Seroepidemiology of Measles, Mumps, Rubella and Varicella in Italian Female School Workers: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
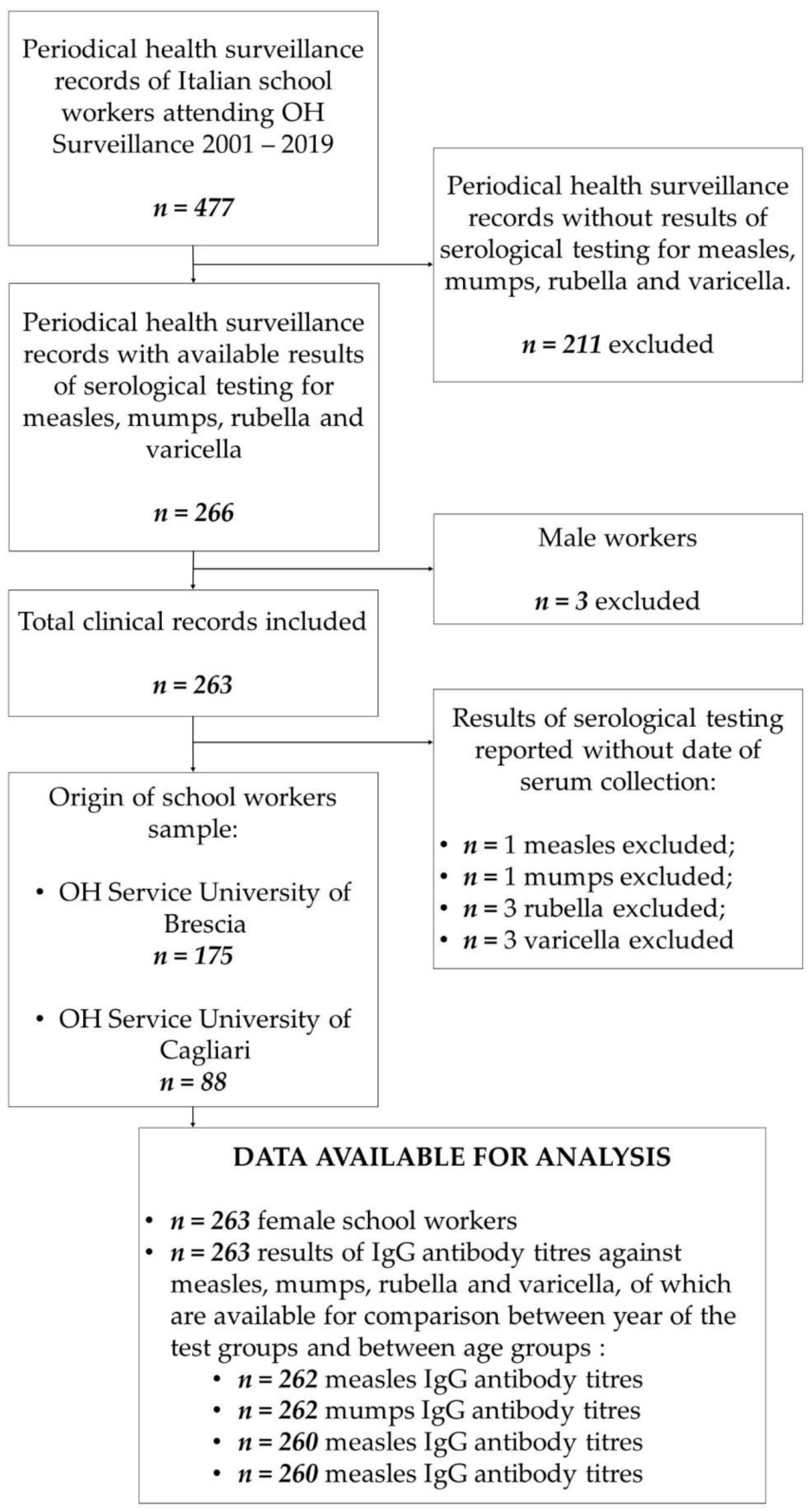
2.2. Study Population
2.3. Laboratory Methods
2.4. Statistical Analysis
3. Results
Distribution of the Seropositivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Centre for Diseases Prevention and Control. Monthly Measles and Rubella Monitoring Report—December 2019; ECDC: Stockholm, Sweden, 2019.
- McClure, C.C.; Cataldi, J.R.; O’Leary, S.T. Vaccine Hesitancy: Where We Are and Where We Are Going. Clin. Ther. 2017, 39, 1550–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubé, E.; Gagnon, D.; Nickels, E.; Jeram, S.; Schuster, M. Mapping vaccine hesitancy—Country-specific characteristics of a global phenomenon. Vaccine 2014, 32, 6649–6654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connor, P.; Jankovic, D.; Muscat, M.; Ben-Mamou, M.; Reef, S.; Papania, M.; Singh, S.; Kaloumenos, T.; Butler, R.; Datta, S. Measles and rubella elimination in the WHO Region for Europe: Progress and challenges. Clin. Microbiol. Infect. 2017, 23, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, C.; Odone, A.; Cella, P.; Iannazzo, S. Childhood vaccine coverage in Italy after the new law on mandatory immunization. Ann Ig 2018, 30, 1–10. [Google Scholar] [CrossRef]
- Filia, A.; Bella, A.; Del Manso, M.; Baggieri, M.; Marchi, A.; Bucci, P.; Magurano, F.; Nicoletti, L.; Rota, M.C. Morbillo & Rosolia News, N. 54 Luglio. Available online: http://www.epicentro.iss.it/problemi/morbillo/bollettino.asp (accessed on 8 August 2019).
- Filia, A.; Bella, A.; Del Manso, M.; Baggieri, M.; Magurano, F.; Rota, M.C. Ongoing outbreak with well over 4,000 measles cases in Italy from January to end August 2017—What is making elimination so difficult? Eurosurveillance 2017, 22, 30614. [Google Scholar] [CrossRef]
- Filia, A.; Bella, A.; Del Manso, M.; Rota, M.C. Morbillo & Rosolia News, Gennaio 2018; EpiCentro—Istituto Superiore di Sanità: Rome, Italy, 2018. [Google Scholar]
- Giambi, C.; Del Manso, M.; Bella, A.; Filia, A.; Rota, M. Rosolia Congenita E in Gravidanza News, Marzo 2018; Epicentro—Istituto Superiore di Sanità: Rome, Italy, 2018. [Google Scholar]
- European Centre for Disease Prevention and Control. Annual Epidemiological Report for 2017; Mumps; ECDC: Stockholm, Sweden, 2020.
- Maria Cristina, R. Varicella—Aspetti Epidemiologici. Available online: https://www.epicentro.iss.it/varicella/epidemiologia (accessed on 10 May 2019).
- Parlamento Italiano. Legge 31 luglio 2017, n. 119: Conversione in Legge, con Modificazioni, del Decreto-Legge 7 Giugno 2017, n. 73, Recante Disposizioni Urgenti in Materia di Prevenzione Vaccinale. Rome, Italy: Gazzetta Ufficiale. 2017. Available online: https://www.gazzettaufficiale.it/eli/id/2017/08/05/17A05515/sg (accessed on 14 October 2021).
- Haagsma, J.A.; Tariq, L.; Heederik, D.J.; Havelaar, A.H. Infectious disease risks associated with occupational exposure: A systematic review of the literature. Occup. Environ. Med. 2012, 69, 140–146. [Google Scholar] [CrossRef] [Green Version]
- Kutter, J.S.; Spronken, M.I.; Fraaij, P.L.; Fouchier, R.A.; Herfst, S. Transmission routes of respiratory viruses among humans. Curr. Opin. Virol. 2018, 28, 142–151. [Google Scholar] [CrossRef]
- International Labour Office. International Standard Classification of Occupations. Structure, Group Definitions and Correspondence Tables; ILO: Geneva, Switzerland, 2012; pp. 141–142. [Google Scholar]
- Luthy, K.E.; Houle, K.; Beckstrand, R.L.; Macintosh, J.; Lakin, R.G. Vaccination Perceptions and Barriers of School Employees: A Pilot Study. J. Sch. Nurs. 2013, 29, 284–293. [Google Scholar] [CrossRef]
- Macintosh, J.; Luthy, K.E.; Beckstrand, R.L.; Eden, L.M.; Orton, J. Vaccination perceptions of school employees in a rural school district. Vaccine 2014, 32, 4766–4771. [Google Scholar] [CrossRef] [Green Version]
- Sydnor, E.; Perl, T.M. Healthcare providers as sources of vaccine-preventable diseases. Vaccine 2014, 32, 4814–4822. [Google Scholar] [CrossRef]
- Koivisto, K.; Puhakka, L.; Lappalainen, M.; Blomqvist, S.; Saxén, H.; Nieminen, T. Immunity against vaccine-preventable diseases in Finnish pediatric healthcare workers in 2015. Vaccine 2017, 35, 1608–1614. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Global Measles and Rubella Strategic Plan: 2012–2020; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- Watson, J.C.; Hadler, S.C.; Dykewicz, C.A.; Reef, S.; Phillips, L. Measles, mumps, and rubella—Vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. Recomm. Rep. Morb. Mortal. Wkly. Rep. Recomm. Rep. 1998, 47, 38–39. [Google Scholar]
- Campagna, M.; Bacis, M.; Belotti, L.; Biggi, N.; Carrer, P.; Cologni, L.; Gattinis, V.; Lodi, V.; Magnavita, N.; Micheloni, G.; et al. Exanthemic diseases (measles, chickenpox, rubella and parotitis). Focus on screening and health surveillance of health workers: Results and perspectives of a multicenter working group. G. Ital. Med. Lav. Ergon. 2010, 32, 298–303. [Google Scholar]
- Porru, S.; Campagna, M.; Arici, C.; Carta, A.; Placidi, D.; Crotti, A.; Parrinello, G.; Alessio, L. Susceptibility to varicella-zoster, measles, rosacea and mumps among health care workers in a Northern Italy hospital. G. Ital. Med. Lav. Ergon. 2007, 29, 407–409. [Google Scholar]
- Copello, F.; Garbarino, S.; Messineo, A.; Campagna, M.; Durando, P. Collaborators Occupational Medicine and Hygiene: Applied research in Italy. J. Prev. Med. Hyg. 2015, 56, E102–E110. [Google Scholar]
- Harris, R.W.; Kehrer, A.F.; Isacson, P. Relationship of occupations to risk of clinical mumps in adults. Am. J. Epidemiol. 1969, 89, 264–270. [Google Scholar] [CrossRef]
- Macintosh, J.L.B.; Luthy, K.E.; Merrill, K.C.; Beckstrand, R.L.; Eden, L.M.; Wright, E.L. Vaccination Perceptions of Urban School Employees. J. Nurse Pract. 2016. [Google Scholar] [CrossRef]
- World Health Organization WHO. WHO Recommendations for Routine Immunization—Summary Tables. Available online: https://www.who.int/immunization/policy/immunization_tables/en/ (accessed on 1 July 2019).
- Ministero della Salute Piano Nazionale per L’eliminazione del Morbillo e Della Rosolia Congenita 2003–2007. Available online: https://www.gazzettaufficiale.it/atto/serie_generale/caricaArticolo?art.progressivo=0&art.idArticolo=1&art.versione=1&art.codiceRedazionale=03A13286&art.dataPubblicazioneGazzetta=2003-12-23&art.idGruppo=0&art.idSottoArticolo1=10&art.idSottoArticolo=1&art. (accessed on 8 August 2019).
- Istituto Superiore di Sanità Gravidanza fisiologica—Aggiornamento 2011. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_1436_allegato.pdf (accessed on 26 July 2019).
- Società Italiana di Igiene Vaccinarsi contro la Parotite—VaccinarSì. Available online: https://www.vaccinarsi.org/scienza-conoscenza/vantaggi-rischi-vaccinazioni/vantaggi/parotite (accessed on 26 July 2019).
- Società Italiana di Igiene Efficacia del Vaccino Contro la Varicella: Quindici Anni di Storia—VaccinarSì. Available online: https://www.vaccinarsi.org/notizie/2014/08/29/vaccino-varicella-quindici-anni-di-storia (accessed on 26 July 2019).
- Filia, A.; Brenna, A.; Panà, A.; Maggio Cavallaro, G.; Massari, M.; Ciofi Degli Atti, M.L. Health burden and economic impact of measles-related hospitalizations in Italy in 2002–2003. BMC Public Health 2007, 7, 169. [Google Scholar] [CrossRef] [Green Version]
- Pontrelli, G.; Bella, A.; Salmaso, S. Indagine Sugli Aspetti Organizzativi Della Campagna Stagionale di Vaccinazione Anti-Influenzale. Available online: https://www.epicentro.iss.it/influenza/pdf/inchiestaasl.pdf (accessed on 8 August 2019).
- Office of Occupational Statistics and Employment Projections Educational Attainment for Workers 25 Years and Older by Detailed Occupation: U.S. Bureau of Labor Statistics. Available online: https://www.bls.gov/emp/tables/educational-attainment.htm (accessed on 6 October 2021).
- Guzman-Holst, A.; DeAntonio, R.; Prado-Cohrs, D.; Juliao, P. Barriers to vaccination in Latin America: A systematic literature review. Vaccine 2020, 38, 470–481. [Google Scholar] [CrossRef]
- Larson, H.J.; Jarrett, C.; Eckersberger, E.; Smith, D.M.D.; Paterson, P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: A systematic review of published literature, 2007–2012. Vaccine 2014, 32, 2150–2159. [Google Scholar] [CrossRef]
- Barrabeig, I.; Rovira, A.; Muñoz, P.; Batalla, J.; Rius, C.; Sánchez, J.A.; Domínguez, À. MMR vaccine effectiveness in an outbreak that involved day-care and primary schools. Vaccine 2011, 29, 8024–8031. [Google Scholar] [CrossRef]
- D’Agaro, P.; Molin, G.D.; Gallo, T.; Rossi, T.; Santon, D.; Busetti, M.; Comar, M.; Campello, C. Epidemiological and molecular assessment of a measles outbreak in a highly vaccinated population of northeast Italy. Epidemiol. Infect. 2011, 139, 1727–1733. [Google Scholar] [CrossRef] [Green Version]
- Carrieri, V.; Madio, L.; Principe, F. Vaccine hesitancy and (fake) news: Quasi-experimental evidence from Italy. Health Econ. 2019, 28, 1377. [Google Scholar] [CrossRef]
- Riccó, M.; Vezzosi, L.; Gualerzi, G.; Balzarini, F.; Mezzoiuso, A.G.; Odone, A.; Signorelli, C. Measles vaccine in the school settings: A cross-sectional study about knowledge, personal beliefs, attitudes and practices of school teachers in northern Italy. Minerva Pediatr. 2018. [Google Scholar] [CrossRef]
- Tafuri, S.; Gallone, M.S.; Cappelli, M.G.; Gallone, M.F.; Larocca, A.M.V.; Germinario, C. A seroprevalence survey on varicella among adults in the vaccination era in Apulia (Italy). Vaccine 2014, 32, 6544–6547. [Google Scholar] [CrossRef]
- Gallone, M.S.; Germinario, C.; Larocca, A.; Tafuri, S. Long time immunogenicity of measles vaccine in the vaccination era: An open question. Hum. Vaccin. Immunother. 2017, 13, 117–119. [Google Scholar] [CrossRef]
- Anichini, G.; Gandolfo, C.; Fabrizi, S.; Miceli, G.B.; Terrosi, C.; Savellini, G.G.; Prathyumnan, S.; Orsi, D.; Battista, G.; Cusi, M.G. Seroprevalence to Measles Virus after Vaccination or Natural Infection in an Adult Population, in Italy. Vaccines 2020, 8, 66. [Google Scholar] [CrossRef] [Green Version]
- Smetana, J.; Chlibek, R.; Hanovcova, I.; Sosovickova, R.; Smetanova, L.; Gal, P.; Dite, P. Decreasing Seroprevalence of Measles Antibodies after Vaccination—Possible Gap in Measles Protection in Adults in the Czech Republic. PLoS ONE 2017, 12, 170257. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, F.P.; Mascipinto, S.; Stefanizzi, P.; De Nitto, S.; Germinario, C.; Tafuri, S. Long-term immunogenicity after measles vaccine vs. wild infection: An Italian retrospective cohort study. Hum. Vaccin. Immunother. 2021, 17, 2078–2084. [Google Scholar] [CrossRef]
- Amanna, I.J.; Carlson, N.E.; Slifka, M.K. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 2007, 357, 1903–1915. [Google Scholar] [CrossRef] [Green Version]
- OECD. Italy—Country Note—Education at a Glance 2018; OECD Indicators; OECD Publishing: Paris, France, 2018; pp. 1–11. [Google Scholar]
- Moss, W.J. Measles. Lancet 2017, 390, 2490–2502. [Google Scholar] [CrossRef]
- Hviid, A.; Rubin, S.; Mühlemann, K. Mumps. Lancet 2008, 371, 932–944. [Google Scholar] [CrossRef]
- Lambert, N.; Strebel, P.; Orenstein, W.; Icenogle, J.; Poland, G.A. Rubella. Lancet 2015, 385, 2297–2307. [Google Scholar] [CrossRef] [Green Version]
- Heininger, U.; Seward, J.F. Varicella. Lancet 2006, 368, 1365–1376. [Google Scholar] [CrossRef]
- Trevisan, A.; Frasson, C.; Morandin, M.; Beggio, M.; Bruno, A.; Davanzo, E.; Marco, L.D.; Simioni, L.; Amato, G. Immunity Against Infectious Diseases Predictive Value of Self-Reported History of Vaccination and Disease. Infect. Control Hosp. Epidemiol. 2007, 28, 564–569. [Google Scholar] [CrossRef]
- Almuneef, M.; Dillon, J.; Abbas, M.F.; Memish, Z. Varicella zoster virus immunity in multinational health care workers of a Saudi Arabian hospital. Am. J. Infect. Control 2003, 31, 375–381. [Google Scholar] [CrossRef]
- OECD. Education at a Glance 2019; OECD Indicators; OECD Publishing: Paris, France, 2019; ISBN 9789264803985. [Google Scholar] [CrossRef]
| Variable | N | (%) | Median Age at the Time of Antibody Determination (IQR) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Birthplace | ||||||||||||
| North Italy | 195 | (74.1) | ||||||||||
| Centre Italy | 5 | (1.9) | ||||||||||
| South Italy and Islands | 53 | (20.2) | ||||||||||
| Foreign Country | 10 | (3.8) | ||||||||||
| Occupation | ||||||||||||
| Teacher | 117 | (44.5) | ||||||||||
| Early childhood educator | 117 | (44.5) | ||||||||||
| Other | 29 | (11) | ||||||||||
| Disease | ||||||||||||
| Measles | 36.2 | (30.0–42.7) | ||||||||||
| Mumps | 36.2 | (29.8–42.7) | ||||||||||
| Rubella | 35.4 | (29.7–42.3) | ||||||||||
| Varicella | 36.2 | (29.9–43.0) | ||||||||||
| Measles | Mumps | Rubella | Varicella | |||||||||
| N | (%) | N | (%) | N | (%) | N | (%) | |||||
| Year | ||||||||||||
| Before 2015 | 145 | (55.1) | 145 | (55.1) | 146 | (55.5) | 144 | (54.8) | ||||
| After 2015 | 117 | (44.5) | 117 | (44.5) | 114 | (43.4) | 116 | (44.1) | ||||
| Age | ||||||||||||
| <30 | 63 | (24.0) | 66 | (25.1) | 66 | (25.1) | 64 | (24.3) | ||||
| 30–39 | 109 | (41.4) | 106 | (40.3) | 108 | (41.1) | 106 | (40.3) | ||||
| 40–49 | 65 | (24.7) | 66 | (25.1) | 63 | (24.0) | 64 | (24.3) | ||||
| ≥50 | 25 | (9.5) | 24 | (9.1) | 23 | (8.7) | 26 | (9.9) | ||||
| Variables | Seropositivity | |||||||
|---|---|---|---|---|---|---|---|---|
| Measles | Mumps | Rubella | Varicella | |||||
| % | (95% CI) | % | (95% CI) | % | (95% CI) | % | (95% CI) | |
| Total sample | 90.5 | (87.0–94.0) | 85.2 | (80.9–89.5) | 94.7 | (92.0–97.4) | 97.3 | (95.3–99.3) |
| Birthplace | ||||||||
| North Italy | 88.2 | (83.7–92.7) | 84.6 | (79.5–89.7) | 97.4 | (95.2–99.6) | 96.9 | (94.1–99.1) |
| Centre Italy | 100 | (100) | 100 | (100) | 60.0 | (17.1–100) | 80.0 | (44.9–100) |
| South Italy and Islands | 98.1 | (94.4–100) | 86.8 | (77.7–95.9) | 88.7 | (80.2–97.2) | 100 | (100) |
| Foreign Country | 90.0 | (71.4–100) | 80.0 | (55.2–100) | 90.0 | (71.4–100) | 100 | (100) |
| p value | 0.11 | 0.84 | 0.001 | 0.11 | ||||
| Occupation | ||||||||
| Teacher | 94.0 | (89.7–98.3) | 93.2 | (88.6–97.8) | 95.7 | (92.0–99.4) | 96.6 | (93.3–99.9) |
| Early childhood educator | 85.5 | (79.1–91.9) | 81.2 | (74.1–88.3) | 92.3 | (87.5–97.1) | 98.3 | (96.0–100) |
| Other occupation | 96.6 | (90.0–100) | 69.0 | (52.2–85.8) | 100 | (100) | 96.6 | (90.0–100) |
| p value | 0.55 | 0.001 | 0.27 | 0.63 | ||||
| Year of test | ||||||||
| Before 2015 | 95.2 | (91.7–98.7) | 82.8 | (76.7–88.9) | 97.9 | (95.6–100) | 97.9 | (95.6–100) |
| After 2015 | 84.6 | (78.1–91.1) | 88.0 | (82.1–93.9) | 90.4 | (85.0–95.8) | 96.6 | (93.3–99.9) |
| p value | 0.005 | 0.30 | 0.01 | 0.70 | ||||
| Age (years) | ||||||||
| <30 | 87.3 | (79.1–95.5) | 93.9 | (88.1–99.7) | 93.3 | (87.3–99.3) | 98.4 | (95.3–100) |
| 30–39 | 88.1 | (82.0–94.2) | 79.2 | (71.5–86.9) | 95.4 | (91.4–99.4) | 94.3 | (89.9–98.7) |
| 40–49 | 93.8 | (87.9–99.7) | 86.4 | (78.1–94.8) | 92.1 | (85.4–98.8) | 100 | (100) |
| ≥50 | 100 | (100) | 83.3 | (68.4–98.2) | 100 | (100) | 100 | (100) |
| p value | 0.18 | 0.07 | 0.56 | 0.14 | ||||
| Variables | Measles | Mumps | Rubella | Varicella | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | p Value | Odds Ratio (95% CI) | p Value | Odds Ratio (95% CI) | p Value | Odds Ratio (95% CI) | p Value | |||||
| Birthplace | ||||||||||||
| North Italy (Reference) | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Rest of Italy | 12.7 | (1.6–101.3) | 0.02 | 1.27 | (0.50—3.22) | 0.62 | 0.20 | (0.06–0.69) | 0.20 | 1.76 | (0.19–16.2) | 0.62 |
| Occupation | ||||||||||||
| Teacher (Reference) | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Early childhood educator | 0.31 | (0.11–0.86) | 0.02 | 0.34 | (0.14–0.80) | 0.01 | 0.38 | (0.11–1.36) | 0.14 | 2.47 | (0.44–14.07) | 0.31 |
| Other | 0.24 | (0.02–2.72) | 0.25 | 0.13 | (0.04–0.48) | 0.002 | N.C. * | N.C. * | N.C. * | 0.10 | (0.01–1.60) | 0.10 |
| Year of test | ||||||||||||
| Before 2015 (Reference) | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| After 2015 | 0.17 | (0.06–0.48) | 0.001 | 0.99 | (0.44–2.23) | 0.98 | 0.38 | (0.95–1.54) | 0.18 | 0.32 | (0.06–1.83) | 0.20 |
| Age (years) | ||||||||||||
| <40 (Reference) | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| ≥40 | 4.22 | (1.11–16.04) | 0.03 | 1.25 | (0.52–3.01) | 0.62 | 1.02 | (0.28–3.76) | 0.97 | N.C. * | N.C. * | N.C. * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frau, N.; Meloni, F.; Fostinelli, J.; Portas, L.; Portoghese, I.; Sala, E.; Pilia, I.; Lecca, L.I.; De Palma, G.; Campagna, M. Seroepidemiology of Measles, Mumps, Rubella and Varicella in Italian Female School Workers: A Cross-Sectional Study. Vaccines 2021, 9, 1191. https://doi.org/10.3390/vaccines9101191
Frau N, Meloni F, Fostinelli J, Portas L, Portoghese I, Sala E, Pilia I, Lecca LI, De Palma G, Campagna M. Seroepidemiology of Measles, Mumps, Rubella and Varicella in Italian Female School Workers: A Cross-Sectional Study. Vaccines. 2021; 9(10):1191. https://doi.org/10.3390/vaccines9101191
Chicago/Turabian StyleFrau, Nicola, Federico Meloni, Jacopo Fostinelli, Laura Portas, Igor Portoghese, Emma Sala, Ilaria Pilia, Luigi Isaia Lecca, Giuseppe De Palma, and Marcello Campagna. 2021. "Seroepidemiology of Measles, Mumps, Rubella and Varicella in Italian Female School Workers: A Cross-Sectional Study" Vaccines 9, no. 10: 1191. https://doi.org/10.3390/vaccines9101191
APA StyleFrau, N., Meloni, F., Fostinelli, J., Portas, L., Portoghese, I., Sala, E., Pilia, I., Lecca, L. I., De Palma, G., & Campagna, M. (2021). Seroepidemiology of Measles, Mumps, Rubella and Varicella in Italian Female School Workers: A Cross-Sectional Study. Vaccines, 9(10), 1191. https://doi.org/10.3390/vaccines9101191







