71, e665–e671. [Google Scholar] [CrossRef] [PubMed]
- Mossong, J.; Hens, N.; Jit, M.; Beutels, P.; Auranen, K.; Mikolajczyk, R.; Massari, M.; Salmaso, S.; Tomba, G.S.; Wallinga, J.; et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008, 5, e74. [Google Scholar] [CrossRef] [PubMed]
- Zagheni, E.; Billari, F.C.; Manfredi, P.; Melegaro, A.; Mossong, J.; Edmunds, W.J. Using time-use data to parameterize models for the spread of close-contact infectious diseases. Am. J. Epidemiol. 2008, 168, 1082–1090. [Google Scholar] [CrossRef] [Green Version]
- Baguelin, M.; Camacho, A.; Flasche, S.; Edmunds, W.J. Extending the elderly- and risk-group programme of vaccination against seasonal influenza in England and Wales: A cost-effectiveness study. BMC Med. 2015, 13, 236. [Google Scholar] [CrossRef] [Green Version]
- Nelder, J.; Mead, R. A simplex method for function minimization. Comput. J. 1965, 7, 308–313. [Google Scholar] [CrossRef]
- de Boer, P.T.; Crepey, P.; Pitman, R.J.; Macabeo, B.; Chit, A.; Postma, M.J. Cost-Effectiveness of Quadrivalent versus Trivalent Influenza Vaccine in the United States. Value Health 2016, 19, 964–975. [Google Scholar] [CrossRef] [Green Version]
- Grosse, S.D. Assessing cost-effectiveness in healthcare: History of the $50,000 per QALY threshold. Expert Rev. Pharm. Outcomes Res. 2008, 8, 165–178. [Google Scholar] [CrossRef]
- Centers for Disease Control & Prevention. Archived CDC Vaccine Price List as of 1 July 2019. Available online: https://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/2019/2019-07-01.html (accessed on 11 August 2021).
- Centers for Disease Control & Prevention. Flu Vaccination Coverage, United States, 2018–2019 Influenza Season. Available online: https://www.cdc.gov/flu/fluvaxview/coverage-1819estimates.htm (accessed on 11 August 2021).
- Rajaram, S.; Van Boxmeer, J.; Leav, B.; Suphaphiphat, P.; Iheanacho, I.; Kistler, K. Retrospective evaluation of mismatch from egg-based isolation of influenza strains compared with cell-based isolation and the possible implications for vaccine effectiveness. Open Forum Infect. Dis. 2018, 5, S69. [Google Scholar] [CrossRef] [Green Version]
- Bruxvoort, K.J.; Luo, Y.; Ackerson, B.; Tanenbaum, H.C.; Sy, L.S.; Gandhi, A.; Tseng, H.F. Comparison of vaccine effectiveness against influenza hospitalization of cell-based and egg-based influenza vaccines, 2017–2018. Vaccine 2019, 37, 5807–5811. [Google Scholar] [CrossRef]
- Belongia, E.A.; McLean, H.Q. Influenza Vaccine Effectiveness: Defining the H3N2 Problem. Clin. Infect. Dis. 2019, 69, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; Ruiz-Aragon, J.; Nasiri, M.; Mould-Quevedo, J.; Rajaram, S. The economic advantages of a cell-based quadrivalent influenza vaccine in the adult population in Europe. The cost-effectiveness evidence in United Kingdom and Spain. Options Control. Influ. 2019, 16, 2238–2244. [Google Scholar]
- Rizzo, C.; Capri, S.; Merler, S. Valutazione economica dell’intro- duzione del nuovo vaccino antinfluenzale quadrivalente da coltura cellulare (Flucelvax® Tetra) nel contesto di cura italiano. Ital. J. Public Health 2019, 8, 5. [Google Scholar]
- Cai, R.; Gerlier, L.; Eichner, M.; Schwehm, M.; Rajaram, S.; Mould-Quevedo, J.; Lamotte, M. Cost-effectiveness of the cell-based quadrivalent versus the standard egg-based quadrivalent influenza vaccine in Germany. J. Med. Econ. 2021, 24, 490–501. [Google Scholar] [CrossRef]
- Baguelin, M.; Jit, M.; Miller, E.; Edmunds, W.J. Health and economic impact of the seasonal influenza vaccination programme in England. Vaccine 2012, 30, 3459–3462. [Google Scholar] [CrossRef]
- Thommes, E.W.; Chit, A.; Meier, G.C.; Bauch, C.T. Examining Ontario’s universal influenza immunization program with a multi-strain dynamic model. Vaccine 2014, 32, 5098–5117. [Google Scholar] [CrossRef]
- Crepey, P.; de Boer, P.T.; Postma, M.J.; Pitman, R. Retrospective public health impact of a quadrivalent influenza vaccine in the United States. Influenza Other Respir Viruses 2015, 9 (Suppl. 1), 39–46. [Google Scholar] [CrossRef]
- Grohskopf, L.A.; Alyanak, E.; Ferdinands, J.M.; Broder, K.R.; Blanton, L.H.; Talbot, H.K.; Fry, A.M. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2021–2022 Influenza Season. MMWR Recomm. Rep. 2021, 70, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Viboud, C.; Boëlle, P.Y.; Cauchemez, S.; Lavenu, A.; Valleron, A.J.; Flahault, A.; Carrat, F. Risk factors of influenza transmission in households. Br. J. Gen. Pract. 2004, 54, 684–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, S.; Daleno, C.; Baldanti, F.; Scala, A.; Campanini, G.; Taroni, F.; Fossali, E.; Pelucchi, C.; Principi, N. Viral shedding in children infected by pandemic A/H1N1/2009 influenza virus. Virol. J. 2011, 8, 349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruf, B.R.; Knuf, M. The burden of seasonal and pandemic influenza in infants and children. Eur. J. Pediatr. 2014, 173, 265–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega-Sanchez, I.R.; Molinari, N.A.; Fairbrother, G.; Szilagyi, P.G.; Edwards, K.M.; Griffin, M.R.; Cassedy, A.; Poehling, K.A.; Bridges, C.; Staat, M.A. Indirect, out-of-pocket and medical costs from influenza-related illness in young children. Vaccine 2012, 30, 4175–4181. [Google Scholar] [CrossRef]


| Match [16] (CI 95%) | Mismatch [16] (CI 95%) | Match (Computed) | rVE [17] | |||
|---|---|---|---|---|---|---|
| Age Group (Years) | A/H1N1pdm09 | B Victoria | B Yamagata | A(H3N2) | A(H3N2) | |
| 18–49 | 48% (18–67%) | 57% (43–69%) | 57% (43–69%) | 14% (0–30%) | 43% | 26.8% (14–37%) |
| 50–64 | 36% (0–67%) | 44% (24–60%) | 44% (24–60%) | 21% (0–41) | 50% | |
| QIVe | QIVc | Difference | ||
|---|---|---|---|---|
| Number of doses | 118,818,800 | 118,818,800 | 0 | |
| Number of cases | 27,240,600 | 21,556,900 | −5,683,700 | |
| Number of GP * visits | 8,595,300 | 6,751,400 | −1,843,900 | |
| Lost workdays | 29,390,500 | 22,983,500 | −6,407,000 | |
| Hospitalizations | 199,916 | 150,191 | −49,725 | |
| Deaths | 22,436 | 16,983 | −5,453 | |
| Life years lost | 371,040 | 277,635 | −93,405 | |
| Life years lost (discounted) | 269,745 | 202,268 | −67,477 | |
| QALY lost due to sickness | 165,410 | 129,151 | −36,259 | |
| QALY lost due to death | 367,118 | 274,906 | −92,212 | |
| QALY lost due to death (discounted) | 266,001 | 199,575 | −66,426 | |
| Total QALY lost | 532,527 | 404,057 | −128,470 | |
| Total QALY lost (discounted) | 431,410 | 328,726 | −102,684 | |
| Cost of GP visits | 1,028,822,400 | 775,428,700 | −253,393,700 | |
| Cost of hospitalizations | 3,794,189,100 | 2,775,660,700 | −1,018,528,400 | |
| Cost of lost workdays | 2,105,378,600 | 1,648,894,900 | −456,483,700 | |
| Vaccine cost | 2,610,747,400 | 3,037,887,500 | 427,140,100 | |
| Total direct costs | 7,433,759,000 | 6,588,976,900 | −844,782,100 | |
| ICER | −10,400 [−17,400; 11,000] (cost saving) | |||
| Medical Costs * | QALY ** Gained | ICER *** | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of Mismatch Years | Median | Lower Bound | Upper Bound | Median | Lower Bound | Upper Bound | Median | Lower Bound | Upper Bound |
| 1 | −136,179,800 | −2,176,061,100 | 821,374,700 | 74,800 | 10,300 | 205,900 | −1800 | −12,000 | 67,900 |
| 2 | −1,570,682,800 | −4,916,394,100 | 577,624,600 | 164,100 | 26,800 | 396,300 | −8600 | −16,000 | 15,000 |
| 3 | −2,749,808,800 | −7,530,202,100 | 351,732,800 | 241,200 | 42,200 | 600,100 | −10,500 | −18,000 | 3400 |
| 4 | −3,999,641,400 | −9,658,482,100 | 182,466,200 | 325,700 | 50,400 | 732,600 | −11,600 | −18,800 | 100 |
| 5 | −5,222,697,300 | −12,426,192,400 | 21,380,200 | 412,000 | 64,600 | 920,200 | −12,200 | −19,400 | −3200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, V.H.; Hilsky, Y.; Mould-Quevedo, J. The Epidemiological and Economic Impact of a Cell-Based Quadrivalent Influenza Vaccine in Adults in the US: A Dynamic Modeling Approach. Vaccines 2021, 9, 1095. https://doi.org/10.3390/vaccines9101095
Nguyen VH, Hilsky Y, Mould-Quevedo J. The Epidemiological and Economic Impact of a Cell-Based Quadrivalent Influenza Vaccine in Adults in the US: A Dynamic Modeling Approach. Vaccines. 2021; 9(10):1095. https://doi.org/10.3390/vaccines9101095
Chicago/Turabian StyleNguyen, Van Hung, Yvonne Hilsky, and Joaquin Mould-Quevedo. 2021. "The Epidemiological and Economic Impact of a Cell-Based Quadrivalent Influenza Vaccine in Adults in the US: A Dynamic Modeling Approach" Vaccines 9, no. 10: 1095. https://doi.org/10.3390/vaccines9101095
APA StyleNguyen, V. H., Hilsky, Y., & Mould-Quevedo, J. (2021). The Epidemiological and Economic Impact of a Cell-Based Quadrivalent Influenza Vaccine in Adults in the US: A Dynamic Modeling Approach. Vaccines, 9(10), 1095. https://doi.org/10.3390/vaccines9101095





