Nanotechnology Interventions in the Management of COVID-19: Prevention, Diagnosis and Virus-Like Particle Vaccines
Abstract
:1. Introduction
2. Search and Inclusion Criteria
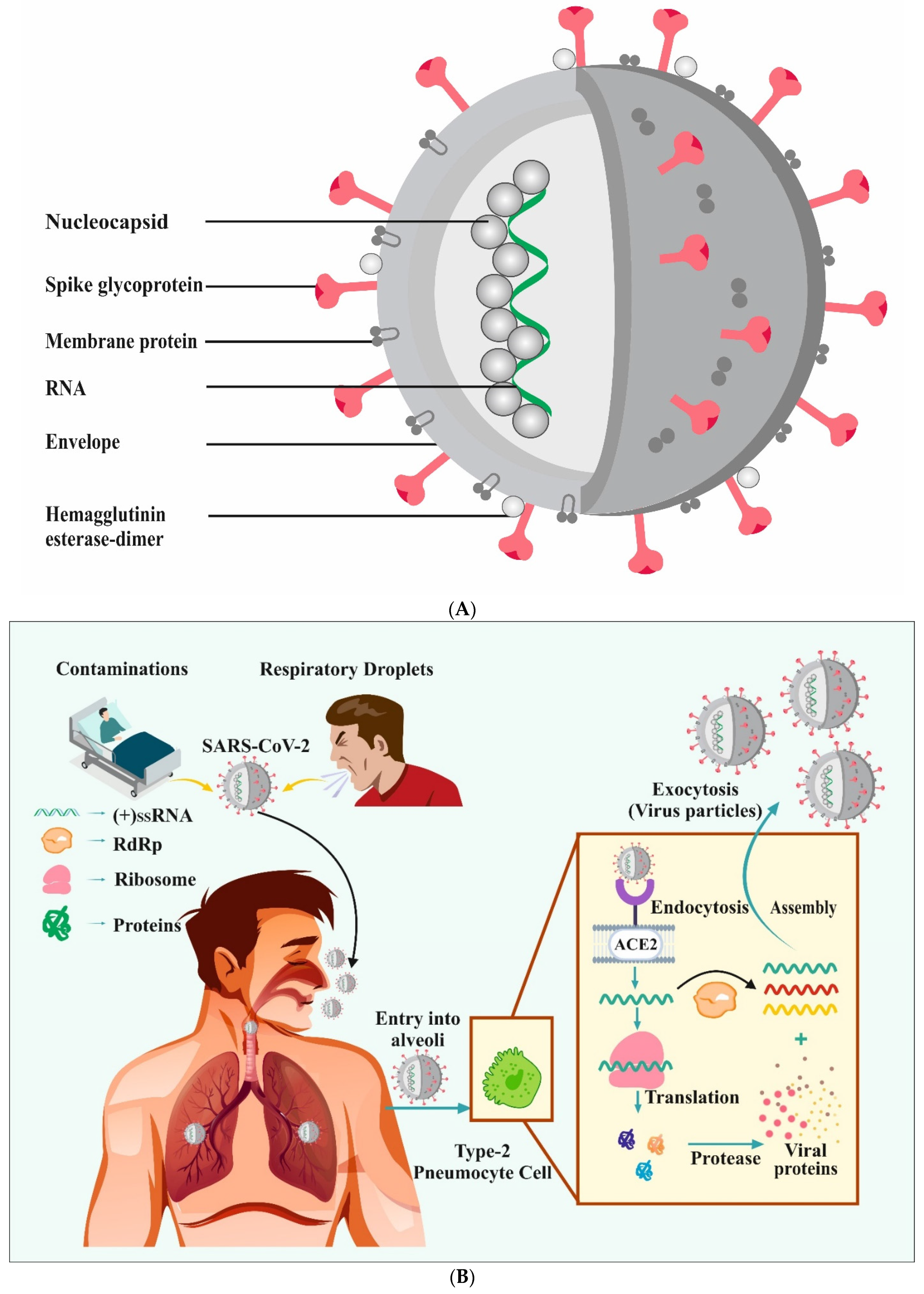
3. COVID-19: A Brief Overview
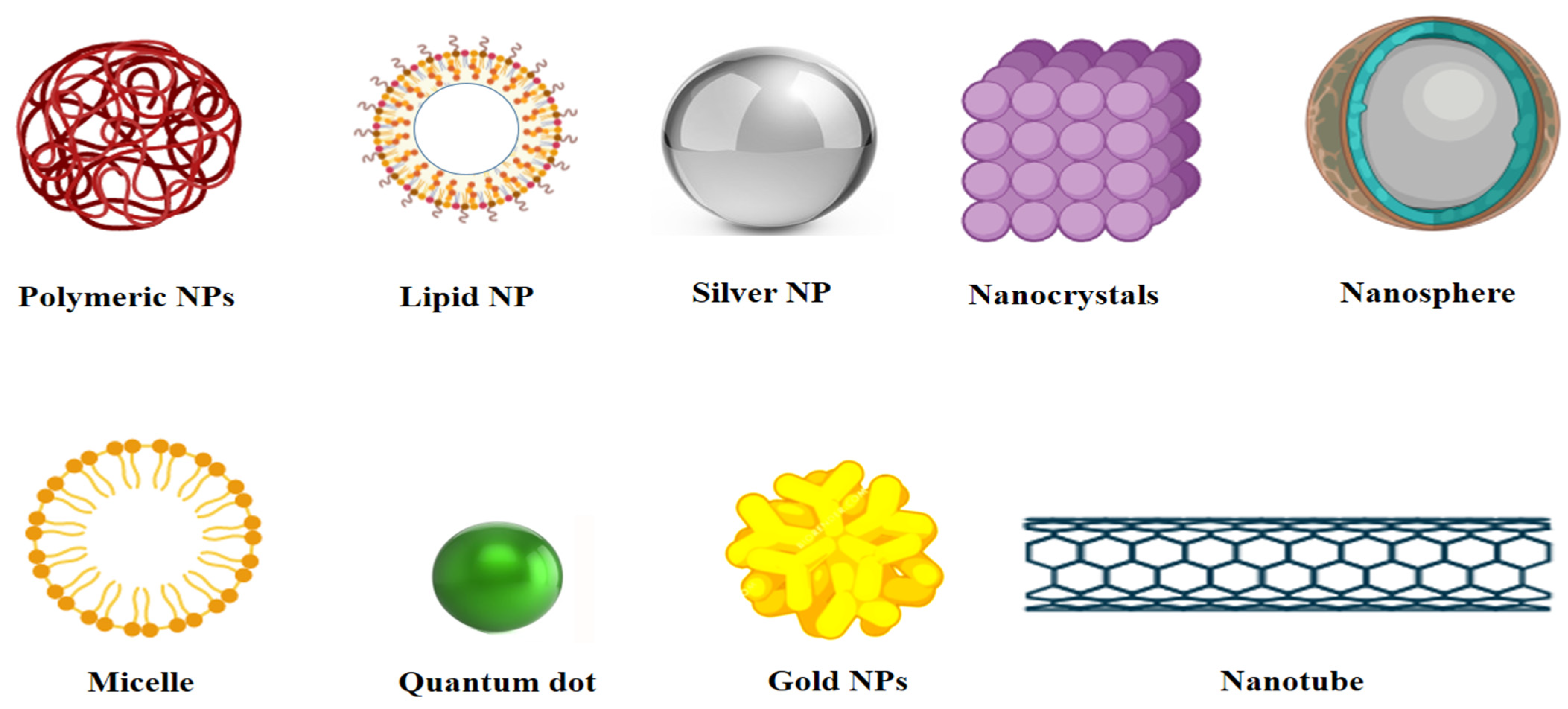
4. Properties and Applications of Different Nanomaterials
5. Role of Nanotechnology in Prevention and Diagnostic Approaches
5.1. Nanotechnology in Prevention: A Brief Overview
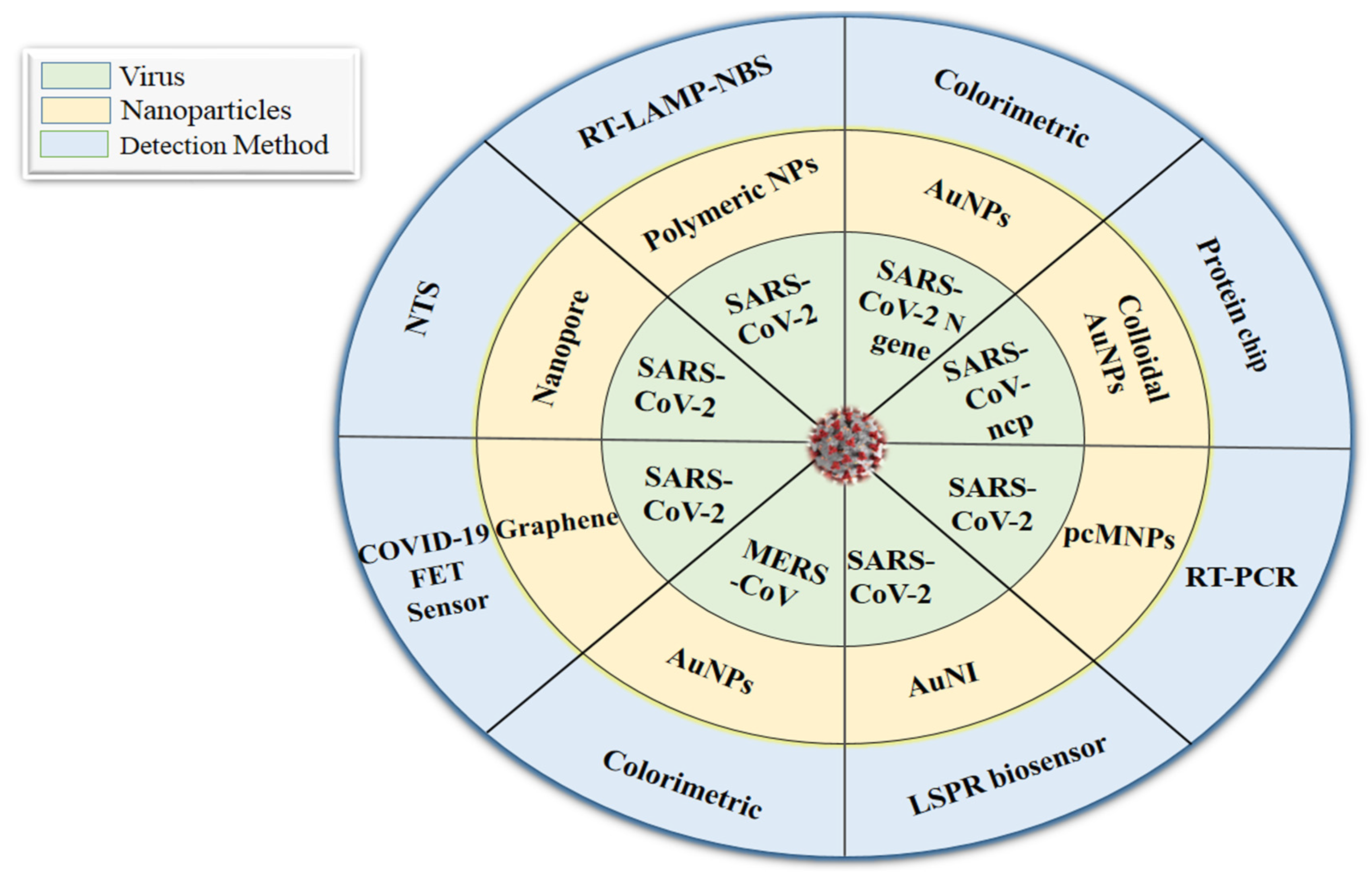
5.2. Nanotechnology-Based Diagnostic Approaches
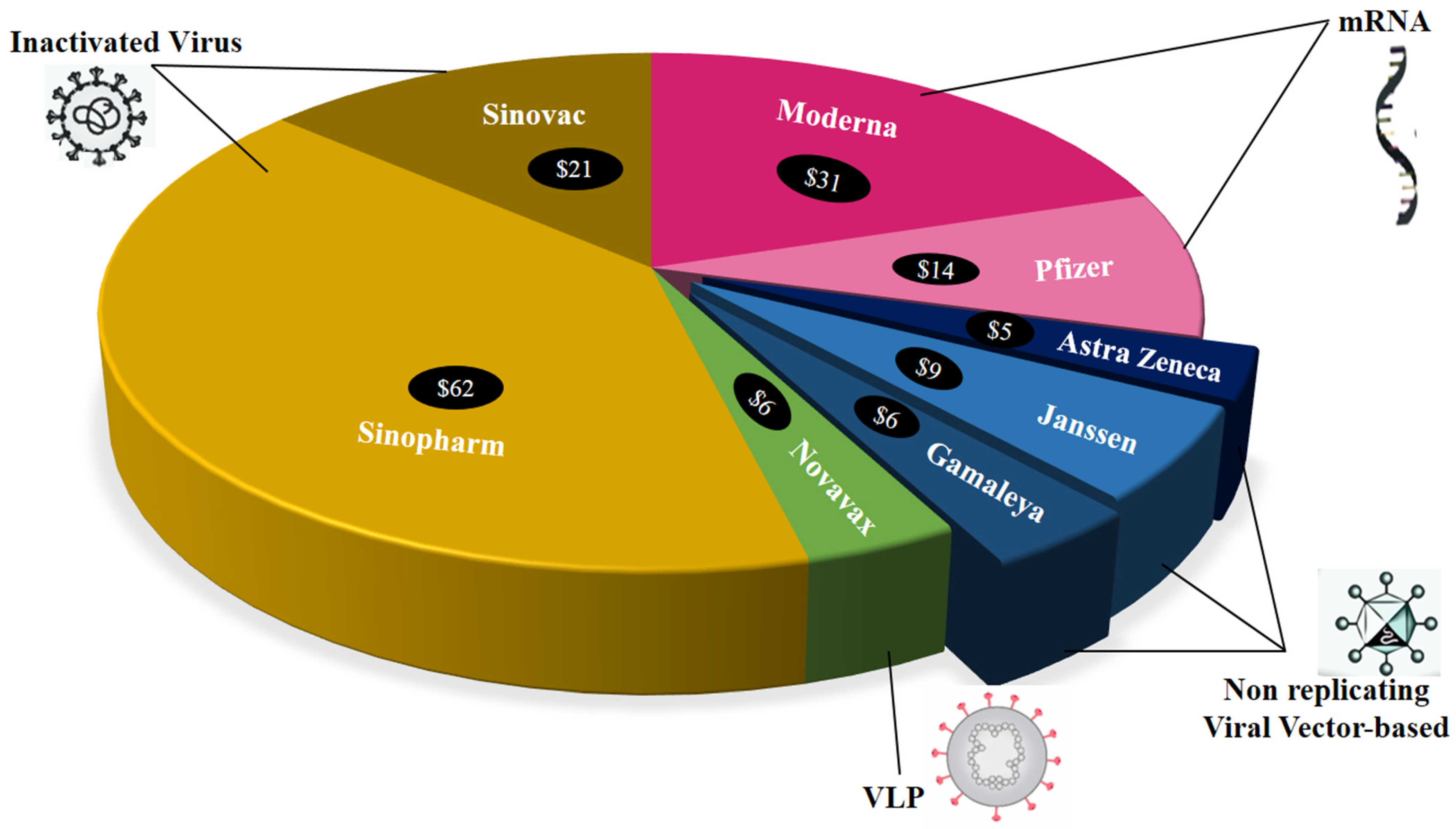
6. Vaccines against COVID-19: The Role of Nanocarriers
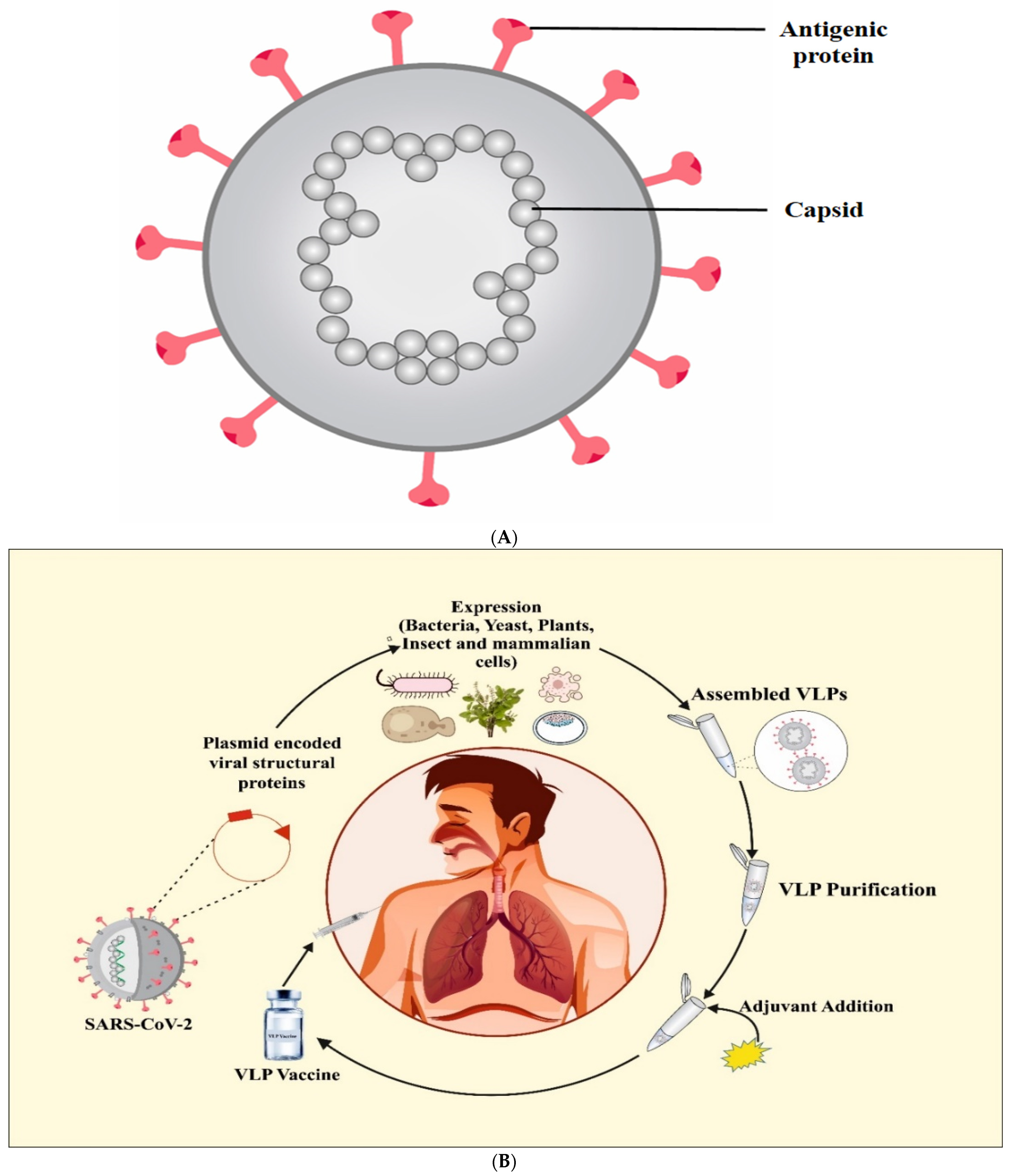
7. Virus-Like Particle (VLP) Vaccines
8. COVID-19 Vaccine Hesitancy: A Major Concern
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Shen, J.; Ye, D.; Yan, X.; Zhang, Y.; Yang, W.; Li, X.; Wang, J.; Zhang, L.; Pan, L. Disinfection technology of hospital wastes and wastewater: Suggestions for disinfection strategy during coronavirus Disease 2019 (COVID-19) pandemic in China. Environ. Pollut. 2020, 262, 114665. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.A.; Mulla, A.H.; Farooqi, T.; Pottoo, F.H.; Anwar, S.; Rengasamy, K.R. Targets and strategies for vaccine development against SARS-CoV-2. Biol. Pharmacol. 2021, 137, 111254. [Google Scholar]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.; Peng, Y.; Huang, B.; Ding, X.; Wang, X.; Niu, P.; Meng, J.; Zhu, Z.; Zhang, Z.; Wang, J.; et al. Genome Composition and Divergence of the Novel Coronavirus (2019-nCoV) Originating in China. Cell Host Microbe 2020, 27, 325–328. [Google Scholar] [CrossRef] [Green Version]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Shih, T.P.; Ko, W.C.; Tang, H.J.; Hsueh, P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef]
- Campos, E.V.R.; Pereira, A.E.S.; De Oliveira, J.L.; Carvalho, L.; Guilger-Casagrande, M.; De Lima, R.; Fraceto, L.F. How can nanotechnology help to combat COVID-19? Opportunities and urgent need. J. Nanobiotechnology 2020, 18, 125. [Google Scholar] [CrossRef]
- Wouters, O.J.; Shadlen, K.C.; Salcher-Konrad, M.; Pollard, A.J.; Larson, H.J.; Teerawattananon, Y.; Jit, M. Challenges in en-suring global access to COVID-19 vaccines: Production, affordability, allocation, and deployment. Lancet 2021, 397, 1023–1034. [Google Scholar] [CrossRef]
- Block, P.; Hoffman, M.; Raabe, I.J.; Dowd, J.B.; Rahal, C.; Kashyap, R.; Mills, M.C. Social network-based distancing strategies to flatten the COVID-19 curve in a post-lockdown world. Nat. Hum. Behav. 2020, 4, 588–596. [Google Scholar] [CrossRef]
- Zumla, P.S.A.; Hui, D.S.; Azhar, E.; A Memish, Z.; Maeurer, M. Reducing mortality from 2019-nCoV: Host-directed therapies should be an option. Lancet 2020, 395, e35–e36. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.W.; Kok, K.H.; Zhu, Z.; Chu, H.; To, K.K.W.; Yuan, S.; Yuen, K.Y. Genomic characterization of the 2019 novel hu-man-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microb. Infect. 2020, 9, 221–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vellingiri, B.; Jayaramayya, K.; Iyer, M.; Narayanasamy, A.; Govindasamy, V.; Giridharan, B.; Ganesan, S.; Venugopal, A.; Venkatesan, D.; Ganesan, H.; et al. COVID-19: A promising cure for the global panic. Sci. Total Environ. 2020, 725, 138277. [Google Scholar] [CrossRef]
- Tyo, K.M.; Lasnik, A.B.; Zhang, L.; Mahmoud, M.; Jenson, A.B.; Fuqua, J.L.; Palmer, K.E.; Steinbach-Rankins, J.M. Sustained-release Griffithsin nanoparticle-fiber composites against HIV-1 and HSV-2 infections. J. Control. Release. 2020, 321, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Mainardes, R.M.; Diedrich, C. The potential role of nanomedicine on COVID-19 therapeutics. Ther. Deliv. 2020, 11, 411–414. [Google Scholar] [CrossRef]
- Anu; Thakur, N.; Kumar, K.; Sharma, K.K. Application of Co-doped copper oxide nanoparticles against different multidrug resistance bacteria. Inorg. Nano-Met. Chem. 2020, 50, 933–943. [Google Scholar] [CrossRef]
- Thakur, N.; Anu; Kumar, K.; Kumar, A. Effect of (Ag, Zn) co-doping on structural, optical and bactericidal properties of CuO nanoparticles synthesized by a microwave-assisted method. Dalton Trans. 2021, 50, 6188–6203. [Google Scholar] [CrossRef]
- Thakur, B.; Kumar, A.; Kumar, D. Green synthesis of titanium dioxide nanoparticles using Azadirachta indica leaf extract and evaluation of their antibacterial activity. S. Afr. J. Bot. 2019, 124, 223–227. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Kumar, D. Evaluation of antimicrobial potential of cadmium sulphide nanoparticles against bacterial pathogens. Int. J. Pharm. Sci. Rev. Res. 2014, 24, 202–206. [Google Scholar]
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse Applications of Nanomedicine. ACS Nano 2017, 11, 2313–2381. [Google Scholar] [CrossRef] [Green Version]
- Balasubramaniam, B.; Prateek; Ranjan, S.; Saraf, M.; Kar, P.; Singh, S.P.; Thakur, V.K.; Singh, A.; Gupta, R.K. Antibacterial and antiviral functional materials: Chemistry and biological activity toward tackling COVID-19-like pandemics. ACS Pharmacol. Transl. Sci. 2020, 4, 8–54. [Google Scholar] [CrossRef]
- Chen, L.; Liang, J. An overview of functional nanoparticles as novel emerging antiviral therapeutic agents. Mater. Sci. Eng. C 2020, 112, 110924. [Google Scholar] [CrossRef]
- Rupp, R.; Rosenthal, S.L.; Stanberry, L.R. VivaGel™ (SPL7013 Gel): A candidate dendrimer—microbicide for the prevention of HIV and HSV infection. Int. J. Nanomed. 2007, 2, 561–566. [Google Scholar]
- Liu, Z.; Shang, C.; Ma, H.; You, M. An upconversion nanoparticle-based photostable FRET system for long-chain DNA sequence detection. Nanotechnology 2020, 31, 235501. [Google Scholar] [CrossRef] [PubMed]
- Joe, Y.H.; Woo, K.; Hwang, J. Fabrication of an anti-viral air filter with SiO2–Ag nanoparticles and performance evaluation in a continuous airflow condition. J. Hazard. Mater. 2014, 280, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Le, T.S.; Dao, T.H.; Nguyen, D.C.; Nguyen, H.C.; Balikhin, I. Air purification equipment combining a filter coated by silver nanoparticles with a nano-TiO2 photocatalyst for use in hospitals. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 015016. [Google Scholar] [CrossRef]
- Adams, J.G.; Walls, R.M. Supporting the Health Care Workforce During the COVID-19 Global Epidemic. JAMA 2020, 323, 1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mejía-Salazar, J.; Oliveira, O.N., Jr. Plasmonic biosensing: Focus review. Chem. Rev. 2018, 118, 10617–10625. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-E.; Kim, K.; Jung, Y.; Kim, J.-H.; Nam, J.-M. Metal Nanoparticles for Virus Detection. ChemNanoMat 2016, 2, 927–936. [Google Scholar] [CrossRef]
- Mokhtarzadeh, A.; Eivazzadeh-Keihan, R.; Pashazadeh, P.; Hejazi, M.; Gharaatifar, N.; Hasanzadeh, M.; Baradaran, B.; de la Guardia, M. Nanomaterial-based biosensors for detection of pathogenic virus. TrAC Trends Anal. Chem. 2017, 97, 445–457. [Google Scholar] [CrossRef]
- Medhi, R.; Srinoi, P.; Ngo, N.; Tran, H.-V.; Lee, T.R. Nanoparticle-Based Strategies to Combat COVID-19. ACS Appl. Nano Mater. 2020, 3, 8557–8580. [Google Scholar] [CrossRef]
- Demento, S.L.; Cui, W.; Criscione, J.M.; Stern, E.; Tulipan, J.; Kaech, S.M.; Fahmy, T.M. Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials 2012, 33, 4957–4964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregory, A.E.; Titball, R.; Williamson, D. Vaccine delivery using nanoparticles. Front. Cell. Infect. Microbiol. 2013, 3, 13. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-G.; Park, J.Y.; Shon, Y.; Kim, G.; Shim, G.; Oh, Y.-K. Nanotechnology and vaccine development. Asian J. Pharm. Sci. 2014, 9, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.Y.; Liu, Y.; Zhang, X.J.; Han, L.; Du, L.B.; Tian, Q.; Xu, Y.C. Potential oxidative stress of gold nanoparticles by induced-NO releasing in serum. J. Am. Chem. Soc. 2009, 131, 40–41. [Google Scholar] [CrossRef] [PubMed]
- Durocher, S.; Rezaee, A.; Hamm, C.; Rangan, C.; Mittler, S.; Mutus, B. Disulfide-Linked, Gold Nanoparticle Based Reagent for Detecting Small Molecular Weight Thiols. J. Am. Chem. Soc. 2009, 131, 2475–2477. [Google Scholar] [CrossRef]
- Kirchner, C.; Liedl, T.; Kudera, S.; Pellegrino, T.; Muñoz Javier, A.; Gaub, H.E.; Stölzle, S.; Fertig, N.; Parak, W.J. Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles. Nano Lett. 2005, 5, 331–338. [Google Scholar] [CrossRef]
- Bai, B.; Hu, Q.; Hu, H.; Zhou, P.; Shi, Z.; Meng, J.; Lu, B.; Huang, Y.; Mao, P.; Wang, H. Virus-Like Particles of SARS-Like Coronavirus Formed by Membrane Proteins from Different Origins Demonstrate Stimulating Activity in Human Dendritic Cells. PLoS ONE 2008, 3, e2685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balke, I.; Zeltins, A. Use of plant viruses and virus-like particles for the creation of novel vaccines. Adv. Drug Deliv. Rev. 2018, 145, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Chroboczek, J.; Szurgot, I.; Szolajska, E. Virus-like particles as vaccine. Acta Biochim. Pol. 2014, 61, 531–539. [Google Scholar] [CrossRef]
- Jain, N.K.; Sahni, N.; Kumru, O.S.; Joshi, S.B.; Volkin, D.B.; Middaugh, C.R. Formulation and stabilization of recombinant protein based virus-like particle vaccines. Adv. Drug Deliv. Rev. 2014, 93, 42–55. [Google Scholar] [CrossRef]
- WHO. WHO Draft Landscape of COVID-19 Candidate Vaccines; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Khan, S.; Liu, J.; Xue, M. Transmission of SARS-CoV-2, Required Developments in Research and Associated Public Health Concerns. Front. Med. 2020, 7, 310. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterization and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J. Virol. 2020, 94, e00127-20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Worldometers-Countries where COVID-19 has Spread. Available online: www.worldometers.info (accessed on 14 July 2021).
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gayle, A.A.; Wilder-Smith, A.; Rocklöv, J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Travel Med. 2020, 27, taaa021. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Hua, J.; Wang, G.; Huang, M.; Hua, S.; Yang, S. A visual approach for the SARS (severe acute respiratory syndrome) out-break data analysis. Int. J. Environ. Res. Public Health 2020, 17, 3973. [Google Scholar] [CrossRef]
- Wang, C.; Horby, P.W.; Hayden, F.G.; Gao, G.F. A novel coronavirus outbreak of global health concern. Lancet 2020, 395, 470–473. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.F.W.; Yuan, S.; Kok, K.H.; To, K.K.W.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.Y.; Poon, R.W.S.; et al. A fa-milial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Pandey, V.; Singh, A.; Siddiqui, S.; Raikwar, A.; Gond, A.K.; Ali, S.; Yadav, M.; Datta, A.; Singh, A. COVID 19: An update of current knowledge. World Acad. Sci. J. 2021, 3, 1–8. [Google Scholar] [CrossRef]
- Huang, H.; Fan, C.; Li, M.; Nie, H.-L.; Wang, F.-B.; Wang, H.; Wang, R.; Xia, J.; Zheng, X.; Zuo, X.; et al. COVID-19: A Call for Physical Scientists and Engineers. ACS Nano 2020, 14, 3747–3754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovato, A.; De Filippis, C. Clinical presentation of COVID-19: A systematic review focusing on upper airway symptoms. Ear Nose Throat J. 2020, 99, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Yao, L.; Wei, T.; Tian, F.; Jin, D.-Y.; Chen, L.; Wang, M. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA 2020, 323, 1406. [Google Scholar] [CrossRef] [Green Version]
- Florindo, H.F.; Kleiner, R.; Vaskovich-Koubi, D.; Acúrcio, R.C.; Carreira, B.; Yeini, E.; Tiram, G.; Liubomirski, Y.; Satchi-Fainaro, R. Immune-mediated approaches against COVID-19. Nat. Nanotechnol. 2020, 15, 630–645. [Google Scholar] [CrossRef]
- Astuti, I.; Ysrafil. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response. Diabetes Metab. Syndr. 2020, 14, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.-C.; Deng, Q.-X.; Dai, S.-X. Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: An evaluation of the evidence. Travel Med. Infect. Dis. 2020, 35, 101647. [Google Scholar] [CrossRef]
- Jiang, S.; Hillyer, C.; Du, L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020, 41, 355–359. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.M.; Yu, X.H.; Tang, S.L.; Tang, C.K. Coronavirus disease 2019 (COVID-19): Current status and future perspectives. Int. J. Antimicrob. Agents 2020, 55, 105951. [Google Scholar] [CrossRef]
- Shereen, M.A.; Khan, S.; Kazmi, A.; Bashir, N.; Siddique, R. COVID-19 infection: Emergence, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020, 24, 91–98. [Google Scholar] [CrossRef]
- Zeng, Q.; Langereis, M.; van Vliet, A.L.W.; Huizinga, E.G.; de Groot, R.J. Structure of coronavirus hemagglutinin-esterase offers insight into corona and influenza virus evolution. Proc. Natl. Acad. Sci. USA 2008, 105, 9065–9069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jha, N.; Jeyaraman, M.; Rachamalla, M.; Ojha, S.; Dua, K.; Chellappan, D.; Muthu, S.; Sharma, A.; Jha, S.; Jain, R.; et al. Current Understanding of Novel Coronavirus: Molecular Pathogenesis, Diagnosis, and Treatment Approaches. Immuno 2021, 1, 4. [Google Scholar] [CrossRef]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [Green Version]
- Kerry, R.G.; Malik, S.; Redda, Y.T.; Sahoo, S.; Patra, J.K.; Majhi, S. Nano-based approach to combat emerging viral (NIPAH virus) infection. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 196–220. [Google Scholar] [CrossRef]
- Comparetti, E.J.; Pedrosa, V.; Kaneno, R. Carbon Nanotube as a Tool for Fighting Cancer. Bioconjugate Chem. 2017, 29, 709–718. [Google Scholar] [CrossRef]
- Alidori, S.; Bowman, R.L.; Yarilin, D.; Romin, Y.; Barlas, A.; Mulvey, J.J.; Fujisawa, S.; Xu, K.; Ruggiero, A.; Riabov, V.; et al. Deconvoluting hepatic processing of carbon nanotubes. Nat. Commun. 2016, 7, 12343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, S.; Ray, S.; Thakur, R. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009, 71, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Makwana, V.; Jain, R.; Patel, K.; Nivsarkar, M.; Joshi, A. Solid lipid nanoparticles (SLN) of Efavirenz as lymph targeting drug delivery system: Elucidation of mechanism of uptake using chylomicron flow blocking approach. Int. J. Pharm. 2015, 495, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Vyas, T.K.; Shahiwala, A.; Amiji, M.M. Improved oral bioavailability and brain transport of Saquinavir upon administration in novel nanoemulsion formulations. Int. J. Pharm. 2008, 347, 93–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zielińska, A.; Carreiró, F.; Oliveira, A.; Neves, A.; Pires, B.; Venkatesh, D.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Owens, D.E.; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Chiappetta, D.A.; Hocht, C.; Taira, C.; Sosnik, A. Efavirenz-loaded polymeric micelles for pediatric anti-HIV pharma-cotherapy with significantly higher oral bioavailability. Nanomedicine 2010, 5, 11–23. [Google Scholar] [CrossRef]
- Li, Q.; Du, Y.-Z.; Yuan, H.; Zhang, X.-G.; Miao, J.; Cui, F.-D.; Hu, F.-Q. Synthesis of Lamivudine stearate and antiviral activity of stearic acid-g-chitosan oligosaccharide polymeric micelles delivery system. Eur. J. Pharm. Sci. 2010, 41, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Taymouri, S.; Jafari, E.; Jahanian-Najafabadi, A.; Taheri, A. Formulation and characterization of cellulose ace-tate butyrate nanoparticles loaded with nevirapine for HIV treatment. J. Drug Deliv. Sci. Technol. 2018, 48, 9–20. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Chen, W.; Ma, X.; Huang, J.; Chen, R. Extended peginterferon alfa-2a (Pegasys) therapy in Chinese pa-tients with HBeAg-negative chronic hepatitis B. J. Med. Virol. 2014, 86, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Wani, T.U.; Raza, S.N.; Khan, N.A. Nanoparticle opsonization: Forces involved and protection by long chain polymers. Polym. Bull. 2019, 77, 3865–3889. [Google Scholar] [CrossRef]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnology 2021, 19, 59. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Coronavirus Disease 2019 (COVID-19)—Transmission; CDC: Atlanta, GA, USA, 2020.
- Matthew, C. A Facemask having One or More Nanofiber Layers. European Patent WO2014143039A1, 18 September 2014. [Google Scholar]
- Francois, L. Nanofiber Filtering Material for Disposable/Reusable Respirators. US Patent US9446547B2, 20 September 2016. [Google Scholar]
- Elston, D.M. Occupational skin disease among health care workers during the coronavirus (COVID-19) epidemic. J. Am. Acad. Dermatol. 2020, 82, 1085–1086. [Google Scholar] [CrossRef]
- Mechael, K. Antimicrobial fabric materials for use in safety masks and personal protection clothing. European Patent WO2016125173A1, 8 November 2016. [Google Scholar]
- NBIC-Reusable Graphene Mask Sterilizes Itself Against Coronavirus with Electrical Charge. Available online: https://statnano.com/news/67559/Reusable-Graphene-Mask-Sterilises-Itself-against-Coronavirus-with-Electrical-Charge (accessed on 16 May 2020).
- Widdowson, N. New Mask Material Can Remove Virus-Size Nanoparticles. Available online: https://phys.org/news/2020-04-mask-material-virus-size-nanoparticles.html (accessed on 18 May 2021).
- Balagna, C.; Perero, S.; Percivalle, E.; Nepita, E.V.; Ferraris, M. Virucidal effect against coronavirus SARS-CoV-2 of a silver nanocluster/silica composite sputtered coating. Open Ceram. 2020, 1, 100006. [Google Scholar] [CrossRef]
- Nanotechnology in Battle Against Coronavirus. Available online: https://statnano.com/nanotechnology-in-battle-against-coronavirus (accessed on 26 May 2021).
- Liu, J.; Chamakura, K.; Perez-Ballestero, R.; Bashir, S. Historical Overview of the First Two Waves of Bactericidal Agents and Development of the Third Wave of Potent Disinfectants. In Nanomaterials for Biomedicine; American Chemical Society: Washington, DC, USA, 2012; pp. 129–154. [Google Scholar] [CrossRef]
- Shahzadi, S.; Zafar, N.; Sharif, R. Antibacterial activity of metallic nanoparticles. In Bacterial Pathogenesis and Antibacterial Control; Kırmusaoğlu, S., Ed.; IntechOpen: London, UK, 2018; pp. 51–72. [Google Scholar]
- Sportelli, M.C.; Izzi, M.; Kukushkina, E.A.; Hossain, S.I.; Picca, R.A.; Ditaranto, N.; Cioffi, N. Can nanotechnology and ma-terials science help the fight against SARS-CoV-2? Nanomaterials 2020, 10, 802. [Google Scholar] [CrossRef] [Green Version]
- Talebian, S.; Wallace, G.G.; Schroeder, A.; Stellacci, F.; Conde, J. Nanotechnology-based disinfectants and sensors for SARS-CoV-2. Nat. Nanotechnol. 2020, 15, 618–621. [Google Scholar] [CrossRef]
- Alpdagtas, S.; Ilhan, E.; Uysal, E.; Sengor, M.; Ustundag, C.B.; Gunduz, O. Evaluation of current diagnostic methods for COVID-19. APL Bioeng. 2020, 4, 041506. [Google Scholar] [CrossRef]
- FIND-Find Evaluations of SARS-COV-2 Assays. 2021. Available online: https://www.finddx.org/covid-19/sarscov2-eval/ (accessed on 15 June 2021).
- WHO. WHO Standard Q Covid-19 Ag Test; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Chaimayo, C.; Kaewnaphan, B.; Tanlieng, N.; Athipanyasilp, N.; Sirijatuphat, R.; Chayakulkeeree, M.; Angkasekwinai, N.; Sutthent, R.; Puangpunngam, N.; Tharmviboonsri, T.; et al. Rapid SARS-CoV-2 antigen detection assay in comparison with real-time RT-PCR assay for laboratory diagnosis of COVID-19 in Thailand. Virol. J. 2020, 17, 177. [Google Scholar] [CrossRef]
- Sheridan, C. Fast, portable tests come online to curb coronavirus pandemic. Nat. Biotechnol. 2020, 38, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Ramdas, K.; Darzi, A.; Jain, S. ‘Test, re-test, re-test’:using inaccurate tests to greatly increase the accuracy of COVID-19 testing. Nat. Med. 2020, 26, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Han, H.; Liu, F.; Lv, Z.; Wu, K.; Liu, Y.; Feng, Y.; Zhu, C. Positive rate of RT-PCR detection of SARS-CoV-2 infection in 4880 cases from one hospital in Wuhan, China, from January to February 2020. Clin. Chim. Acta 2020, 505, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wen, T.; Shi, F.J.; Zeng, X.Y.; Jiao, Y.J. Rapid detection of IgM antibodies against the SARS-CoV-2 virus via colloidal gold nanoparticle-based lateral-flow assay. ACS Omega 2020, 5, 12550–12556. [Google Scholar] [CrossRef] [PubMed]
- Kircher, M.F.; Mahmood, U.; King, R.S.; Weissleder, R.; Josephson, L. A multimodal nanoparticle for preoperative magnetic resonance imaging and intraoperative optical brain tumor delineation. Cancer Res. 2003, 63, 8122–8125. [Google Scholar]
- Nam, J.-M.; Stoeva, S.I.; Mirkin, C.A. Bio-Bar-Code-Based DNA Detection with PCR-like Sensitivity. J. Am. Chem. Soc. 2004, 126, 5932–5933. [Google Scholar] [CrossRef]
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef]
- Neuwelt, E.A.; Várallyay, P.; Bagó, A.G.; Muldoon, L.L.; Nesbit, G.; Nixon, R. Imaging of iron oxide nanoparticles by MR and light microscopy in patients with malignant brain tumours. Neuropathol. Appl. Neurobiol. 2004, 30, 456–471. [Google Scholar] [CrossRef]
- Jamieson, T.; Bakhshi, R.; Petrova, D.; Pocock, R.; Imani, M.; Seifalian, A. Biological applications of quantum dots. Biomaterials 2007, 28, 4717–4732. [Google Scholar] [CrossRef] [PubMed]
- Mansuriya, B.; Altintas, Z. Applications of Graphene Quantum Dots in Biomedical Sensors. Sensors 2020, 20, 1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stringer, R.C.; Schommer, S.; Hoehn, D.; Grant, S.A. Development of an optical biosensor using gold nanoparticles and quantum dots for the detection of Porcine Reproductive and Respiratory Syndrome Virus. Sens. Actuators B Chem. 2008, 134, 427–431. [Google Scholar] [CrossRef]
- Nikaeen, G.; Abbaszadeh, S.; Yousefinejad, S. Application of nanomaterials in treatment, anti-infection and detection of coronaviruses. Nanomedicine 2020, 15, 1501–1512. [Google Scholar] [CrossRef]
- Liu, I.L.; Lin, Y.C.; Lin, Y.C.; Jian, C.Z.; Cheng, I.C.; Chen, H.W. A novel immunochromatographic strip for antigen detec-tion of avian infectious bronchitis virus. Int. J. Mol. Sci. 2019, 20, 2216. [Google Scholar] [CrossRef] [Green Version]
- Teengam, P.; Siangproh, W.; Tuantranont, A.; Vilaivan, T.; Chailapakul, O.; Henry, C.S. Multiplex paper-based colorimetric DNA sensor using pyrrolidinyl peptide nucleic acid-induced AgNPs aggregation for detecting MERS-CoV, MTB, and HPV oligonucleotides. Anal. Chem. 2017, 89, 5428–5435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roh, C.; Jo, S.K. Quantitative and sensitive detection of SARS coronavirus nucleocapsid protein using quantum dots-conjugated RNA aptamer on chip. J. Chem. Technol. Biotechnol. 2011, 86, 1475–1479. [Google Scholar] [CrossRef]
- Layqah, L.A.; Eissa, S. An electrochemical immunosensor for the corona virus associated with the Middle East respiratory syndrome using an array of gold nanoparticle-modified carbon electrodes. Microchim. Acta 2019, 186, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, J.; Yan, M.; Li, H.; Liu, T.; Lin, C.; Huang, S.; Shen, C. Evaluation of Enzyme-Linked Immunoassay and Colloidal Gold-Immunochromatographic Assay Kit for Detection of Novel Coronavirus (SARS-Cov-2) Causing an Outbreak of Pneumonia (COVID-19). medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.H.; Choi, M.; Ku, K.B.; Lee, C.S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal Swab specimens using field-effect transistor-based biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef] [Green Version]
- Moitra, P.; Alafeef, M.; Dighe, K.; Frieman, M.B.; Pan, D. Selective naked-eye detection of SARS-CoV-2 mediated by N gene targeted antisense oligonucleotide capped plasmonic nanoparticles. ACS Nano 2020, 14, 7617–7627. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, X.; Han, L.; Chen, T.; Wang, L.; Li, H.; Li, S.; He, L.; Fu, X.; Chen, S.; et al. Reverse transcription loop-mediated isothermal amplifcation combined with nanoparticles-based biosensor for diagnosis of COVID-19. Biosens. Bioelectron. 2020, 166, 112437. [Google Scholar] [CrossRef]
- Zhao, Z.; Cui, H.; Song, W.; Ru, X.; Zhou, W.; Yu, X. A simple magnetic nano particles-based viral RNA extraction method for efcient detection of SARS-CoV-2. Mol. Biol. 2020, 1–18. [Google Scholar]
- Qiu, G.; Gai, Z.; Tao, Y.; Schmitt, J.; Kullak-Ublick, G.A.; Wang, J. Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano 2020, 14, 5268–5277. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Fu, A.; Hu, B.; Tong, Y.; Liu, R.; Liu, Z.; Gu, J.; Xiang, B.; Liu, J.; Jiang, W.; et al. Nanopore Targeted Sequencing for the Accurate and Comprehensive Detection of SARS-CoV-2 and Other Respiratory Viruses. Small 2020, 16, e2002169. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, G.; Madou, M.J.; Kalra, S.; Chopra, V.; Ghosh, D.; Martinez-Chapa, S.O. Nanotechnology for COVID-19: Thera-peutics and vaccine research. ACS Nano 2020, 14, 7760–7782. [Google Scholar] [CrossRef]
- Hanney, S.R.; Wooding, S.; Sussex, J.; Grant, J. From COVID-19 research to vaccine application: Why might it take 17 months not 17 years and what are the wider lessons? Health Res Policy Syst. 2020, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.M.; Butchart, A.T.; Wheeler, J.R.; Coleman, M.S.; Singer, D.C.; Freed, G.L. Failure-to-success ratios, transition probabilities and phase lengths for prophylactic vaccines versus other pharmaceuticals in the development pipeline. Vaccine 2011, 29, 9414–9416. [Google Scholar] [CrossRef] [PubMed]
- Pronker, E.S.; Weenen, T.C.; Commandeur, H.; Claassen, E.H.J.H.M.; Osterhaus, A.D.M.E. Risk in vaccine research and de-velopment quantified. PLoS ONE 2013, 8, e57755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Struck, M.-M. Vaccine R&D success rates and development times. Nat. Biotechnol. 1996, 14, 591–593. [Google Scholar] [CrossRef]
- Watanabe, Y.; Mendonça, L.; Allen, E.R.; Howe, A.; Lee, M.; Allen, J.D.; Chawla, H.; Pulido, D.; Donnellan, F.; Davies, H.; et al. Native-like SARS-CoV-2 Spike Glycoprotein Expressed by ChAdOx1 nCoV-19/AZD1222 Vaccine. ACS Central Sci. 2021, 7, 594–602. [Google Scholar] [CrossRef] [PubMed]
- European Medicine Agency. COVID-19 Vaccine AstraZeneca; European Medicine Agency: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; I Tukhvatullin, A.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G.; et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. Lancet 2020, 396, 887–897. [Google Scholar] [CrossRef]
- Sputnik-The First Registered Vaccine Against COVID-19. Available online: https://sputnikvaccine.com/ (accessed on 21 June 2021).
- WHO. Status of COVID-19 Vaccines within WHO EUL/PQ Evaluation Process; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- USFDA. USFDA Fact Sheet for Recipients and Caregivers: Emergency Use Authorization (EUA) of the Moderna Covid-19 Vaccine to Prevent Coronavirus Disease 2019 (Covid-19) In Individuals 18 Years Of Age And Older; USFDA: Silver Spring, MD, USA, 2021.
- WHO. COVID-19 Vaccine (Vero Cell), Inactivated (Sinopharm), COVID-19 Vaccine Explainer; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Vijayan, V.; Mohapatra, A.; Uthaman, S.; Park, I.-K. Recent Advances in Nanovaccines Using Biomimetic Immunomodulatory Materials. Pharmaceutics 2019, 11, 534. [Google Scholar] [CrossRef] [Green Version]
- Lung, P.; Yang, J.; Li, Q. Nanoparticle formulated vaccines: Opportunities and challenges. Nanoscale 2020, 12, 5746–5763. [Google Scholar] [CrossRef]
- Espeseth, A.S.; Cejas, P.J.; Citron, M.P.; Wang, D.; DiStefano, D.J.; Callahan, C.; Donnell, G.O.; Galli, J.D.; Swoyer, R.; Touch, S.; et al. Modifed mRNA/lipid nanoparticle-based vaccines ex-pressing respira tory syncytial virus F protein variants are immunogenic and protective in rodent models of RSV infection. NPJ Vaccines 2020, 16, 16. [Google Scholar]
- Sampat, B.N.; Shadlen, K.C. The COVID-19 Innovation System. Health Aff. 2021, 40, 400–409. [Google Scholar] [CrossRef]
- CEPI (Coalition for Epidemic Preparedness Innovations). CEPI-Survey of Global Drug Substance and Drug Product Landscape June 2020; CEPI: Oslo, Norway, 2020. [Google Scholar]
- Khamsi, R. If a coronavirus vaccine arrives, can the world make enough? Nature 2020, 580, 578–580. [Google Scholar] [CrossRef]
- Hill, B.D.; Zak, A.; Khera, E.; Wen, F.; Hill, A.Z.B.D. Engineering Virus-like Particles for Antigen and Drug Delivery. Curr. Protein Pept. Sci. 2017, 19, 112–127. [Google Scholar] [CrossRef]
- Pushko, P.; Pumpens, P.; Grens, E. Development of Virus-Like Particle Technology from Small Highly Symmetric to Large Complex Virus-Like Particle Structures. Intervirology 2013, 56, 141–165. [Google Scholar] [CrossRef] [PubMed]
- Bayer, M.E.; Blumberg, B.S.; Werner, B. Particles associated with Australia Antigen in the Sera of Patients with Leukaemia, Down’s Syndrome and Hepatitis. Nature 1968, 218, 1057–1059. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Gomes, A.C.; Vogel, M.; Bachmann, M.F. Interaction of Viral Capsid-Derived Virus-Like Particles (VLPs) with the Innate Immune System. Vaccines 2018, 6, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, A.L.; Peres, C.; Conniot, J.; de Matos, A.I.N.; Moura, L.; Carreira, B.; Sainz, V.; Scomparin, A.; Satchi-Fainaro, R.; Préat, V.; et al. Nanoparticle impact on innate immune cell pattern-recognition receptors and inflammasomes activation. Semin. Immunol. 2017, 34, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Latham, T.; Galarza, J.M. Formation of wild-type and chimeric infuenza virus-like particles following simultaneous expres-sion of only four structural proteins. J. Virol. 2001, 75, 6154–6165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sailaja, G.; Skountzou, I.; Quan, F.-S.; Compans, R.W.; Kang, S.-M. Human immunodeficiency virus-like particles activate multiple types of immune cells. Virology 2007, 362, 331–341. [Google Scholar] [CrossRef] [Green Version]
- Le, D.T.; Radukic, M.T.; Müller, K.M. Adeno-associated virus capsid protein expression in Escherichia coli and chemically defned capsid assembly. Sci. Rep. 2019, 9, 18631. [Google Scholar] [CrossRef]
- Joe, C.C.; Chatterjee, S.; Lovrecz, G.; Adams, T.E.; Thaysen-Andersen, M.; Walsh, R.; Locarnini, S.A.; Smooker, P.; Netter, H.J. Glycoengineered hepatitis B virus-like particles with enhanced immunogenicity. Vaccine 2020, 38, 3892–3901. [Google Scholar] [CrossRef]
- Zhai, L.; Yadav, R.; Kunda, N.K.; Anderson, D.; Bruckner, E.; Miller, E.K.; Basu, R.; Muttil, P.; Tumban, E. Oral immunization with bacteriophage MS2-L2 VLPs protects against oral and genital infection with multiple HPV types associated with head & neck cancers and cervical cancer. Antivir. Res. 2019, 166, 56–65. [Google Scholar] [CrossRef]
- Shiri, F.; Petersen, K.E.; Romanov, V.; Zou, Q.; Gale, B.K. Characterization and differential retention of Q beta bacterio-phage virus-like particles using cyclical electrical field–flow fractionation and asymmetrical flow field– flow fractionation. Anal. Bioanal. Chem. 2020, 412, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Seong, B.L. Exploiting virus-like particles as innovative vaccines against emerging viral infections. J. Microbiol. 2017, 55, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Galaway, F.A.; Stockley, P.G. MS2 Viruslike Particles: A Robust, Semisynthetic Targeted Drug Delivery Platform. Mol. Pharm. 2012, 10, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Yui, M.; Deo, V.K.; Park, E.Y. Development of Rous sarcoma Virus-like Particles Displaying hCC49 scFv for Specific Targeted Drug Delivery to Human Colon Carcinoma Cells. Pharm. Res. 2015, 32, 3699–3707. [Google Scholar] [CrossRef]
- Pang, H.H.; Chen, P.Y.; Wei, K.C.; Huang, C.W.; Shiue, Y.L.; Huang, C.Y.; Yang, H.W. Convection-enhanced delivery of a vi-rus-like nanotherapeutic agent with dual-modal imaging for besiegement and eradication of brain tumors. Theranostics 2019, 9, 1752–1763. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Zha, L.; Cabral-Miranda, G.; Bachmann, M.F. Major findings and recent advances in virus–like particle (VLP)-based vaccines. Semin. Immunol. 2017, 34, 123–132. [Google Scholar] [CrossRef]
- Li, Y.-D.; Chi, W.-Y.; Su, J.-H.; Ferrall, L.; Hung, C.-F.; Wu, T.-C. Coronavirus vaccine development: From SARS and MERS to COVID-19. J. Biomed. Sci. 2020, 27, 104. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, B.; Islam, S.S.; Zohora, U.S.; Ullah, M.A. Virus like particles—A recent advancement in vaccine development. Korean J. Microbiol. 2019, 55, 327–343. [Google Scholar]
- Qian, C.; Liu, X.; Xu, Q.; Wang, Z.; Chen, J.; Li, T.; Zheng, Q.; Yu, H.; Gu, Y.; Li, S.; et al. Recent Progress on the Versatility of Virus-Like Particles. Vaccines 2020, 8, 139. [Google Scholar] [CrossRef] [Green Version]
- Lokugamage, K.G.; Yoshikawa-Iwata, N.; Ito, N.; Watts, D.M.; Wyde, P.R.; Wang, N.; Newman, P.; Kent Tseng, C.T.; Peters, C.J.; Makino, S. Chimeric coronavirus like particles carrying severe acute respiratory syndrome coronavirus (SCoV) S protein protect mice against challenge with SCoV. Vaccine 2008, 26, 797–808. [Google Scholar] [CrossRef]
- Liu, Y.V.; Massare, M.J.; Barnard, D.L.; Kort, T.; Nathan, M.; Wang, L.; Smith, G. Chimeric severe acute respiratory syn-drome coronavirus (SARS-CoV) S glycoprotein and infuenza matrix 1 efficiently form virus-like particles (VLPs) that pro-tect mice against challenge with SARS-CoV. Vaccine 2011, 29, 6606–6613. [Google Scholar] [CrossRef] [PubMed]
- VBI VACCINES. VBI-2901: Pan-Coronavirus Vaccine Candidate Overview; VBI VACCINES: Cambridge, MA, USA, 2020. [Google Scholar]
- Murphy, J.; Vallières, F.; Bentall, R.P.; Shevlin, M.; McBride, O.; Hartman, T.K.; McKay, R.; Bennett, K.; Mason, L.; Gibson-Miller, J.; et al. Psychological characteristics associated with COVID-19 vaccine hesitancy and resistance in Ireland and the United Kingdom. Nat. Commun. 2021, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Pomares, T.D.; Buttenheim, A.M.; Amin, A.B.; Joyce, C.M.; Porter, R.M.; Bednarczyk, R.A.; Omer, S.B. Association of cogni-tive biases with human papillomavirus vaccine hesitancy: A cross-sectional study. Hum. Vaccines Immunother. 2020, 16, 1018–1023. [Google Scholar] [CrossRef]
- Wismans, A.; Thurik, R.; Baptista, R.; Dejardin, M.; Janssen, F.; Franken, I. Psychological characteristics and the mediating role of the 5C Model in explaining students’ COVID-19 vaccination intention. PLoS ONE 2021, 16, e0255382. [Google Scholar] [CrossRef]
- Sallam, M. COVID-19 Vaccine Hesitancy Worldwide: A Concise Systematic Review of Vaccine Acceptance Rates. Vaccines 2021, 9, 160. [Google Scholar] [CrossRef]
- Ebrahimi, O.V.; Johnson, M.S.; Ebling, S.; Amundsen, O.M.; Halsøy, O.; Hoffart, A.; Skjerdingstad, N.; Johnson, S.U. Risk, Trust, and Flawed Assumptions: Vaccine Hesitancy During the COVID-19 Pandemic. Front. Public Health 2021, 9, 700213. [Google Scholar] [CrossRef] [PubMed]
- Guidry, J.P.; Laestadius, L.I.; Vraga, E.K.; Miller, C.A.; Perrin, P.B.; Burton, C.W.; Ryan, M.; Fuemmeler, B.F.; Carlyle, K.E. Willingness to get the COVID-19 vaccine with and without emergency use authorization. Am. J. Infect. Control. 2020, 49, 137–142. [Google Scholar] [CrossRef]
- Solís Arce, J.S.; Warren, S.S.; Meriggi, N.F.; Scacco, A.; McMurry, N.; Voors, M.; Omer, S.B. COVID-19 vaccine ac-ceptance and hesitancy in low-and middle-income countries. Nat. Med. 2021, 27, 1385–1394. [Google Scholar] [CrossRef]
- Xiao, Q.; Liu, X.; Wang, R.; Mao, Y.; Chen, H.; Li, X.; Liu, X.; Dai, J.; Gao, J.; Fu, H.; et al. Predictors of Willingness to Receive the COVID-19 Vaccine after Emergency Use Authorization: The Role of Coping Appraisal. Vaccines 2021, 9, 967. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balkrishna, A.; Arya, V.; Rohela, A.; Kumar, A.; Verma, R.; Kumar, D.; Nepovimova, E.; Kuca, K.; Thakur, N.; Thakur, N.; et al. Nanotechnology Interventions in the Management of COVID-19: Prevention, Diagnosis and Virus-Like Particle Vaccines. Vaccines 2021, 9, 1129. https://doi.org/10.3390/vaccines9101129
Balkrishna A, Arya V, Rohela A, Kumar A, Verma R, Kumar D, Nepovimova E, Kuca K, Thakur N, Thakur N, et al. Nanotechnology Interventions in the Management of COVID-19: Prevention, Diagnosis and Virus-Like Particle Vaccines. Vaccines. 2021; 9(10):1129. https://doi.org/10.3390/vaccines9101129
Chicago/Turabian StyleBalkrishna, Acharya, Vedpriya Arya, Akansha Rohela, Ashwani Kumar, Rachna Verma, Dinesh Kumar, Eugenie Nepovimova, Kamil Kuca, Naveen Thakur, Nikesh Thakur, and et al. 2021. "Nanotechnology Interventions in the Management of COVID-19: Prevention, Diagnosis and Virus-Like Particle Vaccines" Vaccines 9, no. 10: 1129. https://doi.org/10.3390/vaccines9101129
APA StyleBalkrishna, A., Arya, V., Rohela, A., Kumar, A., Verma, R., Kumar, D., Nepovimova, E., Kuca, K., Thakur, N., Thakur, N., & Kumar, P. (2021). Nanotechnology Interventions in the Management of COVID-19: Prevention, Diagnosis and Virus-Like Particle Vaccines. Vaccines, 9(10), 1129. https://doi.org/10.3390/vaccines9101129









