COVID-19 Vaccine Platforms: Challenges and Safety Contemplations
Abstract
:1. Introduction
2. Contemporary COVID-19 Vaccine Platforms and Allied Safety and Efficacy Concerns
2.1. Inactivated Vaccine
2.2. Live Attenuated Vaccine
2.3. Viral Vector Vaccine
2.4. Nucleic Acid (DNA and RNA)-Based Vaccine
2.5. Protein Subunit and Virus-Like Particles Vaccine
| Platform/Vaccine Type | No. | Vaccine Name | Number of Doses (Dosage) | Dosing Schedule | Route of Administration | Developer/Manufacturer | Construct and/or Targeted SARS-CoV-2 Protein | Current Stage of Clinical Trial (Recruitment Status) | Efficacy * | Current Approvals/Authorizations | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inactivated virus | 1 | CoronaVac | 2 doses (3 μg) | Day 0 + 14 | IM | Sinovac Research and Development Co., Ltd. | Whole inactivated SARS-CoV-2 with aluminum hydroxide adjuvant | Phase IV (Not yet recruiting) | Efficacy from clinical trials: Brazil: 50.7% against symptomatic disease ≥14 d after 2 doses. Turkey: 83.5% against symptomatic disease ≥14 d after 2 doses. Indonesia: 65.3% against symptomatic disease ≥14 d after 2 doses. Efficacy/effectiveness against variants: Chile (predominant circulation of P.1 and B.1.1.7.): 67% against symptomatic disease ≥28 d after 2 doses. Brazil (predominant circulation of P.2 and P.1 lineages): 50.7% and 36.8% against symptomatic disease ≥14 d after 2 doses, respectively. | WHO EUL Approved in 37 countries 1 | [26,36,37,38,109,110,111,112] |
| Inactivated virus | 2 | BBIBP-CorV | 2 doses (4 μg) | Day 0 + 21 | IM | Sinopharm + China National Biotec Group Co + Beijing Institute of Biological Products | Whole inactivated SARS-CoV-2 | Phase IV (Recruiting) | Efficacy from clinical trials in UAE, Bahrain, Egypt, and Jordan: 78.1% against symptomatic disease ≥14 d after 2 doses, and 79% against hospitalization. | WHO EUL Approved in 56 countries 2 | [26,34,39,41,110,113] |
| Inactivated virus | 3 | Inactivated SARS-CoV-2 vaccine (Vero cell) | 2–3 doses (5 μg) | Day 0 + 21 + 42 or 111 or 171 | IM | Sinopharm + China National Biotec Group Co + Wuhan Institute of Biological Products | Whole inactivated SARS-CoV-2 with aluminum hydroxide adjuvant | Phase III (Completed) | Efficacy from clinical trials in UAE, Bahrain, Egypt, and Jordan: 72.8% against symptomatic disease ≥14 d after 2 doses, and 79% against hospitalization. | WHO EUL (Approval pending) China | [26,40,110,114,115] |
| Inactivated virus | 4 | Inactivated SARS-CoV-2 vaccine (Vero cell) | 2 doses (50, 100, or 150 EU) | Day 0 + 14 | IM | Institute of Medical Biology + Chinese Academy of Medical Sciences | Whole inactivated SARS-CoV-2 with Al(OH)3 adjuvant | Phase III (Enrolling by invitation) | NR | Not yet approved in any country | [26,116,117] |
| Inactivated virus | 5 | QazCovid-in | 2 doses | Day 0 + 21 | IM | Research Institute for Biological Safety Problems, Rep of Kazakhstan | Whole inactivated SARS-CoV-2 | Phase III (Active, not recruiting) | Efficacy from clinical trials in the Republic of Kazakhstan: 96% | Republic of Kazakhstan | [26,118,119] |
| Inactivated virus | 6 | BBV152 (COVAXIN) | 2 doses (3 or 6 μg) | Day 0 + 14 | IM | Bharat Biotech International Limited | Whole inactivated SARS-CoV-2 with Algel-IMDG adjuvant | Phase III (Active, not recruiting) | Efficacy from clinical trials: 77.8% against symptomatic disease, 93.4% against severe disease, 63.6% against asymptomatic disease. Efficacy/effectiveness against variants: 65.2% against disease caused by Delta (B.617.2) variant. | WHO EUL (Approval pending) Approved in 9 countries 3 | [26,110,120,121,122,123] |
| Inactivated virus | 7 | Inactivated SARS-CoV-2 vaccine (Vero cell) | 2 doses | Day 0 + 28 | IM | Shenzhen Kangtai Biological Products Co., Ltd. | Whole inactivated SARS-CoV-2 | Phase III (Not yet recruiting) | NR | China | [26,124] |
| Inactivated virus | 8 | VLA2001 | 2 doses | Day 0 + 21 | IM | Valneva, National Institute for Health Research, United Kingdom | Whole inactivated SARS-CoV-2 with high S-protein density, in combination with two adjuvants, alum and CpG 1018 | Phase III (Not yet recruiting) | NR | Not yet approved in any country | [26,125] |
| Inactivated virus | 9 | ERUCOV-VAC (TURKOVAC) | 2 doses (3 μg) | Day 0 + 28 | IM | Erciyes University + Health Institutes of Turkey | Whole inactivated SARS-CoV-2 | Phase III (Recruiting) | NR | Not yet approved in any country | [26,126] |
| Inactivated virus | 10 | COVID-19 inactivated vaccine | 2 doses (5 μg) | Day 0 + 28 | IM | Shifa Pharmed Industrial Co | Whole inactivated SARS-CoV-2 | Phase II–III (Recruitment complete) | NR | Iran | [26,127] |
| Inactivated virus | 11 | FAKHRAVAC (MIVAC) | 2 doses (10 µg) | Day 0 + 14 | IM | Organization of Defensive Innovation and Research | Whole inactivated SARS-CoV-2 | Phase II (Recruiting) | NR | Not yet approved in any country | [26,128] |
| Inactivated virus | 12 | Inactivated (NDV-based) chimeric vaccine | 2 doses | Day 0 + 28 | IM | The Government Pharmaceutical Organization (GPO) + PATH + Dynavax | Whole inactivated NDV chimera stably expressing membrane-anchored SARS-CoV-2 S protein +/− CpG 1018 adjuvant | Phase I–II (NR) | NR | Not yet approved in any country | [26,129] |
| Inactivated virus | 13 | KD-414 | 2 doses | Day 0 + 28 | IM | KM Biologics Co., Ltd. | Whole inactivated SARS-CoV-2 | Phase I–II (Not Recruiting) | NR | Not yet approved in any country | [26,130] |
| Inactivated virus | 14 | Koçak-19 | 2 doses (4 or 6 µg) | Day 0 + 21 | IM | Kocak Farma, Turkey | Whole inactivated SARS-CoV-2 with adjuvant | Phase I (Recruiting) | NR | Not yet approved in any country | [26,131] |
| Inactivated virus | 15 | Adjuvanted inactivated vaccine | 2 doses (10 µg-3M or 20 µg-6M) | Day 0 + 20 | SC | The Scientific and Technological Research Council of Turkey (TÜBITAK) | Whole inactivated SARS-CoV-2 with CpG ODN adjuvant | Phase I (Recruiting) | NR | Not yet approved in any country | [26,132] |
| Inactivated virus | 16 | Live recombinant (rNDV) vector vaccine | 2 doses | Day 0 + 21 | IM or IN | Laboratorio Avi-Mex | Live recombinant NDV vector expressing SARS-CoV-2 S protein | Phase I (Recruiting) | NR | Not yet approved in any country | [26,133] |
| Live-attenuated virus | 1 | COVI-VAC | 1–2 doses | Day 0 or Day 0 + 28 | IN | Codagenix, Inc + Serum Institute of India | Whole SARS-CoV-2 with all viral proteins | Phase I (Active, not recruiting) | NR | Not yet approved in any country | [26,134] |
| Live-attenuated virus | 2 | MV-014-212 | 1 dose | Day 0 | IN | Meissa Vaccines, Inc. | RSV expressing SARS-CoV-2 S protein | Phase I (Recruiting) | NR | Not yet approved in any country | [26,55,135] |
| Viral vector (non-replicating) | 1 | ChAdO x 1 AZD1222 | 2 doses (standard dose: 5 × 1010 viral particles, low dose: 2.2 × 1010 viral particles) | Day 0 + 28 | IM | AstraZeneca + University of Oxford | Chimpanzee adenovirus-vectored vaccine (ChAdOx1) expressing S protein | Phase IV (Recruiting) | Efficacy from clinical trials in UK, Brazil, and South Africa: 66.7%–70.4% overall efficacy ≥14 d after 2 doses, 62.1% after 2 standard doses76.0% after single low dose within 20–90 d, 90.0% after one low dose and one standard dose. Real-world effectiveness: England: 60–75% after 1 dose. Scotland: 88% against hospitalization 28–34 d after 1 dose. U.S: 76% in adults, and 85% in elderly (≥65 y). Efficacy/effectiveness against variants: UK: 70.4% against Alpha (B.1.1.7) variant, 81.5% against non-B.1.1.7 lineages. South Africa: 10.4% against Beta (B.1.351) variant. England: 76.0% after 1 dose, 86.0% after 2 doses against Beta variant. 71.0% after 1 dose, 92.0% after 2 doses against Delta variant. Canada: 68% ≥ 14 d after dose 1 against symptomatic infection caused by Alpha variant. 48% ≥ 14 d after 1 dose against symptomatic infection caused by Beta or Gamma (P.1) variants. 67% ≥ 14 d after 1 dose against symptomatic infection caused by Delta variant. | WHO EUL Approved in 118 countries 4 and issued an Endorsed by ART CARPHA EU recommendation EMA approved | [67,72,73,74,93,110,136,137,138,139,140,141,142,143,144,145] |
| Viral vector (non-replicating) | 2 | Convidicea (Ad5-nCoV) | 1 dose (5 × 1010 viral particles per dose) | Day 0 | IM | CanSino Biological Inc. + Beijing Institute of Biotechnology | Recombinant replication-defective human type 5 adenovirus (Ad5) expressing S protein | Phase IV (Active, not recruiting) | Efficacy from clinical trials in Pakistan, Russia, Argentina, Mexico, and Chile: 68.8% and 65.7% against symptomatic disease ≥14 d and ≥28 d after vaccination, respectively. 95.5% and 91.0% against severe disease ≥14 d and ≥28 d after vaccination, respectively. | WHO EUL (Approval pending) Approved in 8 countries 5 | [26,110,146,147,148,149,150,151] |
| Viral vector (non-replicating) | 3 | Ad26.COV2.S | 1 dose (5 × 1010 viral particles per dose) | Day 0 | IM | Janssen Pharmaceutical | Recombinant replication-incompetent adenovirus serotype 26 (Ad26) vector encoding full-length and stabilized S protein | Phase IV (NR) | Efficacy from clinical trials in Argentina, Brazil, Chile, Colombia, Mexico, Peru, South Africa, and the U.S: 66.3-76.3% and 65.5-83.5% against moderate to severe/critical disease ≥14 d and ≥28d after vaccination, respectively. Real-world efficacy: U.S. and India: 76.7% against infection ≥14 d after vaccination. Efficacy/effectiveness against variants: South Africa (95% predominant B.1.351 variant): 52.0–73.1% and 64.0–81.7% against moderate to severe/critical disease ≥14 d and ≥28 d after vaccination, respectively. Brazil (69% predominant P.2 lineages): 66.2–68.1% and 81.9–87.6% against moderate to severe/critical disease ≥14 d and ≥28 d after vaccination, respectively. | FDA EUA WHO EUL Approved in 55 countries 6 Endorsed by ART EMA approved | [26,69,71,110,145,152,153] |
| Viral vector (non-replicating) | 4 | Gam-COVID-Vac (Sputnik V) | 2 doses (1 × 1011 viral particles per dose) | Day 0 + 21 (first: rAd26-S; second: rAd5-S) | IM | Gamaleya Research Institute + Health Ministry of the Russian Federation | Recombinant Ad26 and recombinant Ad5 encoding full-length S protein (rAd26-S and rAd5-S) | Phase III (Active, not recruiting) | Efficacy from clinical trials: 91.6% overall efficacy against symptomatic disease, 100% against moderate-severe disease, 73.1% after 1 dose, 91.1% after 2 doses. Efficacy/effectiveness against variants: 90% against Delta variant. | WHO EUL (Approval pending) Approved in 69 countries 7 | [26,110,154,155,156,157] |
| Viral vector (non-replicating) | 5 | GRAd-COV2 | 1–2 doses (1 × 1011 viral particles per dose) | Day 0 + 21 | IM | ReiThera + Leukocare + Univercells | Replication defective Simian Adenovirus (GRAd) encoding S protein | Phase II–III (Active, not recruiting) | NR | Not yet approved in any country | [26,158,159,160] |
| Viral vector (non-replicating) | 6 | LV-SMENP-DC | 1 dose (5 × 106 cells of LV-DC vaccine and 1 × 108 antigen-specific CTLs) | Day 0 | SC (LV-DC vaccine) and IV (antigen-specific CTLs) | Shenzhen Geno-Immune Medical Institute | Modified dendritic cells (DC) with lentivirus vectors (LV) expressing minigenes SMENP and immune-modulatory genes. Cytotoxic T-cells (CTLs) are activated by LV-DC, presenting specific viral antigens | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,161] |
| Viral vector (non-replicating) | 7 | hAd5-S-Fusion + N-ETSD vaccine | 1 dose (5 × 1010 IU/ dose SC, 1 × 1010 IU/ dose SL) | Day 0 | SC, oral, or SL | ImmunityBio, Inc. + NantKwest, Inc. | Human second-generation adenovirus 5 (hAd5) encoding S and N antigens | Phase I–II (Not yet recruiting) | NR | Not yet approved in any country | [26,162,163,164] |
| Viral vector (non-replicating) | 8 | AdCLD-CoV19 | 1 dose (2.5 × 1010, 5 × 1010, or 1 × 1011 virus particles per dose) | Day 0 | IM | Cellid Co., Ltd. | Replication-defective human adenovirus type 5/35 vector expressing S protein | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,165] |
| Viral vector (non-replicating) | 9 | COVIVAC | 2 doses (1 × 107 IU, 5 × 107 IU, or 1 × 108 IU per dose) | Day 0 + 28 | IM | Institute of Vaccines and Medical Biologicals, Vietnam | NDV expressing membrane-anchored pre-fusion-stabilized trimeric S protein +/− CpG 1018 adjuvant | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,166] |
| Viral vector (non-replicating) | 10 | MVA-SARS-2-ST | 2 doses (1 × 107 IU, or 1 × 108 IU per dose) | Day 0 + 28 | IM | Universitätsklinikum Hamburg-Eppendorf + German Center for Infection Research | MVA vector expressing stabilized S protein | Phase I–II (Not yet recruiting) | NR | Not yet approved in any country | [26,167] |
| Viral vector (non-replicating) | 11 | MVA-SARS-2-S | 2 doses (1 × 107 IU, or 1 × 108 IU per dose) | Day 0 + 28 | IM | University of Munich (Ludwig-Maximilians) | MVA vector expressing S protein | Phase I (Recruiting) | NR | Not yet approved in any country | [26,168] |
| Viral vector (non-replicating) | 12 | VXA-CoV2-1 | 1–2 doses (1 × 1010 IU, or 1 × 1011 IU per dose) | Day 0 or Day 0 + 28 | Oral | Vaxart | Non-replicating adenovirus vector expressing viral antigens and dsRNA adjuvant | Phase I (Active, not recruiting) | NR | Not yet approved in any country | [26,169,170] |
| Viral vector (non-replicating) | 13 | AdCOVID, | 1–2 doses | Day 0 + NR | IN | Altimmune, Inc. | Adenovirus expressing the RBD of S protein | Phase I (Recruiting) | NR | Not yet approved in any country | [26,171] |
| Viral vector (non-replicating) | 14 | COH04S1 (MVA-SARS-2-S) | 2 doses (1 × 107, 1 × 108, or 2.5 × 108 PFU per dose) | Day 0 + 28 | IM | City of Hope Medical Center + National Cancer Institute | Synthetic MVA carrying small pieces of SARS-CoV-2 DNA (the chemical form of genes) | Phase I (Recruiting) | NR | Not yet approved in any country | [26,172] |
| Viral vector (non-replicating) | 15 | ChAdV68-S ChAdV68-S-TCE (Homologous and heterologous prime-boost schedule) | 2–3 doses (5 × 1010 or 1 × 1011 viral particles of ChAdV68-S, 10 µg or 30 µg SEM) | Day 0 + 28, or Day 0 + 56, or Day 0 + 112, or Day 0 + 56 + 112 | IM | Gritstone Oncology | Chimpanzee Adenovirus serotype 68 (ChAd) and self-amplifying mRNA (SAM) vectors expressing either S protein alone, or S protein with additional T-cell epitopes (TCE) | Phase I (Recruiting) | NR | Not yet approved in any country | [26,173] |
| Viral vector (non-replicating) | 16 | SC-Ad6-1 | 1–2 doses | Day 0 or Day 0 + 21 | IM | Tetherex Pharmaceuticals Corporation | Adenovirus vector vaccine | Phase I (Not yet recruiting) | NR | Not yet approved in any country | [26,174] |
| Viral vector (non-replicating) | 17 | BBV154 | 1–2 doses (1 × 1010 viral particles per dose) | Day 0 or Day 0 + 28 | IN | Bharat Biotech International Limited | S protein | Phase I (Active, not recruiting) | NR | Not yet approved in any country | [26,175] |
| Viral vector (replicating) | 18 | DelNS1-2019-nCoV-RBD-OPT1 | 2 doses (1 × 107 EID50 and 1 × 107.7 EID50) | Day 0 + 28 | IN | University of Hong Kong, Xiamen University + Beijing Wantai Biological Pharmacy | Genetically engineered live attenuated influenza virus vector expressing the RBD of S protein | Phase II (Recruiting) | NR | Not yet approved in any country | [26,176,177] |
| Viral vector (replicating) | 19 | rVSV-SARS-CoV-2-S Vaccine | 2 doses (1 × 105, 1 × 106, 1 × 107, or 1 × 108 PFU/mL) | Day 0 + 28 | IM | Institute for Biological Research | cDNA vector encoding the sequence of the N, P, M, and L genes of the VSV genome, and SARS-CoV-2 S protein | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,178] |
| Viral vector (replicating) | 20 | AV-COVID-19 | 1 dose (0.1, 0.33, or 1.0 mg) | Day 0 | IM | Aivita Biomedical, Inc. + National Institute of Health Research and Development + Ministry of Health Republic of Indonesia | Autologous dendritic cells loaded with antigens from SARS-CoV-2 +/− GM-CSF | Phase I–II (Not yet recruiting) | NR | Not yet approved in any country | [26,179] |
| Viral vector (replicating) | 21 | Covid-19/aAPC vaccine | 3 doses | Day 0 + 14 + 28 | SC | Shenzhen Geno-Immune Medical Institute | Lentivirus vector system expressing viral minigenes to the artificial antigen-presenting cells (aAPCs) | Phase I (Recruiting) | NR | Not yet approved in any country | [26,180] |
| DNA based vaccine | 1 | nCov vaccine (ZyCoV-D) | 3 doses (1 or 2 mg) | Day 0 + 28 + 56 | ID | Zydus Cadila | S protein | Phase III (Not recruiting) | Efficacy from clinical trials in India: 66.6% | Not yet approved in any country | [26,81,181,182] |
| DNA based vaccine | 2 | INO-4800+ electroporation | 2 doses (1 mg) | Day 0 + 28 | ID | Inovio Pharmaceuticals + International Vaccine Institute + Advaccine Biopharmaceutical Co., Ltd. | S1 and S2 subunits of SARS-CoV-2 S protein | Phase II–III (Active, not recruiting) | NR | Not yet approved in any country | [26,183,184] |
| DNA based vaccine | 3 | AG0301-COVID19 | 2 doses (2 mg) | Day 0 + 14 | IM | AnGes + Takara Bio + Osaka University | S protein | Phase II–III (Active, not recruiting) | NR | Not yet approved in any country | [26,185] |
| DNA based vaccine | 4 | GX-19 | 2 doses | Day 0 + 28 | IM | Genexine Consortium | S protein | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,186] |
| DNA based vaccine | 5 | Covigenix VAX-001 | 2 doses | Day 0 + 14 | IM | Entos Pharmaceuticals Inc. | Full-length S protein | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,187] |
| DNA based vaccine | 6 | GLS-5310 | 2 doses (0.6 or 1.2 mg) | Day 0 + 56 or Day 0 + 84 | ID | GeneOne Life Science, Inc. | S protein and a second antigenic target of SARS-CoV-2 | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,188,189] |
| DNA based vaccine | 7 | COVID-eVax | 2 doses (0.5, 1, or 2 mg) | Day 0 + 28 | IM | Takis + Rottapharm Biotech | RBD of S protein | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,190] |
| DNA based vaccine | 8 | CORVax | 2 doses | Day 0 + 14 | ID | Providence Health and Services | S protein +/− the combination of electroporated IL-12p70 plasmid | Phase I (Active, not recruiting) | NR | Not yet approved in any country | [26,191] |
| DNA based vaccine | 9 | bacTRL | 1–2 doses | Day 0 or Day 0 + 28 | Oral | Symvivo Corporation | S protein | Phase I (Active, not recruiting) | NR | Not yet approved in any country | [26,192] |
| DNA based vaccine | 10 | COVIGEN (COVALIA) | 2 doses (0.8, 2, or 4 mg) | Day 0 + 28 | IM or ID | University of Sydney, Bionet Co., Ltd. | S protein | Phase I (Not yet recruiting) | NR | Not yet approved in any country | [26,193] |
| RNA vaccine | 1 | mRNA-1273 | 2 doses (100 μg) | Day 0 + 28 | IM | Moderna + National Institute of Allergy and Infectious Diseases (NIAID) | Full-length S protein with proline substitutions | Phase IV (Recruiting) | Efficacy from clinical trials in the U.S.: 92.1% against symptomatic disease ≥14 d after 1 dose, 94.1% ≥ 14 d after 2 doses, and 100% against severe disease. Real-world efficacy: U.S.: 80% ≥ 14 d after 1 dose and 90% ≥ 14 d after 2 doses. 83% ≥ 14 d after 1 dose and 82% after 2 doses. 88.7% against infection ≥ 36 d after 1 dose. Canada: 72% against infection after 1 dose and 94% after 2 doses. Efficacy/ effectiveness against variants: Qatar: 88.1% ≥ 14 d after 1 dose, 100% after 2 doses against Alpha variant. 61.3% ≥ 14 d after 1 dose, 96.4% after 2 doses against Betavariant. Canada: 83% ≥ 14 d after 1 dose and 92% ≥ 7 d after 2 doses against symptomatic infection caused by Alpha variant. 77% ≥ 14 d after 1 dose against symptomatic infection caused by Beta or Gammavariants. 72% ≥ 14 d after 1 dose against symptomatic infection caused by Delta variant. | FDA EUAWHO EUL Approved in 57 countries 9EMA approved | [26,73,88,92,94,110,137,145,194,195,196,197,198] |
| RNA vaccine | 2 | BNT162b2 (3 LNP-mRNAs), also known as “Comirnaty” | 2 doses (30 μg) | Day 0 + 21 | IM | Pfizer/BioNTech + Fosun Pharma | Full-length S protein with proline substitutions | Phase IV (Recruiting) | Efficacy from clinical trials: 52.4% after 1 dose and 94.6% ≥ 7 d after 2 doses in adults. Real-world efficacy: England: 60–70% against infection after 1 dose, 85–90% after 2 doses in elderly (≥80 y). 72% against infection ≥21 d after 1 dose, and 86% ≥ 7 d after 2 doses. 91% against infection 15–28 d after 1 dose. UK: 70% ≥ 21 d after 1 dose, 85% ≥ 7 d after 2 doses. Denmark: 17% ≥ 14 d after 1 dose, 64–90% ≥ 7 d after 2 doses. Scotland: 91% against hospitalization 28–34 d after 1 dose. U.S.: 80% ≥ 14 d after 1 dose, 93% ≥ 14 d after 2 doses. 88.7% against infection ≥ 36 d after 1 dose. Sweden: 42% against infection ≥ 14 d after 1 dose, 86% ≥ 7 d after 2 doses. Canada: 59% ≥ 14 d after 1 dose and 91% after 2 doses. Qatar: 39.4% against disease after 1 dose and 97.4% ≥ 14 d after 2 doses. Efficacy/ effectiveness against variants: England: 83.0% against hospitalization after 1 dose, 95.0% after 2 doses against Alpha variant. 94.0% against hospitalization after 1 dose, 96.0% after 2 doses against Deltavariant. Canada: 89% ≥ 7 d after 2 doses against symptomatic infection caused by Alpha variant. 60% ≥ 14 d after 1 dose and 84% ≥ 7 d against symptomatic infection caused by Beta or Gammavariants. 56% ≥ 14 d after 1 dose and 87% ≥ 7 d against symptomatic infection caused by Delta variant. Qatar: 29.5% after 1 dose and 89.5% ≥ 14 d after 2 doses against infection caused by Alpha variant. 16.9% after 1 dose and 75.0% after 2 doses against infection caused by Beta variant. | FDA EUA WHO EUL Approved in 93 countries 10 CARPHA EU recommendation EMA approved | [26,73,89,92,93,110,141,142,145,196,197,199,200,201,202,203,204,205,206,207,208,209] |
| RNA vaccine | 3 | CVnCoV (CureVac) | 2 doses (12 μg) | Day 0 + 28 | IM | CureVac AG | LNP-encapsulated mRNA vaccine encoding the full-length, pre-fusion stabilized S protein | Phase III (Active, not recruiting) | Efficacy from clinical trials conducted in 10 countries in Latin America and Europe: 47% against symptomatic disease across all age groups and 15 variants, 53% against any disease severity, 77% against moderate and severe disease. | WHO EUL (Pending approval) Not yet approved in any country | [26,110,210,211,212] |
| RNA vaccine | 4 | ARCoV or ARCoVax | 1 dose (15 μg) | Day 0 | IM | Academy of Military Science (AMS), Walvax Biotechnology and Suzhou Abogen Biosciences | LNP-encapsulated mRNA vaccine encoding the RBD of S protein | Phase III (Not yet recruiting) | NR | Not yet approved in any country | [26,213,214] |
| RNA vaccine | 5 | mRNA-1273.211 | 1 dose (50 μg) | Day 0 | IM | ModernaTX, Inc. | A multivalent booster candidate combining mRNA-1273 + mRNA-1273.351 | Phase II-III (Active, not recruiting) | NR | Not yet approved in any country | [26,215] |
| RNA vaccine | 6 | mRNA-1273.351 | 1–2 doses (20 or 50 μg) | Day 0, or Day 0 + 28, or Day 56 after 2nd dose of mRNA-1273 | IM | Moderna + NIAID | Full-length prefusion stabilized S protein of SARS-CoV-2 B.1.351 variant | Phase II (Active, not recruiting) | NR | Not yet approved in any country | [26,216,217,218] |
| RNA vaccine | 7 | ARCT-021 | 1–2 doses ± booster dose (5 or 7.5 μg) | Day 0, or Day 0 + 28, or Day 0 + 28 ± 208 (booster) | IM | Arcturus Therapeutics | S protein | Phase II (Two trials: one is recruiting, and the other is active, not recruiting) | NR | Not yet approved in any country | [26,219,220,221] |
| RNA vaccine | 8 | MRT5500 | 2 doses (15, 45, or 135 µg) | Day 0 + 21 | IM | Sanofi Pasteur and Translate Bio | S protein | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,222,223,224] |
| RNA vaccine | 9 | DS-5670a | 2 doses (10, 30, 60 or 100 µg) | Day 0 + 21 | IM | Daiichi Sankyo Co., Ltd. | NR | Phase I–II (Active, not recruiting) | NR | Not yet approved in any country | [26,225,226] |
| RNA vaccine | 10 | EXG-5003 | 1 dose | Day 0 | ID | Elixirgen Therapeutics, Inc | Temperature-sensitive ssRNA vaccine expressing the RBD of S protein | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,227] |
| RNA vaccine | 11 | LNP-nCoVsaRNA (COVAC1) | 2 doses (0.1–10.0 µg) | ND | IM | Imperial College London | S protein | Phase I (No longer recruiting) | NR | Not yet approved in any country | [26,228,229] |
| RNA vaccine | 12 | ChulaCov19 mRNA vaccine | 2 doses (10, 25, 50, or 100 µg) | Day 0 + 21 | IM | Chulalongkorn University | S protein | Phase I (Not yet recruiting) | NR | Not yet approved in any country | [26,230,231] |
| RNA vaccine | 13 | PTX-COVID19-B | 2 doses (16, 40, or 100 μg) | Day 0 + 28 | IM | Providence Therapeutics | Full-length membrane-anchored S protein | Phase I (Active, not recruiting) | NR | Not yet approved in any country | [26,232,233] |
| RNA vaccine | 14 | CoV2 SAM (LNP) | 2 doses (1.0 μg) | Day 0 + 30 | IM | GSK | S protein | Phase I (Active, not recruiting) | NR | Not yet approved in any country | [26,234] |
| RNA vaccine | 15 | HDT-301 | 2 doses (1, 5, or 25 μg) | Day 0 + 28 | IM | SENAI CIMATEC | Full-length S protein | Phase I (Not yet recruiting) | NR | Not yet approved in any country | [26,235] |
| RNA vaccine | 16 | mRNA-1283 | 1–2 doses (10, 30, or 100 μg) | Day 0 or Day 0 + 28 | IM | ModernaTX, Inc. | RBD and NTD of S protein | Phase I (Recruiting) | NR | Not yet approved in any country | [26,236,237] |
| RNA vaccine | 17 | SW-0123 | 2 doses | NR | IM | Shanghai East Hospital + Stemirna Therapeutics | NR | Phase I (Recruiting) | NR | Not yet approved in any country | [26,238,239] |
| RNA vaccine | 18 | LNP-nCOV saRNA-02 (COVAC-Uganda) | 2 doses (5.0 µg) | Day 0 + 28 | IM | MRC/UVRI and LSHTM Uganda Research Unit | S protein | Phase I (Not yet recruiting) | NR | Not yet approved in any country | [26,240] |
| Protein subunit | 1 | NVX-CoV2373 | 2 doses (5 µg) | Day 0 + 21 | IM | Novavax | S protein with Matrix-M adjuvant | Phase III (Recruiting) | Efficacy from clinical trials: UK: 89.7% against symptomatic disease ≥7 d after 2 doses. Real-world efficacy: U.S.: 100% against mild and severe disease. Efficacy/effectiveness against variants: UK: 86.2% against Alpha variant, 96.4% against non-B.1.1.7 variants. South Africa: 51.0% against Beta variant after 2 doses. 85.6% against symptomatic disease caused by Alpha variant. 60% against any disease severity in predominantly circulating Beta variant. U.S.: 93% against Alpha, Beta, and other VOCs/ VOIs. | WHO EUL (Approval pending) Not yet approved in any country | [26,102,103,110,241,242,243] |
| Protein subunit | 2 | ZF2001 (Recombinant SARS-CoV-2 vaccine) | 3 doses (25 µg) | Day 0 + 30 + 93 | IM | Anhui Zhifei Longcom Biopharmaceutical + Institute of Microbiology, Chinese Academy of Sciences | RBD-Dimer with alum adjuvant | Phase III (Recruiting) | NR | China (EUA), Uzbekistan | [26,244,245] |
| Protein subunit | 3 | VAT00008 | 2 doses | Day 0 + 21 | IM | Sanofi Pasteur + GSK | Monovalent and bivalent S protein with adjuvant | Phase III (Not yet recruiting) | NR | Not yet approved in any country | [26,246,247] |
| Protein subunit | 4 | FINLAY-FR-2 | 2 doses (25 μg) + booster dose (FINLAY-FR-1A, 50 μg)) | Day 0 + 28 Day 56 (booster dose) | IM | Instituto Finlay de Vacunas | FINLAY-FR-2: chemically conjugated RBD to tetanus toxoid plus adjuvant FINLAY-FR-1A: dimeric RBD + alum adjuvant | Phase III (Pending) | 62% | Not yet approved in any country | [26,248,249,250] |
| Protein subunit | 5 | Recombinant SARS-CoV-2 vaccine (Sf9 Cell) | 3 doses | Day 0 + 28 + 42 | IM | West China Hospital + Sichuan University | RBD with alum adjuvant | Phase III (Enrolling by invitation) | NR | Not yet approved in any country | [26,251] |
| Protein subunit | 6 | EpiVacCorona | 2 doses | Day 0 + 21 | IM | Federal Budgetary Research Institution State Research Center of Virology and Biotechnology | Peptide antigens of SARS-CoV-2 proteins with alum adjuvant | Phase III (Active, not recruiting) | Efficacy from clinical trials: 100% | Russia, Turkmenistan | [26,252,253] |
| Protein subunit | 7 | CIGB-66 | 3 doses (50 µg RBD + 0.3 mg aluminum hydroxide) | Day 0 + 14 + 28 or Day 0 + 28 + 56 | IM | Center for Genetic Engineering and Biotechnology (CIGB) | RBD with aluminum hydroxide adjuvant | Phase III (Pending) | Efficacy from clinical trials: 91.6% | Not yet approved in any country | [26,254,255] |
| Protein subunit | 8 | NanoCovax | 2 doses (25 µg) | Day 0 + 28 | IM | Nanogen Pharmaceutical Biotechnology | Recombinant S protein with alum adjuvant | Phase III (Recruiting) | NR | Not yet approved in any country | [26,256] |
| Protein subunit | 9 | SCB-2019 | 2 doses (30 μg) | Day 0 + 21 | IM | Clover Biopharmaceuticals Inc. + GSK + Dynavax | Trimeric S protein with CpG 1018 and Alum adjuvants | Phase II–III (Not yet recruiting) | NR | Not yet approved in any country | [26,257,258,259] |
| Protein subunit | 10 | UB-612 | 2 doses (100 µg) | Day 0 + 28 | IM | Vaxxinity, Inc. + Diagnósticos da América S/A (DASA) | RBD of S protein | Phase II–III (Not yet recruiting) | NR | Not yet approved in any country | [26,260] |
| Protein subunit | 11 | FINLAY-FR-1 | 2 doses (10 or 20 μg) | Day 0 + 28 | IM | Instituto Finlay de Vacunas | RBD with adjuvant | Phase II (Pending) | NR | Not yet approved in any country | [26,261] |
| Protein subunit | 12 | COVAX-19 | 2 doses (25 μg) | Day 0 + 21 | IM | Vaxine Pty Ltd. + CinnaGen Co. | Recombinant S protein with Advax-CpG adjuvant | Phase II (Recruiting) | NR | Not yet approved in any country | [26,262] |
| Protein subunit | 13 | MVC-COV1901 | 2 doses (5, 15, or 25 μg) | Day 0 + 28 | IM | Medigen Vaccine Biologics + Dynavax + NIAID | Recombinant S protein with CpG 1018 and alum adjuvants | Phase II (Active, not recruiting for adults, recruiting for elderly) | NR | Not yet approved in any country | [26,263,264,265] |
| Protein subunit | 14 | Razi Cov Pars | 3 doses | Day 0 + 21 (IM) + 51 (IN) | IM and IN | Razi Vaccine and Serum Research Institute | Recombinant S protein | Phase II (Complete) | NR | Not yet approved in any country | [26,266] |
| Protein subunit | 15 | V-01 | 2 doses (10 or 25 μg) | Day 0 + 21 | IM | Guangdong Provincial Center for Disease Control and Prevention/ Gaozhou Center for Disease Control and Prevention | Recombinant S protein | Phase II (Not yet recruiting) | NR | Not yet approved in any country | [26,267] |
| Protein subunit | 16 | CIGB-669 | 3 doses (50 µg RBD + 40 µg AgnHB) | Day 0 + 14 + 28 or Day 0 + 28 + 56 | IN | Center for Genetic Engineering and Biotechnology (CIGB) | Recombinant RBD with AgnHB | Phase I–II (Pending) | NR | Not yet approved in any country | [26,268] |
| Protein subunit | 17 | KBP-COVID-19 | 2 doses (15 μg in phase I, 45 μg in phase II) | Day 0 + 21 | IM | Kentucky Bioprocessing Inc. | RBD of S protein | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,269,270] |
| Protein subunit | 18 | BECOV2 | 2 doses | Day 0 + 28 | IM | Biological E. Limited | Recombinant RBD | Phase I–II (Closed) | NR | Not yet approved in any country | [26,271] |
| Protein subunit | 19 | S-268019 | 2 doses | Day 0 + 21 | IM | Shionogi | Recombinant S protein | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,272] |
| Protein subunit | 20 | AKS-452 | 1–2 doses (22.5, 45, or 90 µg) | NR | SC or IM | University Medical Center Groningen + Akston Biosciences Inc. | RBD-Fc fusion protein | Phase I–II (Enrolling by invitation) | NR | Not yet approved in any country | [26,273] |
| Protein subunit | 21 | COVAC-1 and COVAC-2 | 2 doses (25, 50, or 100 µg) | Day 0 + 28 | IM | University of Saskatchewan | S1 protein with SWE adjuvant | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,274] |
| Protein subunit | 22 | GBP510 | 2 doses (10, or 25 µg) | Day 0 + 28 | IM | SK Bioscience Co., Ltd. And CEPI | Recombinant RBD with AS03 aluminum hydroxide adjuvant | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,275] |
| Protein subunit | 23 | QazCoVac-P | 1–2 doses | Day 0 + 21 | IM | Research Institute for Biological Safety Problems | Phase I–II (Active, not recruiting) | NR | Not yet approved in any country | [26,276] | |
| Protein subunit | 24 | EuCorVac-19 | 2 doses | Day 0 + 21 | IM | POP Biotechnologies and EuBiologics Co., Ltd | Recombinant S protein with an adjuvant | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,277] |
| Protein subunit | 25 | Recombinant SARS-CoV-2 Vaccine (CHO cell) | 3 doses | Day 0 + 30 + 60 | IM | National Vaccine and Serum Institute, China | Recombinant SARS-CoV-2 | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,278] |
| Protein subunit | 26 | SARS-CoV-2 Sclamp vaccine | 2 doses (5, 15, or 45 μg) | Day 0 + 28 | IM | University of Queensland + Syneos Health + CEPI | Recombinant S protein with MF59 adjuvant | Phase I (Recruiting) | NR | Not yet approved in any country | [26,279,280,281] |
| Protein subunit | 27 | IMP CoVac-1 | 1 dose (500 µL) | Day 0 | SC | University Hospital Tuebingen | SARS-CoV-2 HLA-DR peptides | Phase I (Recruiting) | NR | Not yet approved in any country | [26,282] |
| Protein subunit | 28 | AdimrSC-2f | NR | NR | NR | Adimmune Corporation | Recombinant RBD with alum adjuvant | Phase I (Recruiting) | NR | Not yet approved in any country | [26,283] |
| Protein subunit | 29 | NBP2001 | 2 doses (30 or 50 μg) | Day 0 + 28 | IM | SK Bioscience Co., Ltd. | Recombinant RBD protein with alum adjuvant | Phase I (Active, not recruiting) | NR | Not yet approved in any country | [26,284] |
| Protein subunit | 30 | ReCOV | 2 doses (20 or 40 μg) | Day 0 + 21 | IM | Jiangsu Rec-Biotechnology | Recombinant two-component S and RBD protein | Phase I (Not yet recruiting) | NR | Not yet approved in any country | [26,285] |
| Protein subunit | 31 | Spike-Ferritin-Nanoparticle (SpFN) | 2–3 doses (25 or 50 μg) | Day 0 + 28 + 180 | IM | Walter Reed Army Institute of Research (WRAIR) | S proteins with a liposomal formulation QS21 (ALFQ) adjuvant | Phase I (Recruiting) | NR | Not yet approved in any country | [26,286,287,288] |
| Protein subunit | 32 | CoVepiT | 1–2 doses | Day 0 or Day 0 + 21 | SC | OSE Immunotherapeutics | Target 11 viral protein (S, M, N, and several non-structural proteins) | Phase I (Recruiting) | NR | Not yet approved in any country | [26,289] |
| Protein subunit | 33 | CoV2-OGEN1 | 1–2 doses (50, 100, or 200 μg) | Day 0 or Day 0 + 14 | Oral | VaxForm | Recombinant RBD protein | Phase I (Not yet recruiting) | NR | Not yet approved in any country | [26,290] |
| Virus-like particle | 1 | CoVLP | 2 doses (3.75 µg) | Day 0 + 21 | IM | Medicago Inc. | Trimeric S protein with AS03 adjuvant | Phase II–III (Recruiting) | NR | Not yet approved in any country | [26,291,292] |
| Virus-like particle | 2 | RBD SARS-CoV-2 HBsAg VLP | 2 doses (5 or 25 µg) | Day 0 + 28 | IM | Serum Institute of India + Accelagen Pty + SpyBiotech | RBD conjugated to the hepatitis B surface antigen | Phase I–II (Recruiting) | NR | Not yet approved in any country | [26,293] |
| Virus-like particle | 3 | VBI-2902a | 2 doses (5 or 10 μg) | Day 0 + 28 | IM | VBI Vaccines Inc. | Enveloped S glycoprotein with aluminum phosphate adjuvant | Phase I–II (Active, not recruiting) | NR | Not yet approved in any country | [26,294] |
| Virus-like particle | 4 | SARS-CoV-2 VLP Vaccine | 2 doses | NR | SC | The Scientific and Technological Research Council of Turkey | SARS-CoV-2 VLP adjuvanted with alum and CpG ODN-K3 | Phase I (Recruiting) | NR | Not yet approved in any country | [26,295] |
| Virus-like particle | 5 | ABNCoV2 | 2 doses | Day 0 + 28 | IM | Radboud University | capsid virus-like particle (cVLP) +/− adjuvant MF59 | Phase I (Recruiting) | NR | Not yet approved in any country | [26,296] |
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 1 July 2020).
- Mariano, G.; Farthing, R.J.; Lale-Farjat, S.L.M.; Bergeron, J.R.C. Structural Characterization of SARS-CoV-2: Where We Are, and Where We Need to Be. Front. Mol. Biosci. 2020, 7, 605236. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, A.A.T.; Fatima, K.; Mohammad, T.; Fatima, U.; Singh, I.K.; Singh, A.; Atif, S.M.; Hariprasad, G.; Hasan, G.M.; Hassan, M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165878. [Google Scholar] [CrossRef] [PubMed]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Tortorici, M.A.; Veesler, D. Structural insights into coronavirus entry. Adv. Virus Res. 2019, 105, 93–116. [Google Scholar] [CrossRef]
- Hu, T.; Liu, Y.; Zhao, M.; Zhuang, Q.; Xu, L.; He, Q. A comparison of COVID-19, SARS and MERS. PeerJ 2020, 8, e9725. [Google Scholar] [CrossRef]
- Wu, Z.; Harrich, D.; Li, Z.; Hu, D.; Li, D. The unique features of SARS-CoV-2 transmission: Comparison with SARS-CoV, MERS-CoV and 2009 H1N1 pandemic influenza virus. Rev. Med. Virol. 2021, 31, e2171. [Google Scholar] [CrossRef]
- Petersen, E.; Koopmans, M.; Go, U.; Hamer, D.H.; Petrosillo, N.; Castelli, F.; Storgaard, M.; Al Khalili, S.; Simonsen, L. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect. Dis. 2020, 20, e238–e244. [Google Scholar] [CrossRef]
- Cyprian, F.; Sohail, M.U.; Abdelhafez, I.; Salman, S.; Attique, Z.; Kamareddine, L.; Al-Asmakh, M. SARS-CoV-2 and immune-microbiome interactions: Lessons from respiratory viral infections. Int. J. Infect. Dis. 2021, 105, 540–550. [Google Scholar] [CrossRef]
- Güner, R.; Hasanoğlu, I.; Aktaş, F. COVID-19: Prevention and control measures in community. Turk. J. Med. Sci. 2020, 50, 571–577. [Google Scholar] [CrossRef]
- Fontanet, A.; Cauchemez, S. COVID-19 herd immunity: Where are we? Nat. Rev. Immunol. 2020, 20, 583–584. [Google Scholar] [CrossRef]
- Spellberg, B.; Nielsen, T.B.; Casadevall, A. Antibodies, Immunity, and COVID-19. JAMA Intern. Med. 2021, 181, 460–462. [Google Scholar] [CrossRef]
- Sharma, O.; Sultan, A.A.; Ding, H.; Triggle, C.R. A Review of the Progress and Challenges of Developing a Vaccine for COVID-19. Front. Immunol. 2020, 11, 585354. [Google Scholar] [CrossRef]
- Jung, F.; Krieger, V.; Hufert, F.; Küpper, J.-H. Herd immunity or suppression strategy to combat COVID-19. Clin. Hemorheol. Microcirc. 2020, 75, 13–17. [Google Scholar] [CrossRef]
- Randolph, H.E.; Barreiro, L.B. Herd Immunity: Understanding COVID-19. Immunity 2020, 52, 737–741. [Google Scholar] [CrossRef]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef]
- National Institutes of Health. Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV). Available online: https://www.nih.gov/research-training/medical-research-initiatives/activ#:~:text=On%20April%2017%2C%202020%20the,most%20promising%20treatments%20and%20vaccines (accessed on 17 April 2020).
- Forni, G.; Mantovani, A.; Forni, G.; Mantovani, A.; Moretta, L.; Rappuoli, R.; Rezza, G.; Bagnasco, A.; Barsacchi, G.; Bussolati, G.; et al. COVID-19 vaccines: Where we stand and challenges ahead. Cell Death Differ. 2021, 28, 626–639. [Google Scholar] [CrossRef]
- Martin, J.E.; Louder, M.K.; Holman, L.A.; Gordon, I.J.; Enama, M.E.; Larkin, B.D.; Andrews, C.A.; Vogel, L.; Koup, R.A.; Roederer, M.; et al. A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a Phase I clinical trial. Vaccine 2008, 26, 6338–6343. [Google Scholar] [CrossRef]
- Lin, J.T.; Zhang, J.S.; Su, N.; Xu, J.G.; Wang, N.; Chen, J.T.; Chen, X.; Liu, Y.X.; Gao, H.; Jia, Y.P.; et al. Safety and immunogenicity from a phase I trial of inactivated severe acute respiratory syndrome coronavirus vaccine. Antivir. Ther. 2007, 12, 1107–1113. [Google Scholar]
- Li, Y.-D.; Chi, W.-Y.; Su, J.-H.; Ferrall, L.; Hung, C.-F.; Wu, T.C. Coronavirus vaccine development: From SARS and MERS to COVID-19. J. Biomed. Sci. 2020, 27, 104. [Google Scholar] [CrossRef]
- Wang, J.; Peng, Y.; Xu, H.; Cui, Z.; Williams, R.O., 3rd. The COVID-19 Vaccine Race: Challenges and Opportunities in Vaccine Formulation. AAPS PharmSciTech 2020, 21, 225. [Google Scholar] [CrossRef]
- Lurie, N.; Saville, M.; Hatchett, R.; Halton, J. Developing Covid-19 Vaccines at Pandemic Speed. N. Engl. J. Med. 2020, 382, 1969–1973. [Google Scholar] [CrossRef]
- Clem, A.S. Fundamentals of vaccine immunology. J. Glob. Infect. Dis. 2011, 3, 73–78. [Google Scholar] [CrossRef]
- Poland, G.A.; Ovsyannikova, I.G.; Crooke, S.N.; Kennedy, R.B. SARS-CoV-2 Vaccine Development: Current Status. Mayo Clin. Proc. 2020, 95, 2172–2188. [Google Scholar] [CrossRef]
- WHO. Draft Landscape of COVID-19 Candidate Vaccines. 2020. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 29 June 2021).
- Murdin, A.D.; Barreto, L.; Plotkin, S. Inactivated poliovirus vaccine: Past and present experience. Vaccine 1996, 14, 735–746. [Google Scholar] [CrossRef]
- Vellozzi, C.; Burwen, D.R.; Dobardzic, A.; Ball, R.; Walton, K.; Haber, P. Safety of trivalent inactivated influenza vaccines in adults: Background for pandemic influenza vaccine safety monitoring. Vaccine 2009, 27, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Q.; Bao, L.; Mao, H.; Wang, L.; Xu, K.; Yang, M.; Li, Y.; Zhu, L.; Wang, N.; Lv, Z.; et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020, 369, 77–81. [Google Scholar] [CrossRef]
- Briggs, D.J.; Nagarajan, T.; Rupprecht, C.E. Chapter 13—Rabies Vaccines. In Rabies, 3rd ed.; Jackson, A.C., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 497–526. [Google Scholar]
- André, F.; Van Damme, P.; Safary, A.; Banatvala, J. Inactivated hepatitis A vaccine: Immunogenicity, efficacy, safety and review of official recommendations for use. Expert Rev. Vaccines 2002, 1, 9–23. [Google Scholar] [CrossRef]
- Graham, R.L.; Donaldson, E.F.; Baric, R.S. A decade after SARS: Strategies for controlling emerging coronaviruses. Nat. Rev. Microbiol. 2013, 11, 836–848. [Google Scholar] [CrossRef] [Green Version]
- Sanders, B.; Koldijk, M.; Schuitemaker, H. Inactivated Viral Vaccines. In Vaccine Analysis: Strategies, Principles, and Control; Springer: Berlin/Heidelberg, Germany, 2014; pp. 45–80. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Huang, B.; Deng, W.; Quan, Y.; Wang, W.; Xu, W.; Zhao, Y.; Li, N.; Zhang, J.; et al. Development of an Inactivated Vaccine Candidate, BBIBP-CorV, with Potent Protection against SARS-CoV-2. Cell 2020, 182, 713–721.e9. [Google Scholar] [CrossRef]
- Burrell, C.J.; Howard, C.R.; Murphy, F.A. Chapter 11—Vaccines and Vaccination. In Fenner and White’s Medical Virology, 5th ed.; Academic Press: London, UK, 2017; pp. 155–167. [Google Scholar]
- NIH. A Study to Assess the Safety and Immunogenicity of the Coronavac Vaccine Against COVID-19; NIH: Bethseda, MD, USA, 2021. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04756830?term=NCT04756830&draw=2&rank=1 (accessed on 18 February 2021).
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef]
- Palacios, R.; Batista, A.P.; Albuquerque, C.S.N.; Patiño, E.G.; Santos, J.D.P.; Conde, M.T.R.P.; Piorelli, R.D.; Júnior, L.C.P.; Raboni, S.M.; Ramos, F.; et al. Efficacy and Safety of a COVID-19 Inactivated Vaccine in Healthcare Professionals in Brazil: The PROFISCOV Study; SSRN: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef]
- Al Kaabi, N.; Zhang, Y.; Xia, S.; Yang, Y.; Al Qahtani, M.M.; Abdulrazzaq, N.; Al Nusair, M.; Hassany, M.; Jawad, J.S.; Abdalla, J.; et al. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults: A Randomized Clinical Trial. JAMA 2021, 326, 35–45. [Google Scholar] [CrossRef] [PubMed]
- NIH. A Immuno-bridging and Immunization Schedules Study of COVID-19 Vaccine (Vero Cell), Inactivated (COVID-19). 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04863638 (accessed on 29 June 2021).
- Nathanson, N.; Langmuir, A.D. The cutter incident. poliomyelitis following formaldehyde- inactivated poliovirus vaccination in the united states during the spring of 1955. II. Relationship of poliomyelitis to cutter vaccine. Am. J. Hyg. 1963, 78, 29–60. [Google Scholar] [CrossRef] [PubMed]
- Roper, R.L.; Rehm, K.E. SARS vaccines: Where are we? Expert Rev. Vaccines 2009, 8, 887–898. [Google Scholar] [CrossRef]
- Agrawal, A.S.; Tao, X.; Algaissi, A.; Garron, T.; Narayanan, K.; Peng, B.H.; Couch, R.B.; Tseng, C.T. Immunization with inactivated Middle East Respiratory Syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Hum. Vaccines Immunother. 2016, 12, 2351–2356. [Google Scholar] [CrossRef]
- Bolles, M.; Deming, D.; Long, K.; Agnihothram, S.; Whitmore, A.; Ferris, M.; Funkhouser, W.; Gralinski, L.; Totura, A.; Heise, M.; et al. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J. Virol. 2011, 85, 12201–12215. [Google Scholar] [CrossRef] [Green Version]
- Tseng, C.-T.; Sbrana, E.; Iwata-Yoshikawa, N.; Newman, P.C.; Garron, T.; Atmar, R.L.; Peters, C.J.; Couch, R.B. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS ONE 2012, 7, e35421. [Google Scholar] [CrossRef]
- Castilow, E.M.; Olson, M.R.; Varga, S.M. Understanding respiratory syncytial virus (RSV) vaccine-enhanced disease. Immunol. Res. 2007, 39, 225–239. [Google Scholar] [CrossRef]
- Kapikian, A.Z.; Mitchell, R.H.; Chanock, R.M.; Shvedoff, R.A.; Stewart, C.E. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am. J. Epidemiol. 1969, 89, 405–421. [Google Scholar] [CrossRef]
- Murphy, B.R.; Walsh, E.E. Formalin-inactivated respiratory syncytial virus vaccine induces antibodies to the fusion glycoprotein that are deficient in fusion-inhibiting activity. J. Clin. Microbiol. 1988, 26, 1595–1597. [Google Scholar] [CrossRef] [Green Version]
- Vignuzzi, M.; Wendt, E.; Andino, R. Engineering attenuated virus vaccines by controlling replication fidelity. Nat. Med. 2008, 14, 154–161. [Google Scholar] [CrossRef]
- Mak, T.W.; Saunders, M.E. 23—Vaccines and Clinical Immunization. In The Immune Response; Mak, T.W., Saunders, M.E., Eds.; Academic Press: Burlington, NJ, USA, 2006; pp. 695–749. [Google Scholar]
- Tiboni, M.; Casettari, L.; Illum, L. Nasal vaccination against SARS-CoV-2: Synergistic or alternative to intramuscular vaccines? Int. J. Pharm. 2021, 603, 120686. [Google Scholar] [CrossRef]
- Groenke, N.; Trimpert, J.; Merz, S.; Conradie, A.M.; Wyler, E.; Zhang, H.; Hazapis, O.-G.; Rausch, S.; Landthaler, M.; Osterrieder, N.; et al. Mechanism of Virus Attenuation by Codon Pair Deoptimization. Cell Rep. 2020, 31, 107586. [Google Scholar] [CrossRef]
- Coleman, J.R.; Papamichail, D.; Skiena, S.; Futcher, B.; Wimmer, E.; Mueller, S. Virus Attenuation by Genome-Scale Changes in Codon Pair Bias. Science 2008, 320, 1784–1787. [Google Scholar] [CrossRef] [Green Version]
- News, G.E.B. Meissa Vaccines—MV-014-212. Available online: https://www.genengnews.com/covid-19-candidates/meissa-vaccines-mv-014-212/ (accessed on 30 June 2021).
- Mueller, S.; Stauft, C.B.; Kalkeri, R.; Koidei, F.; Kushnir, A.; Tasker, S.; Coleman, J.R. A codon-pair deoptimized live-attenuated vaccine against respiratory syncytial virus is immunogenic and efficacious in non-human primates. Vaccine 2020, 38, 2943–2948. [Google Scholar] [CrossRef]
- Dong, Y.; Dai, T.; Wei, Y.; Zhang, L.; Zheng, M.; Zhou, F. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduct. Target. Ther. 2020, 5, 237. [Google Scholar] [CrossRef]
- Yadav, D.K.; Yadav, N.; Khurana, S.M.P. Chapter 26—Vaccines: Present Status and Applications. In Animal Biotechnology; Verma, A.S., Singh, A., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 491–508. [Google Scholar]
- WHO. MODULE 2 Types of Vaccine and Adverse Reactions. Available online: https://vaccine-safety-training.org/live-attenuated-vaccines.html (accessed on 14 February 2021).
- Levin, M.J.; Song, L.-Y.; Fenton, T.; Nachman, S.; Patterson, J.; Walker, R.; Kemble, G.; Allende, M.; Hultquist, M.; Yi, T.; et al. Shedding of live vaccine virus, comparative safety, and influenza-specific antibody responses after administration of live attenuated and inactivated trivalent influenza vaccines to HIV-infected children. Vaccine 2008, 26, 4210–4217. [Google Scholar] [CrossRef] [Green Version]
- Dudek, T.; Knipe, D.M. Replication-defective viruses as vaccines and vaccine vectors. Virology 2006, 344, 230–239. [Google Scholar] [CrossRef] [Green Version]
- Robert-Guroff, M. Replicating and non-replicating viral vectors for vaccine development. Curr. Opin. Biotechnol. 2007, 18, 546–556. [Google Scholar] [CrossRef]
- Henao-Restrepo, A.M.; Camacho, A.; Longini, I.M.; Watson, C.H.; Edmunds, W.J.; Egger, M.; Carroll, M.W.; Dean, N.E.; Diatta, I.; Doumbia, M.; et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: Final results from the Guinea ring vaccination, open-label, cluster-randomised trial. Lancet 2017, 389, 505–518. [Google Scholar] [CrossRef] [Green Version]
- Ura, T.; Okuda, K.; Shimada, M. Developments in Viral Vector-Based Vaccines. Vaccines 2014, 2, 624–641. [Google Scholar] [CrossRef] [Green Version]
- Van Riel, D.; de Wit, E. Next-generation vaccine platforms for COVID-19. Nat. Mater. 2020, 19, 810–812. [Google Scholar] [CrossRef]
- Gao, W.; Tamin, A.; Soloff, A.; D’Aiuto, L.; Nwanegbo, E.; Robbins, P.D.; Bellini, W.J.; Barratt-Boyes, S.; Gambotto, A. Effects of a SARS-associated coronavirus vaccine in monkeys. Lancet 2003, 362, 1895–1896. [Google Scholar] [CrossRef] [Green Version]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Zhu, F.C.; Li, Y.H.; Guan, X.H.; Hou, L.H.; Wang, W.J.; Li, J.X.; Wu, S.P.; Wang, B.S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Jones, I.; Roy, P. Sputnik V COVID-19 vaccine candidate appears safe and effective. Lancet 2021, 397, 642–643. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Janssen COVID-19 Vaccine. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/janssen-covid-19-vaccine (accessed on 30 June 2021).
- Madhi, S.A.; Baillie, V.; Cutland, C.L.; Voysey, M.; Koen, A.L.; Fairlie, L.; Padayachee, S.D.; Dheda, K.; Barnabas, S.L.; Bhorat, Q.E.; et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021, 384, 1885–1898. [Google Scholar] [CrossRef]
- Nasreen, S.; He, S.; Chung, H.; Brown, K.A.; Gubbay, J.B.; Buchan, S.A.; Wilson, S.E.; Sundaram, M.E.; Fell, D.B.; Chen, B.; et al. Effectiveness of COVID-19 vaccines against variants of concern, Canada. medRxiv 2021. [Google Scholar] [CrossRef]
- Emary, K.R.W.; Golubchik, T.; Aley, P.K.; Ariani, C.V.; Angus, B.; Bibi, S.; Blane, B.; Bonsall, D.; Cicconi, P.; Charlton, S.; et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): An exploratory analysis of a randomised controlled trial. Lancet 2021, 397, 1351–1362. [Google Scholar] [CrossRef]
- Barouch, D.H.; Kik, S.V.; Weverling, G.J.; Dilan, R.; King, S.L.; Maxfield, L.F.; Clark, S.; Ng’ang’a, D.; Brandariz, K.L.; Abbink, P.; et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine 2011, 29, 5203–5209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatulin, A.I.; Dzharullaeva, A.S.; Tukhvatulina, N.M.; Shcheblyakov, D.V.; Shmarov, M.M.; Tokarskaya, E.A.; Simakova, Y.V.; Egorova, D.A.; et al. Safety and immunogenicity of GamEvac-Combi, a heterologous VSV- and Ad5-vectored Ebola vaccine: An open phase I/II trial in healthy adults in Russia. Hum. Vaccines Immunother. 2017, 13, 613–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Restifo, N.P.; Ying, H.; Hwang, L.; Leitner, W.W. The promise of nucleic acid vaccines. Gene Ther. 2000, 7, 89–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Kream, R.M.; Stefano, G.B. An Evidence Based Perspective on mRNA-SARS-CoV-2 Vaccine Development. Med. Sci. Monit. 2020, 26, e924700. [Google Scholar] [CrossRef] [Green Version]
- Reichmuth, A.M.; Oberli, M.A.; Jaklenec, A.; Langer, R.; Blankschtein, D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016, 7, 319–334. [Google Scholar] [CrossRef] [Green Version]
- Dey, A.; Chozhavel Rajanathan, T.M.; Chandra, H.; Pericherla, H.P.R.; Kumar, S.; Choonia, H.S.; Bajpai, M.; Singh, A.K.; Sinha, A.; Saini, G.; et al. Immunogenic Potential of DNA Vaccine candidate, ZyCoV-D against SARS-CoV-2 in Animal Models. bioRxiv 2021, 30, 4108–4116. [Google Scholar] [CrossRef]
- Hobernik, D.; Bros, M. DNA Vaccines-How Far From Clinical Use? Int. J. Mol. Sci. 2018, 19, 3605. [Google Scholar] [CrossRef] [Green Version]
- Wolff, J.A.; Ludtke, J.J.; Acsadi, G.; Williams, P.; Jani, A. Long-term persistence of plasmid DNA and foreign gone expression in mouse muscle. Hum. Mol. Genet. 1992, 1, 363–369. [Google Scholar] [CrossRef]
- Wang, Z.; Troilo, P.; Wang, X.; Griffiths, T.; Pacchione, S.; Barnum, A.; Harper, L.; Pauley, C.; Niu, Z.; Denisova, L. Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther. 2004, 11, 711–721. [Google Scholar] [CrossRef]
- Manam, S.; Ledwith, B.J.; Barnum, A.B.; Troilo, P.J.; Pauley, C.J.; Harper, L.B.; Griffiths II, T.G.; Niu, Z.; Denisova, L.; Follmer, T.T. Plasmid DNA vaccines: Tissue distribution and effects of DNA sequence, adjuvants and delivery method on integration into host DNA. Intervirology 2000, 43, 273–281. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Pfizer-BioNTech COVID-19 Vaccine. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine (accessed on 30 June 2021).
- U.S. Food and Drug Administration. Moderna COVID-19 Vaccine. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine (accessed on 30 June 2021).
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2020, 384, 403–416. [Google Scholar] [CrossRef]
- Skowronski, D.M.; de Serres, G. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2021, 384, 1576–1577. [Google Scholar] [CrossRef]
- Conversation, T. 4 Things about mRNA COVID Vaccines Researchers Still Want to Find out. 2021. Available online: https://theconversation.com/4-things-about-mrna-covid-vaccines-researchers-still-want-to-find-out-154160 (accessed on 3 July 2021).
- Xie, X.; Liu, Y.; Liu, J.; Zhang, X.; Zou, J.; Fontes-Garfias, C.R.; Xia, H.; Swanson, K.A.; Cutler, M.; Cooper, D.; et al. Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera. Nat. Med. 2021, 27, 620–621. [Google Scholar] [CrossRef]
- Abu-Raddad, L.J.; Chemaitelly, H.; Butt, A.A. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N. Engl. J. Med. 2021, 385, 187–189. [Google Scholar] [CrossRef]
- PHE. Effectiveness of COVID-19 Vaccines against Hospital Admission with the Delta (B.1.617.2) Variant. Available online: https://khub.net/web/phe-national/public-library/-/document_library/v2WsRK3ZlEig/view_file/479607329?_com_liferay_document_library_web_portlet_DLPortlet_INSTANCE_v2WsRK3ZlEig_redirect=https%3A%2F%2Fkhub.net%3A443%2Fweb%2Fphe-national%2Fpublic-library%2F-%2Fdocument_library%2Fv2WsRK3ZlEig%2Fview%2F479607266 (accessed on 14 June 2021).
- Chemaitelly, H.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; Tang, P.; Hasan, M.R.; Malek, J.A.; Coyle, P.; Ayoub, H.H.; Al Kanaani, Z.; et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat. Med. 2021, 27, 1614–1621. [Google Scholar] [CrossRef]
- Zakhartchouk, A.N.; Liu, Q.; Petric, M.; Babiuk, L.A. Augmentation of immune responses to SARS coronavirus by a combination of DNA and whole killed virus vaccines. Vaccine 2005, 23, 4385–4391. [Google Scholar] [CrossRef]
- Du, L.; He, Y.; Jiang, S.; Zheng, B.-J. Development of subunit vaccines against severe acute respiratory syndrome. Drugs Today 2008, 44, 63–74. [Google Scholar]
- Enjuanes, L.; Zuñiga, S.; Castaño-Rodriguez, C.; Gutierrez-Alvarez, J.; Canton, J.; Sola, I. Molecular Basis of Coronavirus Virulence and Vaccine Development. Adv. Virus Res. 2016, 96, 245–286. [Google Scholar] [CrossRef]
- Lidder, P.; Sonnino, A. Chapter 1—Biotechnologies for the Management of Genetic Resources for Food and Agriculture. In Advances in Genetics; Goodwin, S.F., Friedmann, T., Dunlap, J.C., Eds.; Academic Press: Cambridge, MA, USA, 2012; Volume 78, pp. 1–167. [Google Scholar]
- WHO. WHO Vaccine Safety Basics. Available online: https://vaccine-safety-training.org/subunit-vaccines.html (accessed on 30 June 2021).
- Lan, J.; Yao, Y.; Deng, Y.; Chen, H.; Lu, G.; Wang, W.; Bao, L.; Deng, W.; Wei, Q.; Gao, G.F.; et al. Recombinant Receptor Binding Domain Protein Induces Partial Protective Immunity in Rhesus Macaques Against Middle East Respiratory Syndrome Coronavirus Challenge. EBioMedicine 2015, 2, 1438–1446. [Google Scholar] [CrossRef] [Green Version]
- Bisht, H.; Roberts, A.; Vogel, L.; Subbarao, K.; Moss, B. Neutralizing antibody and protective immunity to SARS coronavirus infection of mice induced by a soluble recombinant polypeptide containing an N-terminal segment of the spike glycoprotein. Virology 2005, 334, 160–165. [Google Scholar] [CrossRef] [Green Version]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef]
- Medicine, N. Novavax Vaccine Results: How Effective is it against Variants? 2021. Available online: https://www.nebraskamed.com/COVID/novavax-vaccine-results-how-effective-is-it-against-variants (accessed on 12 July 2021).
- Fuenmayor, J.; Gòdia, F.; Cervera, L. Production of virus-like particles for vaccines. New Biotechnol. 2017, 39 (Pt B), 174–180. [Google Scholar] [CrossRef]
- Cai, X.; Zheng, W.; Pan, S.; Zhang, S.; Xie, Y.; Guo, H.; Wang, G.; Li, Z.; Luo, M. A virus-like particle of the hepatitis B virus preS antigen elicits robust neutralizing antibodies and T cell responses in mice. Antiviral. Res. 2018, 149, 48–57. [Google Scholar] [CrossRef]
- Bright, R.A.; Carter, D.M.; Daniluk, S.; Toapanta, F.R.; Ahmad, A.; Gavrilov, V.; Massare, M.; Pushko, P.; Mytle, N.; Rowe, T.; et al. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine 2007, 25, 3871–3878. [Google Scholar] [CrossRef]
- Harper, D.M.; Franco, E.L.; Wheeler, C.; Ferris, D.G.; Jenkins, D.; Schuind, A.; Zahaf, T.; Innis, B.; Naud, P.; De Carvalho, N.S.; et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: A randomised controlled trial. Lancet 2004, 364, 1757–1765. [Google Scholar] [CrossRef]
- Lokugamage, K.G.; Yoshikawa-Iwata, N.; Ito, N.; Watts, D.M.; Wyde, P.R.; Wang, N.; Newman, P.; Kent Tseng, C.T.; Peters, C.J.; Makino, S. Chimeric coronavirus-like particles carrying severe acute respiratory syndrome coronavirus (SCoV) S protein protect mice against challenge with SCoV. Vaccine 2008, 26, 797–808. [Google Scholar] [CrossRef]
- WHO. Interim Recommendations for Use of the Inactivated COVID-19 Vaccine, CoronaVac, Developed by Sinovac. Available online: https://apps.who.int/iris/bitstream/handle/10665/341454/WHO-2019-nCoV-vaccines-SAGE-recommendation-Sinovac-CoronaVac-2021.1-eng.pdf (accessed on 24 May 2021).
- WHO. Status of COVID-19 Vaccines within WHO EUL/PQ Evaluation Process. Available online: https://extranet.who.int/pqweb/sites/default/files/documents/Status_COVID_VAX_02July2021.pdf (accessed on 2 July 2021).
- WHO. Background Document on the Inactivated Vaccine Sinovac-CoronaVac against COVID-19. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-Sinovac-CoronaVac-background-2021.1 (accessed on 1 June 2021).
- Ramasamy, M.N.; Jessop, L.J. CoronaVac: More data for regulators and policy makers. Lancet 2021, 398, 186–188. [Google Scholar] [CrossRef]
- WHO. Interim Recommendations for Use of the Inactivated COVID-19 Vaccine BIBP Developed by China National Biotec Group (CNBG), Sinopharm. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-BIBP-2021.1 (accessed on 7 May 2021).
- Xia, S.; Duan, K.; Zhang, Y.; Zhao, D.; Zhang, H.; Xie, Z.; Li, X.; Peng, C.; Zhang, Y.; Zhang, W.; et al. Effect of an Inactivated Vaccine Against SARS-CoV-2 on Safety and Immunogenicity Outcomes: Interim Analysis of 2 Randomized Clinical Trials. JAMA 2020, 324, 951–960. [Google Scholar] [CrossRef]
- Registry, C.C.T. A Phase III Clinical Trial for Inactivated Novel Coronavirus Pneumonia (COVID-19) Vaccine (Vero Cells). Available online: http://www.chictr.org.cn/showprojen.aspx?proj=56651 (accessed on 4 July 2021).
- Pu, J.; Yu, Q.; Yin, Z.; Zhang, Y.; Li, X.; Li, D.; Chen, H.; Long, R.; Zhao, Z.; Mou, T.; et al. An in-depth investigation of the safety and immunogenicity of an inactivated SARS-CoV-2 vaccine. medRxiv 2020. [Google Scholar] [CrossRef]
- NIH. The Efficacy, Safety and Immunogenicity Study of Inactivated SARS-CoV-2 Vaccine for Preventing Against COVID-19. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04659239?term=vaccination&cond=covid&draw=3 (accessed on 10 February 2021).
- NIH. Immunogenicity, Efficacy and Safety of QazCovid-in® COVID-19 Vaccine. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04691908?id=NCT04639466+OR+NCT04659941+OR+NCT04691947+OR+NCT04651790+OR+NCT04659239+OR+NCT04648800+OR+NCT04691908+OR+NCT04656613+OR+NCT04672395+OR+NCT04673149+OR+NCT04671017+OR+NCT04685603+OR+NCT04664309+OR+NCT04686773+OR+NCT04681092+OR+NCT04662697+OR+NCT04652102+OR+NCT04665258+OR+NCT04649021+OR+NCT04686409+OR+NCT04690387+OR+NCT04666012+OR+NCT04649151+OR+NCT04655625+OR+NCT04684446+OR+NCT04668339+OR+NCT04683224+OR+NCT04674189+OR+NCT04690816+OR+NCT04679909&draw=2&rank=2&load=cart (accessed on 4 May 2021).
- Institute, E.R. QazCovid-in. Available online: https://economy.kz/en/Novosti_ekonomiki_Kazahstana/id=1588 (accessed on 20 December 2020).
- Ella, R.; Vadrevu, K.M.; Jogdand, H.; Prasad, S.; Reddy, S.; Sarangi, V.; Ganneru, B.; Sapkal, G.; Yadav, P.; Abraham, P.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: A double-blind, randomised, phase 1 trial. Lancet Infect. Dis. 2021, 21, 637–646. [Google Scholar] [CrossRef]
- Biotech, B. COVAXIN®—India’s First Indigenous COVID-19 Vaccine. Available online: https://www.bharatbiotech.com/covaxin.html (accessed on 4 July 2021).
- NIH. An Efficacy and Safety Clinical Trial of an Investigational COVID-19 Vaccine (BBV152) in Adult Volunteers. Available online: https://clinicaltrials.gov/ct2/show/NCT04641481 (accessed on 19 March 2021).
- Ella, R.; Reddy, S.; Blackwelder, W.; Potdar, V.; Yadav, P.; Sarangi, V.; Aileni, V.K.; Kanungo, S.; Rai, S.; Reddy, P.; et al. Efficacy, safety, and lot to lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): A, double-blind, randomised, controlled phase 3 trial. medRxiv 2021. [Google Scholar] [CrossRef]
- NIH. A Study to Evaluate the Efficacy, Safety and Immunogenicity of SARS-CoV-2 Vaccine (Vero Cells), Inactivated in Healthy Adults Aged 18 Years and Older (COVID-19). Available online: https://clinicaltrials.gov/ct2/show/NCT04852705?term=vaccine&recrs=abdf&cond=COVID-19&phase=0123&sort=nwst&draw=2 (accessed on 22 April 2021).
- NIH. Study To Compare the Immunogenicity Against COVID-19, of VLA2001 Vaccine to AZD1222 Vaccine (COV-COMPARE). Available online: https://www.clinicaltrials.gov/ct2/show/NCT04864561#contacts (accessed on 8 June 2021).
- NIH. Efficacy, Immunogenicity, and Safety of the Inactivated COVID-19 Vaccine (TURKOVAC) Versus the CoronaVac Vaccine. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04942405 (accessed on 28 June 2021).
- IRCT. A Double-Blind, Randomized, Placebo-Controlled Phase II/III Clinical Trial to Evaluate the Safety and Efficacy of COVID-19 Inactivated Vaccine (Shifa-Pharmed) in a Population Aged 18 to 75 Years. Available online: https://en.irct.ir/trial/54881 (accessed on 4 July 2021).
- IRCT. Phase 2 Trial of Safety and Immunogenicity of 10 Micro Gram Inactivated SARS-CoV-2 Vaccine (FAKHRAVAC), Two Doses Two Weeks Apart in Adults Aged 18–70 Years: A Randomized, Double-Blind, Placebo-Controlled, Clinical Trial. Available online: https://en.irct.ir/trial/56027 (accessed on 5 July 2021).
- Sun, W.; McCroskery, S.; Liu, W.-C.; Leist, S.R.; Liu, Y.; Albrecht, R.A.; Slamanig, S.; Oliva, J.; Amanat, F.; Schäfer, A.; et al. A Newcastle Disease Virus (NDV) Expressing a Membrane-Anchored Spike as a Cost-Effective Inactivated SARS-CoV-2 Vaccine. Vaccines 2020, 8, 711. [Google Scholar] [CrossRef] [PubMed]
- JRCT. Placebo-Controlled, Multicenter, Double-Blind, Randomized, Parallel-Group, Comparative Study to Evaluate the Safety and Immunogenicity of KD-414, a Vaccine Against COVID-19, in Healthy Adults Aged ≥20 Years to <65 Years, and Healthy Elderly Subjects Aged ≥65 Years. Available online: https://jrct.niph.go.jp/en-latest-detail/jRCT2071200106 (accessed on 5 July 2021).
- NIH. Safety and Immunogenicity of the Inactivated Koçak-19 Inaktif Adjuvanlı COVID-19 Vaccine Compared to Placebo. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04838080?term=NCT04838080&draw=2&rank=1 (accessed on 13 April 2021).
- NIH. Study of a Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) Adjuvanted Inactivated Vaccine in Healthy Adults (COVID-19). Available online: https://www.clinicaltrials.gov/ct2/show/NCT04866069 (accessed on 6 May 2021).
- NIH. Study of a Live rNDV Based Vaccine against COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04871737 (accessed on 27 May 2021).
- NIH. Safety and Immunogenicity of COVI-VAC, a Live Attenuated Vaccine against COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04619628 (accessed on 6 November 2020).
- NIH. Safety and Immunogenicity of an Intranasal RSV Vaccine Expressing SARS-CoV-2 Spike Protein (COVID-19 Vaccine) in Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT04798001?term=covid-19+vaccine&draw=2 (accessed on 21 May 2021).
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- NIH. National Cohort Study of Effectiveness and Safety of SARS-CoV-2/COVID-19 Vaccines (ENFORCE) (ENFORCE). Available online: https://www.clinicaltrials.gov/ct2/show/NCT04760132?term=vaccine%2C+phase+4&cond=Covid19&draw=2 (accessed on 26 February 2021).
- Africa CDC. Africa Regulatory Taskforce Has Endorsed the Emergency Used Listing for Two Versions of the AstraZeneca-Oxford Vaccine. Available online: https://africacdc.org/download/africa-regulatory-taskforce-has-endorsed-the-emergency-used-listing-for-two-versions-of-the-astrazeneca-oxford-vaccine-astrazeneca-skbio-in-south-korea-and-serum-institute-of-india/ (accessed on 7 June 2021).
- CARPHA. CARPHA COVID-19 Vaccine Update. Available online: https://carpha.org/Portals/0/Documents/COVID-19%20Vaccine%20Updates/CARPHA%20COVID-19%20Vaccine%20Update%20011%20March%2022,%202021.pdf (accessed on 22 March 2021).
- Voysey, M.; Costa Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; Tessier, E.; Simmons, R.; Cottrell, S.; Roberts, R.; O’Doherty, M.; et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: Test negative case-control study. BMJ 2021, 373, n1088. [Google Scholar] [CrossRef]
- Vasileiou, E.; Simpson, C.R.; Shi, T.; Kerr, S.; Agrawal, U.; Akbari, A.; Bedston, S.; Beggs, J.; Bradley, D.; Chuter, A.; et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: A national prospective cohort study. Lancet 2021, 397, 1646–1657. [Google Scholar] [CrossRef]
- BBC. Covid vaccine: AstraZeneca Updates US Vaccine Efficacy Results. Available online: https://www.bbc.com/news/world-us-canada-56521166 (accessed on 25 March 2021).
- Madhi, S.A.; Baillie, V.; Cutland, C.L.; Voysey, M.; Koen, A.L.; Fairlie, L.; Padayachee, S.D.; Dheda, K.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 (AZD1222) Covid-19 vaccine against the B.1.351 variant in South Africa. medRxiv 2021. [Google Scholar] [CrossRef]
- EMA. COVID-19 Vaccines. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/covid-19-vaccines (accessed on 6 July 2021).
- NIH. Study on Sequential Immunization of Inactivated SARS-CoV-2 Vaccine and Recombinant SARS-CoV-2 Vaccine (Ad5 Vector). Available online: https://www.clinicaltrials.gov/ct2/show/NCT04892459?term=NCT04892459&draw=2&rank=1 (accessed on 30 June 2021).
- NIH. Clinical Trial of Recombinant Novel Coronavirus Vaccine (Adenovirus Type 5 Vector) Against COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04540419 (accessed on 16 February 2021).
- REUTERS. CanSinoBIO’s COVID-19 Vaccine 65.7% Effective in Global Trials, Pakistan Official Says. Available online: https://www.reuters.com/article/us-health-coronavirus-vaccine-pakistan-idUSKBN2A81N0 (accessed on 8 February 2021).
- Wu, S.; Zhong, G.; Zhang, J.; Shuai, L.; Zhang, Z.; Wen, Z.; Wang, B.; Zhao, Z.; Song, X.; Chen, Y.; et al. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun. 2020, 11, 4081. [Google Scholar] [CrossRef]
- Zhu, F.-C.; Guan, X.-H.; Li, Y.-H.; Huang, J.-Y.; Jiang, T.; Hou, L.-H.; Li, J.-X.; Yang, B.-F.; Wang, L.; Wang, W.-J.; et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef]
- Vaccinations, P. Convidicea Vaccine. Available online: https://www.precisionvaccinations.com/vaccines/convidicea-vaccine (accessed on 30 June 2021).
- Africa CDC. African Union and the Africa CDC’s Africa Regulatory Taskforce Has Endorsed the Emergency Used Authorization for Janssen COVID-19 Vaccine. Available online: https://africacdc.org/download/african-union-and-the-africa-centers-for-disease-control-and-preventions-africa-regulatory-taskforce-has-endorsed-the-emergency-used-authorization-for-janssen-covid-19-vaccine-2/ (accessed on 7 June 2021).
- Corchado-Garcia, J.; Puyraimond-Zemmour, D.; Hughes, T.; Cristea-Platon, T.; Lenehan, P.; Pawlowski, C.; Bade, S.; O’Horo, J.C.; Gores, G.J.; Williams, A.W.; et al. Real-world effectiveness of Ad26.COV2.S adenoviral vector vaccine for COVID-19. medRxiv 2021. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatulin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G.; et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. Lancet 2020, 396, 887–897. [Google Scholar] [CrossRef]
- NIH. Clinical Trial of Efficacy, Safety, and Immunogenicity of Gam-COVID-Vac Vaccine Against COVID-19 (RESIST). Available online: https://clinicaltrials.gov/ct2/show/NCT04530396?term=vaccine&cond=covid-19&draw=3 (accessed on 22 January 2021).
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- World News. Sputnik V Gives 90% Protection against Delta Strain of Covid-19: Scientist. In Hindustan Times; World News: New Delhi, India, 2021. [Google Scholar]
- NIH. Study of GRAd-COV2 for the Prevention of COVID-19 in Adults (COVITAR). Available online: https://clinicaltrials.gov/ct2/show/NCT04791423 (accessed on 14 April 2021).
- Capone, S.; Raggioli, A.; Gentile, M.; Battella, S.; Lahm, A.; Sommella, A.; Contino, A.M.; Urbanowicz, R.A.; Scala, R.; Barra, F.; et al. Immunogenicity of a new gorilla adenovirus vaccine candidate for COVID-19. Mol. Ther. 2021, 29, 2412–2423. [Google Scholar] [CrossRef]
- Lanini, S.; Capone, S.; Antinori, A.; Milleri, S.; Nicastri, E.; Camerini, R.; Agrati, C.; Castilletti, C.; Mori, F.; Sacchi, A.; et al. GRAd-COV2, a gorilla adenovirus based candidate vaccine against COVID-19, is safe and immunogenic in young and older adults. medRxiv 2021. [Google Scholar] [CrossRef]
- NIH. Immunity and Safety of Covid-19 Synthetic Minigene Vaccine. Available online: https://clinicaltrials.gov/ct2/show/NCT04276896 (accessed on 19 March 2021).
- NIH. COVID-19 Supplemental Vaccine Boost to Enhance T Cell Protection in Those Who Have Already Received EUA S-Based Vaccines. Available online: https://clinicaltrials.gov/ct2/show/NCT04843722 (accessed on 10 June 2021).
- ImmunityBio. Fighting a war on two fronts: ImmunityBio targets cancer and COVID-19. Biopharma Deal. 2021. Available online: https://www.nature.com/articles/d43747-020-00963-y (accessed on 8 June 2021).
- Rice, A.; Verma, M.; Shin, A.; Zakin, L.; Sieling, P.; Tanaka, S.; Adisetiyo, H.; Taft, J.; Patel, R.; Buta, S.; et al. A Next Generation Bivalent Human Ad5 COVID-19 Vaccine Delivering Both Spike and Nucleocapsid Antigens Elicits Th1 Dominant CD4+, CD8+ T-cell and Neutralizing Antibody Responses. bioRxiv 2020. [Google Scholar] [CrossRef]
- NIH. Safety and Immunogenicity Study of AdCLD-CoV19: A COVID-19 Preventive Vaccine in Healthy Volunteers. Available online: https://clinicaltrials.gov/ct2/show/NCT04666012 (accessed on 29 March 2021).
- NIH. A Phase 1/2 Safety and Immunogenicity Trial of COVID-19 Vaccine COVIVAC. Available online: https://clinicaltrials.gov/ct2/show/NCT04830800 (accessed on 5 April 2021).
- NIH. Safety, Tolerability and Immunogenicity of the Candidate Vaccine MVA-SARS-2-ST Against COVID-19 (MVA-SARS-2-ST). Available online: https://www.clinicaltrials.gov/ct2/show/NCT04895449?term=NCT04895449&draw=2&rank=1 (accessed on 20 May 2021).
- NIH. Safety, Tolerability and Immunogenicity of the Candidate Vaccine MVA-SARS-2-S Against COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04569383?term=vaccine&cond=covid-19&draw=5 (accessed on 1 December 2020).
- NIH. Safety and Immunogenicity Trial of an Oral SARS-CoV-2 Vaccine (VXA-CoV2-1) for Prevention of COVID-19 in Healthy Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT04563702 (accessed on 8 April 2021).
- Moore, A.C.; Dora, E.G.; Peinovich, N.; Tucker, K.P.; Lin, K.; Cortese, M.; Tucker, S.N. Pre-clinical studies of a recombinant adenoviral mucosal vaccine to prevent SARS-CoV-2 infection. bioRxiv 2020. [Google Scholar] [CrossRef]
- NIH. Safety and Immunogenicity of AdCOVID in Healthy Adults (COVID-19 Vaccine Study). Available online: https://clinicaltrials.gov/ct2/show/NCT04679909 (accessed on 24 February 2021).
- NIH. A Synthetic MVA-Based SARS-CoV-2 Vaccine, COH04S1, for the Prevention of COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04639466 (accessed on 7 January 2021).
- NIH. Chimpanzee Adenovirus and Self-Amplifying mRNA Prime-Boost Prophylactic Vaccines against SARS-CoV-2 in Healthy Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT04776317 (accessed on 2 July 2021).
- NIH. A Phase 1, First-in-Human Study of the Investigational COVID-19 Vaccine SC-Ad6-1 in Healthy Volunteers. Available online: https://clinicaltrials.gov/ct2/show/NCT04839042 (accessed on 27 April 2021).
- NIH. Safety and Immunogenicity of an Intranasal SARS-CoV-2 Vaccine (BBV154) for COVID-19. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04751682?term=vaccine&cond=Coronavirus&sfpd_s=01%2F01%2F2021&draw=2 (accessed on 22 June 2021).
- ChiCTR. The Nasal Spray Influenza Virus Vector New Coronary Pneumonia (COVID-19) Vaccine (DelNS1-2019-nCoV-RBD-OPT1) Phase II Clinical Trial. 2020. Available online: http://www.chictr.org.cn/showproj.aspx?proj=63754 (accessed on 7 August 2021).
- NIH. A Study to Evaluate Safety and Immunogenicity of DelNS1-nCoV-RBD LAIV for COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04809389 (accessed on 3 May 2021).
- Yahalom-Ronen, Y.; Tamir, H.; Melamed, S.; Politi, B.; Shifman, O.; Achdout, H.; Vitner, E.B.; Israeli, O.; Milrot, E.; Stein, D.; et al. A single dose of recombinant VSV-ΔG-spike vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun. 2020, 11, 6402. [Google Scholar] [CrossRef]
- NIH. Phase I-II Trial of Dendritic Cell Vaccine to Prevent COVID-19 in Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT04386252 (accessed on 8 June 2021).
- NIH. Safety and Immunity of Covid-19 aAPC Vaccine. Available online: https://clinicaltrials.gov/ct2/show/study/NCT04299724 (accessed on 9 March 2021).
- CTRI. A Prospective, Randomized, Adaptive, Phase I/II Clinical Study to Evaluate the Safety and Immunogenicity of Novel Corona Virus -2019-nCov Vaccine Candidate of M/s Cadila Healthcare Limited by Intradermal Route in Healthy Subjects. Available online: http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=45306&EncHid=&userName=Zydus (accessed on 8 November 2020).
- Das, S. Zydus’ Covid-19 vaccine shows 66.6% efficacy, seeks DCGI approval. In Business Standard; Fiancial Press: New Delhi, India, 2021. [Google Scholar]
- Mammen, M.P.; Tebas, P.; Agnes, J.; Giffear, M.; Kraynyak, K.A.; Blackwood, E.; Amante, D.; Reuschel, E.L.; Purwar, M.; Christensen-Quick, A.; et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: A preliminary report of a randomized, blinded, placebo-controlled, Phase 2 clinical trial in adults at high risk of viral exposure. medRxiv 2021. [Google Scholar] [CrossRef]
- NIH. Safety, Immunogenicity, and Efficacy of INO-4800 for COVID-19 in Healthy Seronegative Adults at High Risk of SARS-CoV-2 Exposure. Available online: https://clinicaltrials.gov/ct2/show/NCT04642638 (accessed on 29 March 2021).
- NIH. Phase II/III Study of COVID-19 DNA Vaccine (AG0302-COVID19). Available online: https://www.clinicaltrials.gov/ct2/show/NCT04655625?term=vaccination&cond=covid&draw=1 (accessed on 8 April 2021).
- NIH. Safety and Immunogenicity Study of GX-19, a COVID-19 Preventive DNA Vaccine in Healthy Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT04445389?term=vaccine&cond=covid-19&draw=3 (accessed on 28 July 2020).
- NIH. A Clinical Trial of a Prophylactic Plasmid DNA Vaccine for COVID-19 [Covigenix VAX-001] in Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT04591184 (accessed on 27 April 2021).
- Sciences, G.L. Nucleic Acid Vaccines. Available online: http://www.genels.com/en/sub/technology/vaccine.asp (accessed on 1 September 2021).
- NIH. GLS-5310 Vaccine for the Prevention of SARS-CoV-2 (COVID-19). Available online: https://www.clinicaltrials.gov/ct2/show/NCT04673149?term=NCT04673149&draw=2&rank=1 (accessed on 24 December 2020).
- NIH. Safety and Immunogenicity of COVID-eVax, a Candidate Plasmid DNA Vaccine for COVID-19, in Healthy Adult Volunteers. Available online: https://clinicaltrials.gov/ct2/show/NCT04788459 (accessed on 16 March 2020).
- NIH. CORVax12: SARS-CoV-2 Spike (S) Protein Plasmid DNA Vaccine Trial for COVID-19 (SARS-CoV-2) (CORVax12). Available online: https://clinicaltrials.gov/ct2/show/NCT04627675 (accessed on 3 June 2021).
- NIH. Evaluating the Safety, Tolerability and Immunogenicity of bacTRL-Spike Vaccine for Prevention of COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04334980 (accessed on 4 March 2021).
- NIH. The Safety and Immunogenicity of a DNA-based Vaccine (COVIGEN) in Healthy Volunteers (COVALIA). Available online: https://clinicaltrials.gov/ct2/show/NCT04742842 (accessed on 8 February 2020).
- Food and Drug Administration (FDA). Vaccines and Related Biological Products Advisory Committee Meeting December 17, 2020; Food and Drug Administration (FDA): White Oak, MD, USA, 2020. [Google Scholar]
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.L.; Yoon, S.K.; Meece, J.; Olsho, L.E.W.; Caban-Martinez, A.J.; Fowlkes, A.; Lutrick, K.; et al. Interim Estimates of Vaccine Effectiveness of BNT162b2 and mRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Health Care Personnel, First Responders, and Other Essential and Frontline Workers—Eight U.S. Locations, December 2020–March 2021. MMWR Morb. Mortal. Wkly Rep. 2021, 70, 495–500. [Google Scholar] [CrossRef]
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.; Yoon, S.K.; Meece, J.; Olsho, L.E.W.; Caban-Martinez, A.J.; Fowlkes, A.L.; Lutrick, K.; et al. Prevention and Attenuation of COVID-19 by BNT162b2 and mRNA-1273 Vaccines. medRxiv 2021. [Google Scholar] [CrossRef]
- Chung, H.; He, S.; Nasreen, S.; Sundaram, M.E.; Buchan, S.A.; Wilson, S.E.; Chen, B.; Calzavara, A.; Fell, D.B.; Austin, P.C.; et al. Effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe COVID-19 outcomes in Ontario, Canada. medRxiv 2021. [Google Scholar] [CrossRef]
- NIH. A Study to Evaluate Efficacy, Safety, and Immunogenicity of mRNA-1273 Vaccine in Adults Aged 18 Years and Older to Prevent COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04470427?term=vaccine&cond=covid-19&draw=5 (accessed on 10 June 2021).
- Hall, V.J.; Foulkes, S.; Saei, A.; Andrews, N.; Oguti, B.; Charlett, A.; Wellington, E.; Stowe, J.; Gillson, N.; Atti, A.; et al. Effectiveness of BNT162b2 mRNA Vaccine Against Infection and COVID-19 Vaccine Coverage in Healthcare Workers in England, Multicentre Prospective Cohort Study (the SIREN Study). SSRN 2021. [Google Scholar] [CrossRef]
- Pawlowski, C.; Lenehan, P.; Puranik, A.; Agarwal, V.; Venkatakrishnan, A.J.; Niesen, M.J.M.; O’Horo, J.C.; Badley, A.D.; Halamka, J.; Soundararajan, V. FDA-authorized COVID-19 vaccines are effective per real-world evidence synthesized across a multi-state health system. medRxiv 2021. [Google Scholar] [CrossRef]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Björk, J.; Inghammar, M.; Moghaddassi, M.; Rasmussen, M.; Malmqvist, U.; Kahn, F. Effectiveness of the BNT162b2 vaccine in preventing COVID-19 in the working age population—First results from a cohort study in Southern Sweden. medRxiv 2021. [Google Scholar] [CrossRef]
- Public Health England. Annex A: Report to JCVI on estimated efficacy of a single dose of Pfizer BioNTech (BNT162b2 mRNA) vaccine and of a single dose of ChAdOx1 vaccine (AZD1222). Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/949505/annex-a-phe-report-to-jcvi-on-estimated-efficacy-of-single-vaccine-dose.pdf (accessed on 22 December 2020).
- Chodick, G.; Tene, L.; Patalon, T.; Gazit, S.; Ben Tov, A.; Cohen, D.; Muhsen, K. Assessment of Effectiveness of 1 Dose of BNT162b2 Vaccine for SARS-CoV-2 Infection 13 to 24 Days After Immunization. JAMA Netw. Open 2021, 4, e2115985. [Google Scholar] [CrossRef]
- Moustsen-Helms, I.R.; Emborg, H.-D.; Nielsen, J.; Nielsen, K.F.; Krause, T.G.; Mølbak, K.; Møller, K.L.; Berthelsen, A.-S.N.; Valentiner-Branth, P. Vaccine effectiveness after 1st and 2nd dose of the BNT162b2 mRNA Covid-19 Vaccine in long-term care facility residents and healthcare workers—A Danish cohort study. medRxiv 2021. [Google Scholar] [CrossRef]
- Chodick, G.; Tene, L.; Patalon, T.; Gazit, S.; Tov, A.B.; Cohen, D.; Muhsen, K. The effectiveness of the first dose of BNT162b2 vaccine in reducing SARS-CoV-2 infection 13-24 days after immunization: Real-world evidence. medRxiv.
- CARPHA. Carpha Situation Report NO. 145. Available online: https://carpha.org/Portals/0/Documents/COVID%20Situation%20Reports/Situation%20Report%20145%20-%20April%201,%202021.pdf (accessed on 1 April 2021).
- Creech, C.B.; Walker, S.C.; Samuels, R.J. SARS-CoV-2 Vaccines. JAMA 2021, 325, 1318–1320. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- NIH. A Study to Evaluate the Safety and Immunogenicity of Vaccine CVnCoV in Healthy Adults in Germany for COVID-19. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04674189?id=NCT04639466+OR+NCT04655625+OR+NCT04662697+OR+NCT04683224+OR+NCT04668339+OR+NCT04674189+OR+NCT04665258+OR+NCT04646590+OR+NCT04642638+OR+NCT04656613+OR+NCT04648800+OR+NCT04649515+OR+NCT04677660+OR+NCT04668625+OR+NCT04649021+OR+NCT04649151+OR+NCT04659486+OR+NCT04664075&draw=2&rank=3&load=cart (accessed on 18 June 2021).
- CureVac. CureVac’s mRNA-based vaccine candidate against COVID-19. Available online: https://www.curevac.com/en/covid-19/ (accessed on 16 June 2021).
- Rauch, S.; Roth, N.; Schwendt, K.; Fotin-Mleczek, M.; Mueller, S.O.; Petsch, B. mRNA-based SARS-CoV-2 vaccine candidate CVnCoV induces high levels of virus-neutralising antibodies and mediates protection in rodents. NPJ Vaccines 2021, 6, 57. [Google Scholar] [CrossRef]
- NIH. A Phase III Clinical Study of a SARS-CoV-2 Messenger Ribonucleic Acid (mRNA) Vaccine Candidate Against COVID-19 in Population Aged 18 Years and Above. Available online: https://clinicaltrials.gov/ct2/show/NCT04847102 (accessed on 17 May 2021).
- Zhang, N.-N.; Li, X.-F.; Deng, Y.-Q.; Zhao, H.; Huang, Y.-J.; Yang, G.; Huang, W.-J.; Gao, P.; Zhou, C.; Zhang, R.-R.; et al. A Thermostable mRNA Vaccine against COVID-19. Cell 2020, 182, 1271–1283. [Google Scholar] [CrossRef]
- NIH. A Study to Evaluate the Immunogenicity and Safety of mRNA-1273.211 Vaccine for COVID-19 Variants. Available online: https://clinicaltrials.gov/ct2/show/NCT04927065 (accessed on 15 June 2021).
- Wu, K.; Choi, A.; Koch, M.; Ma, L.; Hill, A.; Nunna, N.; Huang, W.; Oestreicher, J.; Colpitts, T.; Bennett, H.; et al. Preliminary Analysis of Safety and Immunogenicity of a SARS-CoV-2 Variant Vaccine Booster. medRxiv 2021. [Google Scholar] [CrossRef]
- NIH. Dose-Confirmation Study to Evaluate the Safety, Reactogenicity, and Immunogenicity of mRNA-1273 COVID-19 Vaccine in Adults Aged 18 Years and Older. Available online: https://clinicaltrials.gov/ct2/show/NCT04405076 (accessed on 5 May 2021).
- NIH. Safety and Immunogenicity Study of a SARS-CoV-2 (COVID-19) Variant Vaccine (mRNA-1273.351) in Naïve and Previously Vaccinated Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT04785144 (accessed on 2 July 2021).
- NIH. A Trial Evaluating the Safety and Effects of an RNA Vaccine ARCT-021 in Healthy Adults. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04668339?term=vaccination&cond=covid&draw=1 (accessed on 29 March 2021).
- NIH. Open Label Extension Study to Assess the Safety and Long-Term Immunogenicity of ARCT-021. Available online: https://clinicaltrials.gov/ct2/show/NCT04728347 (accessed on 28 January 2021).
- Holdings, A.T. Arcturus—A Clinical-Stage mRNA Therapeutics and Vaccines Company. Biopharma Deal. 2021. Available online: https://www.nature.com/articles/d43747-021-00073-3 (accessed on 10 June 2021).
- NIH. Study of mRNA Vaccine Formulation against COVID-19 in Healthy Adults 18 Years of Age and Older (VAW00001). Available online: https://clinicaltrials.gov/ct2/show/NCT04798027?term=Sanofi&cond=COVID-19&draw=2&rank=2 (accessed on 29 June 2021).
- Kalnin, K.V.; Plitnik, T.; Kishko, M.; Zhang, J.; Zhang, D.; Beauvais, A.; Anosova, N.G.; Tibbitts, T.; DiNapoli, J.; Ulinski, G.; et al. Immunogenicity and efficacy of mRNA COVID-19 vaccine MRT5500 in preclinical animal models. NPJ Vaccines 2021, 6, 61. [Google Scholar] [CrossRef]
- Sanofi. Sanofi and Translate Bio Initiate Phase 1/2 Clinical Trial of mRNA COVID-19 Vaccine Candidate. Available online: https://www.sanofi.com/en/media-room/press-releases/2021/2021-03-12-07-00-00-2191846# (accessed on 12 March 2021).
- NIH. Study of DS-5670a (COVID-19 Vaccine) in Japanese Healthy Adults and Elderly Subjects. Available online: https://clinicaltrials.gov/ct2/show/NCT04821674 (accessed on 8 July 2021).
- Yan, Z.P.; Yang, M.; Lai, C.L. COVID-19 Vaccines: A Review of the Safety and Efficacy of Current Clinical Trials. Pharmaceuticals 2021, 14, 406. [Google Scholar] [CrossRef]
- NIH. Safety and Immunogenicity of EXG-5003. Available online: https://clinicaltrials.gov/ct2/show/NCT04863131 (accessed on 30 April 2021).
- ISRCTN Registry. Clinical Trial to Assess the Safety of a Coronavirus Vaccine in Healthy Men and Women. 2020. Available online: https://www.isrctn.com/ISRCTN17072692 (accessed on 7 September 2021).
- Imperial College London. COVAC1: How the Trial Works. Available online: https://www.imperial.ac.uk/covid-19-vaccine-trial/trial-info/ (accessed on 7 June 2021).
- Wise, N. The Latest Development in ChulaCov19 Vaccine. Available online: https://www.newswise.com/coronavirus/the-latest-development-in-chulacov19-vaccine (accessed on 30 March 2021).
- NIH. ChulaCov19 mRNA Vaccine in Healthy Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT04566276 (accessed on 6 October 2020).
- NIH. PTX-COVID19-B, an mRNA Humoral Vaccine, is Intended for Prevention of COVID-19 in a General Population. This Study is Designed to Evaluate Safety, Tolerability, and Immunogenicity of PTX-COVID19-B Vaccine in Healthy Seronegative Adults Aged 18–64. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04765436?id=NCT04747821+OR+NCT04756830+OR+NCT04733807+OR+NCT04728828+OR+NCT04760704+OR+NCT04765436&draw=2&rank=1&load=cart (accessed on 30 June 2021).
- Susha Cheriyedath, M.S. SARS-CoV-2 mRNA Vaccine PTX-COVID19-B Safe and Highly Immunogenic in Preclinical Study. Available online: https://www.news-medical.net/news/20210518/SARS-CoV-2-mRNA-vaccine-PTX-COVID19-B-safe-and-highly-immunogenic-in-preclinical-study.aspx (accessed on 18 May 2021).
- NIH. A Study of the Safety of and Immune Response to Varying Doses of a Vaccine Against COVID-19 in Healthy Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT04758962 (accessed on 21 June 2021).
- NIH. Phase 1 Study to Assess Safety, Reactogenicity and Immunogenicity of the HDT-301 Vaccine Against COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04844268 (accessed on 19 April 2021).
- NIH. A Study to Evaluate Safety, Reactogenicity, and Immunogenicity of mRNA-1283 and mRNA-1273 Vaccines in Healthy Adults Between 18 Years and 55 Years of Age to Prevent COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04813796 (accessed on 10 May 2021).
- Moderna, Inc. First Participants Dosed in Phase 1 Study Evaluating mRNA-1283, Moderna’s Next Generation COVID-19 Vaccine. Available online: https://investors.modernatx.com/news-releases/news-release-details/first-participants-dosed-phase-1-study-evaluating-mrna-1283 (accessed on 15 March 2021).
- ChiCTR. Randomized, Double-Blind, Placebo-Controlled Phase I Clinical Trial to Evaluate the Safety and Immunogenicity of mRNACOVID-19 Vaccine in Healthy Susceptible Populations Aged 18 Years and Older People. Available online: http://www.chictr.org.cn/showprojen.aspx?proj=126046 (accessed on 7 September 2021).
- BioWorld. Stemirna Raises Nearly $200M to Advance mRNA COVID-19 Vaccine. Available online: https://www.bioworld.com/articles/507855-stemirna-raises-nearly-200m-to-advance-mrna-covid-19-vaccine?v=preview (accessed on 4 June 2021).
- NIH. Safety and Immunogenicity of LNP-nCOV saRNA-02 Vaccine against SARS-CoV-2, the Causative Agent of COVID-19 (COVAC-Uganda). Available online: https://www.clinicaltrials.gov/ct2/show/NCT04934111?term=vaccine&type=Intr&cond=Covid19&draw=2 (accessed on 22 June 2021).
- NIH. A Study to Evaluate the Efficacy, Immune Response, and Safety of a COVID-19 Vaccine in Adults ≥18 Years With a Pediatric Expansion in Adolescents (12–17 Years) at Risk for SARS-CoV-2. Available online: https://clinicaltrials.gov/ct2/show/NCT04611802 (accessed on 6 May 2021).
- Shinde, V.; Bhikha, S.; Hoosain, Z.; Archary, M.; Bhorat, Q.; Fairlie, L.; Lalloo, U.; Masilela, M.S.L.; Moodley, D.; Hanley, S.; et al. Efficacy of NVX-CoV2373 Covid-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021, 384, 1899–1909. [Google Scholar] [CrossRef]
- Novavax, Inc. Novavax COVID-19 Vaccine Demonstrates 89.3% Efficacy in UK Phase 3 Trial. 2021. Available online: https://ir.novavax.com/news-releases/news-release-details/novavax-covid-19-vaccine-demonstrates-893-efficacy-uk-phase-3 (accessed on 7 September 2021).
- Yang, S.; Li, Y.; Dai, L.; Wang, J.; He, P.; Li, C.; Fang, X.; Wang, C.; Zhao, X.; Huang, E.; et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: Two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect. Dis. 2021, 21, 1107–1119. [Google Scholar] [CrossRef]
- NIH. A Phase III Clinical Trial to Determine the Safety and Efficacy of ZF2001 for Prevention of COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04646590 (accessed on 7 May 2021).
- NIH. Study of Monovalent and Bivalent Recombinant Protein Vaccines Against COVID-19 in Adults 18 Years of Age and Older (VAT00008). 2021. Available online: https://clinicaltrials.gov/ct2/show/study/NCT04904549 (accessed on 11 June 2021).
- Sanofi. Sanofi and GSK Initiate Global Phase 3 Clinical Efficacy Study of COVID-19 Vaccine Candidate. Available online: https://www.sanofi.com/en/media-room/press-releases/2021/2021-05-27-07-30-00-2236989 (accessed on 7 September 2021).
- ZAWYA. Cuba Encouraged by Early Efficacy Results of Homegrown COVID-19 Vaccine. Available online: https://www.zawya.com/mena/en/economy/story/Cuba_encouraged_by_early_efficacy_results_of_homegrown_COVID19_vaccine-TR20210620nL2N2O2003X1/ (accessed on 20 June 2021).
- RPCEC. SOBERANA 02-FaseIII. Available online: https://rpcec.sld.cu/en/trials/RPCEC00000354-En (accessed on 7 September 2021).
- Chang-Monteagudo, A.; Ochoa-Azze, R.; Climent-Ruiz, Y.; Macías-Abraham, C.; Rodríguez-Noda, L.; Valenzuela-Silva, C.; Sánchez-Ramírez, B.; Perez-Nicado, R.; González-Mugica, R.; Hernández-García, T.; et al. A single dose of SARS-CoV-2 FINLAY-FR-1A dimeric-RBD recombinant vaccine enhances neutralization response in COVID-19 convalescents, with excellent safety profile. A preliminary report of an open-label phase 1 clinical trial. medRxiv 2021. [Google Scholar] [CrossRef]
- NIH. A Global Phase III Clinical Trial of Recombinant COVID- 19 Vaccine (Sf9 Cells). Available online: https://clinicaltrials.gov/ct2/show/NCT04887207 (accessed on 30 June 2021).
- NIH. Study of the Tolerability, Safety, Immunogenicity and Preventive Efficacy of the EpiVacCorona Vaccine for the Prevention of COVID-19. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04780035?term=vaccine&cond=Covid19&draw=2 (accessed on 3 March 2021).
- News, D. Two More Russian Vaccines: What We Do and Don’t Know. Available online: https://www.dw.com/en/two-more-russian-vaccines-what-we-do-and-dont-know/a-56811025 (accessed on 3 March 2021).
- Mahase, E. Covid-19: Russian vaccine efficacy is 91.6%, show phase III trial results. BMJ 2021, 372, n309. [Google Scholar] [CrossRef]
- RPCEC. ABDALA Clinical Study—Phase III. Available online: https://rpcec.sld.cu/trials/RPCEC00000359-En (accessed on 8 September 2021).
- NIH. Study to Evaluate the Safety, Immunogenicity, and Efficacy of Nanocovax Vaccine Against COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04922788 (accessed on 8 September 2021).
- NIH. A Controlled Phase 2/3 Study of Adjuvanted Recombinant SARS-CoV-2 Trimeric S-protein Vaccine (SCB-2019) for the Prevention of COVID-19 (SCB-2019). Available online: https://clinicaltrials.gov/ct2/show/NCT04672395 (accessed on 11 June 2021).
- Liang, J.G.; Su, D.; Song, T.-Z.; Zeng, Y.; Huang, W.; Wu, J.; Xu, R.; Luo, P.; Yang, X.; Zhang, X.; et al. S-Trimer, a COVID-19 subunit vaccine candidate, induces protective immunity in nonhuman primates. Nat. Commun. 2021, 12, 1346. [Google Scholar] [CrossRef]
- Richmond, P.; Hatchuel, L.; Dong, M.; Ma, B.; Hu, B.; Smolenov, I.; Li, P.; Liang, P.; Han, H.H.; Liang, J.; et al. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: A phase 1, randomised, double-blind, placebo-controlled trial. Lancet 2021, 397, 682–694. [Google Scholar] [CrossRef]
- NIH. A Study to Evaluate the Safety, Immunogenicity, and Efficacy of UB-612 COVID-19 Vaccine. Available online: https://www.clinicaltrials.gov/ct2/show/study/NCT04683224?id=NCT04639466+OR+NCT04655625+OR+NCT04662697+OR+NCT04683224+OR+NCT04668339+OR+NCT04674189+OR+NCT04665258+OR+NCT04646590+OR+NCT04642638+OR+NCT04656613+OR+NCT04648800+OR+NCT04649515+OR+NCT04677660+OR+NCT04668625+OR+NCT04649021+OR+NCT04649151+OR+NCT04659486+OR+NCT04664075&draw=2&rank=1&load=cart (accessed on 24 December 2020).
- RPCEC. SOBERANA 01. Available online: https://rpcec.sld.cu/en/trials/RPCEC00000332-En (accessed on 3 July 2021).
- IRCT. A phase II, Randomized, Two-armed, Double-Blind, Placebo Controlled Trial to Evaluate Efficacy and Safety of an Adjuvanted Recombinant SARS-CoV-2 Spike (S) Protein Subunit Vaccine (SpikoGen®) Produced by CinnaGen Co. (Two doses of 25 µg with Dosing Interval of 21 days). 2021. Available online: https://www.irct.ir/trial/56287 (accessed on 3 July 2021).
- NIH. A Study to Evaluate MVC-COV1901 Vaccine Against COVID-19 in Adult (COVID-19). Available online: https://clinicaltrials.gov/ct2/show/NCT04695652 (accessed on 30 March 2021).
- NIH. A Study to Evaluate MVC-COV1901 Vaccine Against COVID-19 in Elderly Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT04822025 (accessed on 15 June 2021).
- Hsieh, S.-M.; Liu, W.-D.; Huang, Y.-S.; Lin, Y.-J.; Hsieh, E.-F.; Lian, W.-C.; Chen, C.; Janssen, R.; Shih, S.-R.; Huang, C.-G.; et al. Safety and immunogenicity of a Recombinant Stabilized Prefusion SARS-CoV-2 Spike Protein Vaccine (MVC COV1901) Adjuvanted with CpG 1018 and Aluminum Hydroxide in healthy adults: A Phase 1, dose-escalation study. EClinicalMedicine 2021, 38, 100989. [Google Scholar] [CrossRef]
- IRCT. Phase II, Safety and Immunogenicity of RAZI SARS-CoV-2 Recombinant Spike Protein Vaccine (RAZI Cov Pars) in Adults Aged 18–70 Years; a Randomised, Double Blind, parallel 2 Arms Clinical Trial. 2021. Available online: https://en.irct.ir/trial/55238 (accessed on 8 September 2021).
- ChiCTR. A Randomized, Double-Blind, Placebo-Controlled Phase II Clinical Trial to Evaluate the Immunogenicity and Safety of Recombinant SARS-CoV-2 Fusion Protein Vaccine (V-01) in Healthy Subjects. 2021. Available online: http://www.chictr.org.cn/showproj.aspx?proj=124702 (accessed on 9 July 2021).
- RPCEC. MAMBISA Study. 2021. Available online: https://rpcec.sld.cu/en/trials/RPCEC00000345-En (accessed on 9 July 2021).
- EurekAlert. BAT Progresses COVID-19 Candidate Vaccine into Phase I Human Clinical Trials. Available online: https://www.eurekalert.org/pub_releases/2020-12/raba-bpc121520.php (accessed on 1 July 2021).
- NIH. KBP-201 COVID-19 Vaccine Trial in Healthy Volunteers. Available online: https://clinicaltrials.gov/ct2/show/NCT04473690 (accessed on 10 June 2021).
- Biological E’s Novel Covid-19 Vaccine of SARS-CoV-2 for Protection against Covid-19 Disease. Available online: http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=48329 (accessed on 10 November 2020).
- JRCT. A Phase 1/2, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study of S-268019 in Japanese Adult Participants. Available online: https://jrct.niph.go.jp/en-latest-detail/jRCT2051200092 (accessed on 9 December 2020).
- NIH. Anti-COVID19 AKS-452—ACT Study (ACT). Available online: https://clinicaltrials.gov/ct2/show/NCT04681092?term=NCT04681092&draw=2&rank=1 (accessed on 23 April 2021).
- NIH. A Clinical Trial of COVAC-2 in Healthy Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT04702178 (accessed on 1 April 2021).
- NIH. Safety and Immunogenicity Study of SARS-CoV-2 Nanoparticle Vaccine (GBP510) Adjuvanted with Aluminum Hydroxide (COVID-19). Available online: https://clinicaltrials.gov/ct2/show/NCT04742738 (accessed on 8 February 2021).
- NIH. Reactogenicity, Safety and Immunogenicity of QazCoVac-P COVID-19 Vaccine. Available online: https://clinicaltrials.gov/ct2/show/NCT04930003?term=vaccine&recrs=adf&cond=COVID-19&phase=0123&sort=nwst&draw=2 (accessed on 18 June 2021).
- NIH. Safety, Tolerance and Immunogenicity of EuCorVac-19 for the Prevention of COVID-19 in Healthy Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT04783311 (accessed on 26 April 2021).
- NIH. A Clinical Trial to Evaluate the Recombinant SARS-CoV-2 Vaccine (CHO Cell) for COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04869592 (accessed on 7 May 2021).
- NIH. A Study on the Safety, Tolerability and Immune Response of SARS-CoV-2 Sclamp (COVID-19) Vaccine in Healthy Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT04495933 (accessed on 23 April 2021).
- Watterson, D.; Wijesundara, D.K.; Modhiran, N.; Mordant, F.L.; Li, Z.; Avumegah, M.S.; McMillan, C.L.; Lackenby, J.; Guilfoyle, K.; van Amerongen, G.; et al. Preclinical development of a molecular clamp-stabilised subunit vaccine for severe acute respiratory syndrome coronavirus 2. Clin. Transl. Immunol. 2021, 10, e1269. [Google Scholar] [CrossRef] [PubMed]
- Chappell, K.J.; Mordant, F.L.; Li, Z.; Wijesundara, D.K.; Ellenberg, P.; Lackenby, J.A.; Cheung, S.T.M.; Modhiran, N.; Avumegah, M.S.; Henderson, C.L.; et al. Safety and immunogenicity of an MF59-adjuvanted spike glycoprotein-clamp vaccine for SARS-CoV-2: A randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infect. Dis. 2021, 21, 1383–1394. [Google Scholar] [CrossRef]
- NIH. Safety and Immunogenicity Trial of Multi-peptide Vaccination to Prevent COVID-19 Infection in Adults (pVAC). Available online: https://clinicaltrials.gov/ct2/show/NCT04546841?term=vaccine&cond=covid-19&draw=2 (accessed on 1 December 2020).
- NIH. A Study to Evaluate the Safety and Immunogenicity of COVID-19 (AdimrSC-2f) Vaccine. Available online: https://clinicaltrials.gov/ct2/show/study/NCT04522089 (accessed on 13 January 2021).
- NIH. Safety and Immunogenicity of a SARS-CoV-2 Vaccine (NBP2001) in Healthy Adults (COVID-19). Available online: https://www.clinicaltrials.gov/ct2/show/NCT04760743 (accessed on 18 February 2021).
- NIH. Safety, Reactogenicity and Immunogenicity Study of ReCOV. Available online: https://clinicaltrials.gov/ct2/show/NCT04818801 (accessed on 26 March 2021).
- NIH. SARS-COV-2-Spike-Ferritin-Nanoparticle (SpFN) Vaccine With ALFQ Adjuvant for Prevention of COVID-19 in Healthy Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT04784767 (accessed on 20 April 2021).
- Joyce, M.G.; King, H.A.D.; Naouar, I.E.; Ahmed, A.; Peachman, K.K.; Cincotta, C.M.; Subra, C.; Chen, R.E.; Thomas, P.V.; Chen, W.-H.; et al. Efficacy of a Broadly Neutralizing SARS-CoV-2 Ferritin Nanoparticle Vaccine in Nonhuman Primates. bioRxiv 2021. [Google Scholar] [CrossRef]
- Carmen, J.M.; Shrivastava, S.; Lu, Z.; Anderson, A.; Morrison, E.B.; Sankhala, R.S.; Chen, W.-H.; Chang, W.C.; Bolton, J.S.; Matyas, G.R.; et al. A spike-ferritin nanoparticle vaccine induces robust innate immune activity and drives polyfunctional SARS-CoV-2-specific T cells. bioRxiv 2021. [Google Scholar] [CrossRef]
- NIH. To Evaluate the Safety, and Immunogenicity of Vaccine Candidate against COVID-19, in Healthy Adults (COVEPIT 3). Available online: https://clinicaltrials.gov/ct2/show/NCT04885361 (accessed on 9 June 2021).
- NIH. First-in-Human Study Of Orally Administered CoV2-OGEN1 in Healthy Subjects. Available online: https://clinicaltrials.gov/ct2/show/NCT04893512 (accessed on 7 June 2021).
- NIH. Study of a Recombinant Coronavirus-Like Particle COVID-19 Vaccine in Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT04636697 (accessed on 24 May 2021).
- Ward, B.J.; Gobeil, P.; Séguin, A.; Atkins, J.; Boulay, I.; Charbonneau, P.-Y.; Couture, M.; D’Aoust, M.-A.; Dhaliwall, J.; Finkle, C.; et al. Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19. Nat. Med. 2021, 27, 1071–1078. [Google Scholar] [CrossRef]
- ACT. A Phase 1/2 Randomized, Placebo-Controlled, Multi-Centre Study to Evaluate the Safety and Immunogenicity of COVID-19 Vaccine in Healthy Adults. 2020. Available online: https://www.australianclinicaltrials.gov.au/anzctr/trial/ACTRN12620000817943 (accessed on 4 July 2021).
- NIH. Safety, Tolerability, and Immunogenicity of the COVID-19 Vaccine Candidate (VBI-2902a). Available online: https://clinicaltrials.gov/ct2/show/NCT04636697https://clinicaltrials.gov/ct2/show/NCT04773665 (accessed on 21 April 2021).
- NIH. Study of a Severe Acute Respiratory Syndrome CoV-2 (SARS-CoV-2) Virus-Like Particle (VLP) Vaccine in Healthy Adults (COVID-19). Available online: https://clinicaltrials.gov/ct2/show/NCT04818281?cond=NCT04818281&draw=2 (accessed on 8 April 2021).
- NIH. Safety and Tolerability of COVID-19 Vaccine (ABNCoV2) (COUGH-1). Available online: https://clinicaltrials.gov/ct2/show/NCT04839146 (accessed on 9 April 2021).
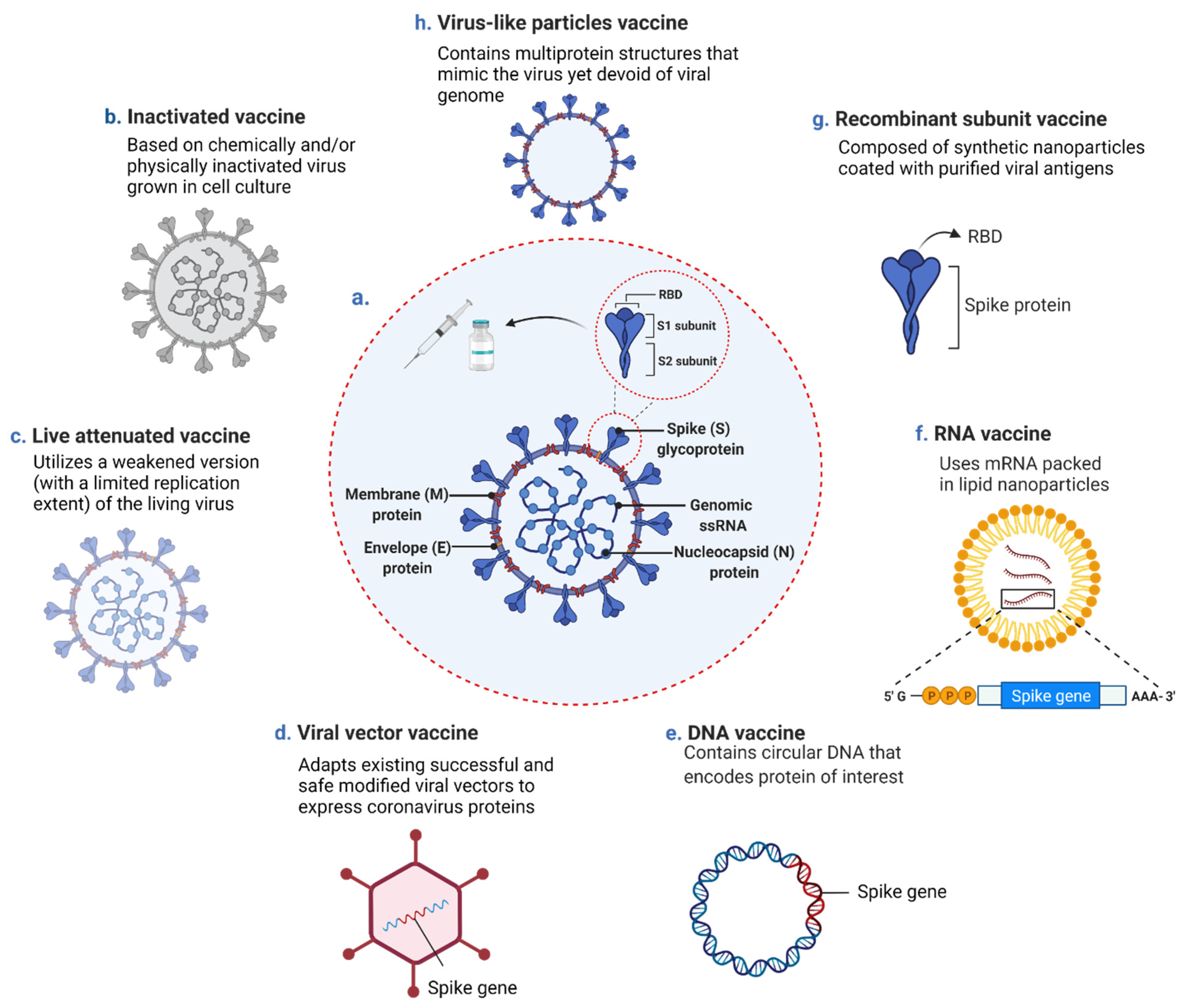
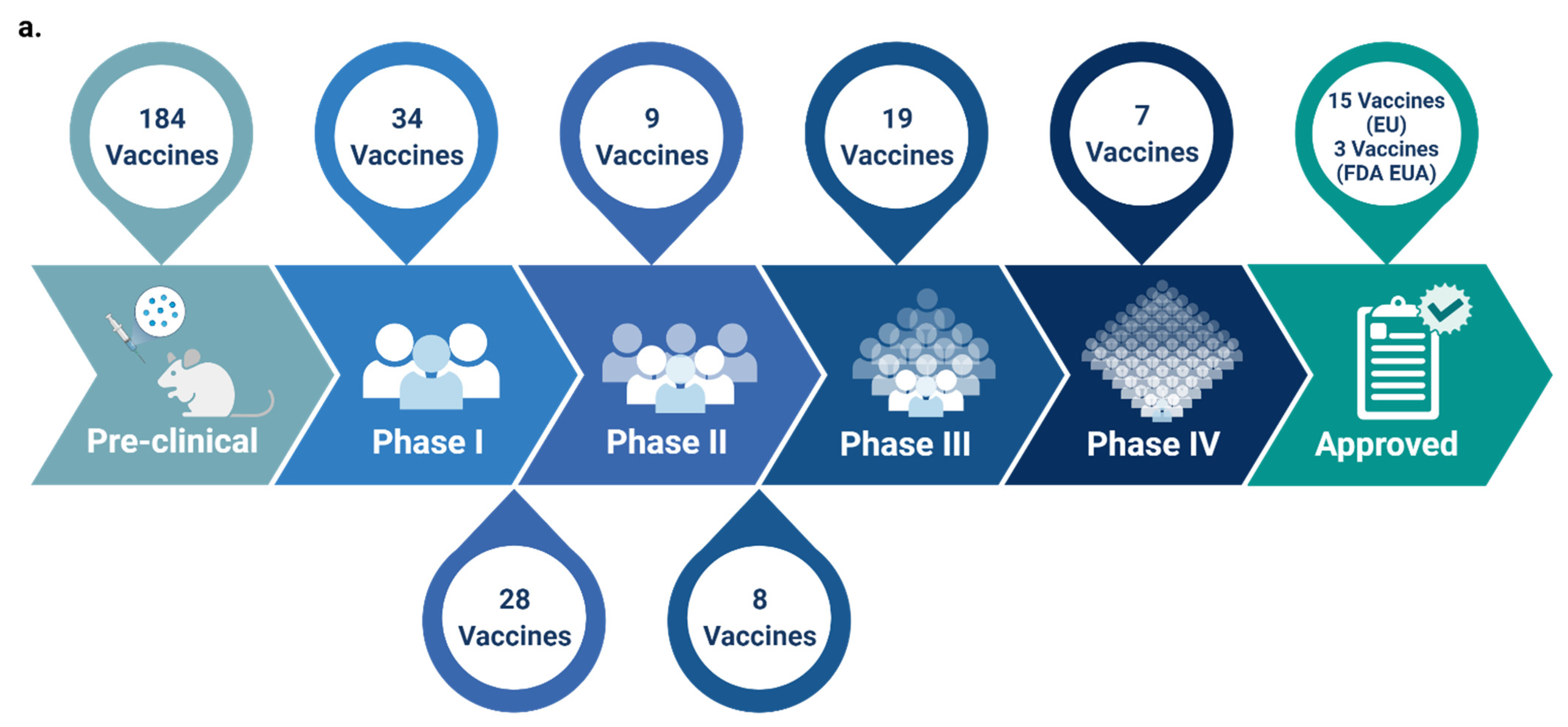

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Jighefee, H.T.; Najjar, H.; Ahmed, M.N.; Qush, A.; Awwad, S.; Kamareddine, L. COVID-19 Vaccine Platforms: Challenges and Safety Contemplations. Vaccines 2021, 9, 1196. https://doi.org/10.3390/vaccines9101196
Al-Jighefee HT, Najjar H, Ahmed MN, Qush A, Awwad S, Kamareddine L. COVID-19 Vaccine Platforms: Challenges and Safety Contemplations. Vaccines. 2021; 9(10):1196. https://doi.org/10.3390/vaccines9101196
Chicago/Turabian StyleAl-Jighefee, Hadeel T., Hoda Najjar, Muna Nizar Ahmed, Abeer Qush, Sara Awwad, and Layla Kamareddine. 2021. "COVID-19 Vaccine Platforms: Challenges and Safety Contemplations" Vaccines 9, no. 10: 1196. https://doi.org/10.3390/vaccines9101196






