Riboflavin as a Mucosal Adjuvant for Nasal Influenza Vaccine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statements
2.2. Experimental Animals and Materials
2.3. Preparation of H1N1 WIV
2.4. Immunogenicity Study
2.5. Virus Challenge
2.6. Isolation and Culture of DCs
2.7. Phenotype and Migration Assay of DCs
2.8. Cytokine Assay of DCs
2.9. Allogenic Mixed Lymphocyte Reaction (MLR) Assay
2.10. Western Blot
2.11. Statistical Analysis
3. Results
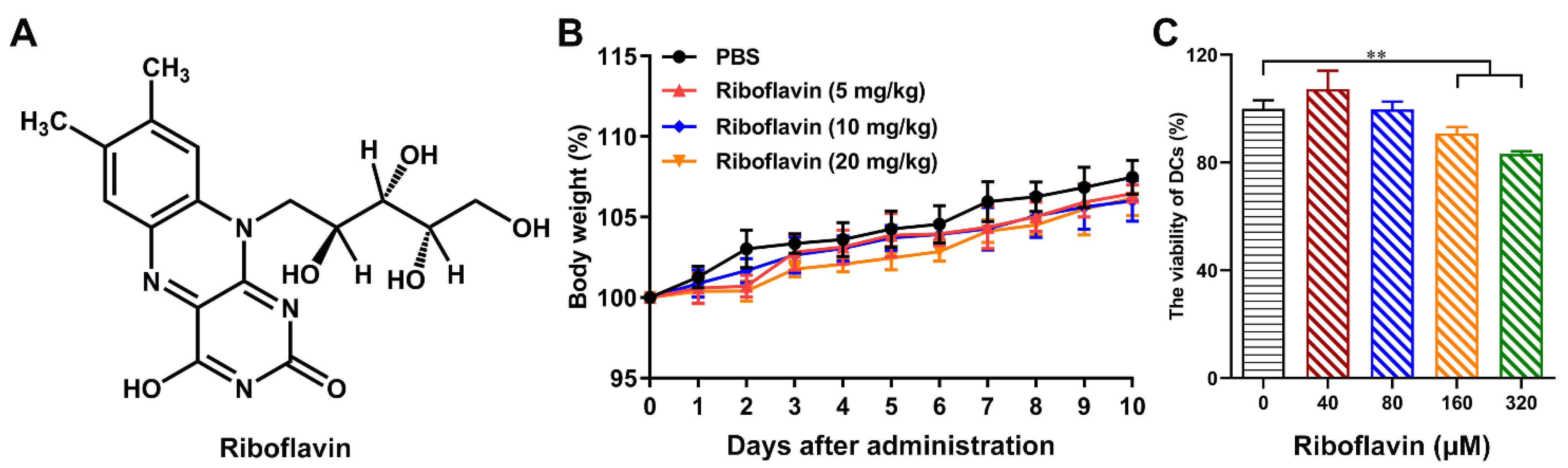
3.1. Riboflavin Enabled Inactivated Influenza Vaccine to Trigger the Local Mucosal and Systemic Immune Responses
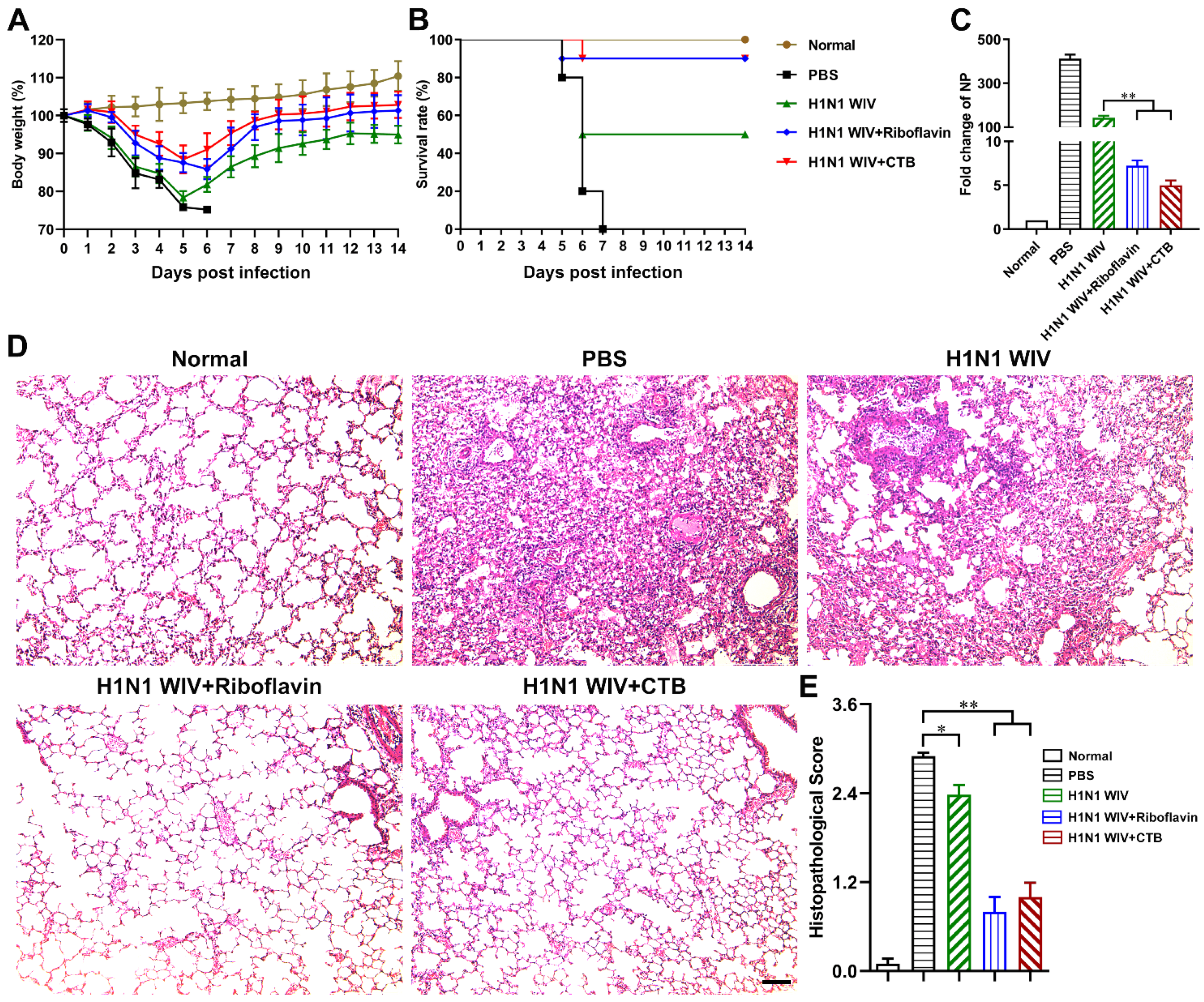
3.2. Riboflavin Strongly Improved the Protection Levels of Influenza Vaccine against H1N1 Influenza Virus Attack
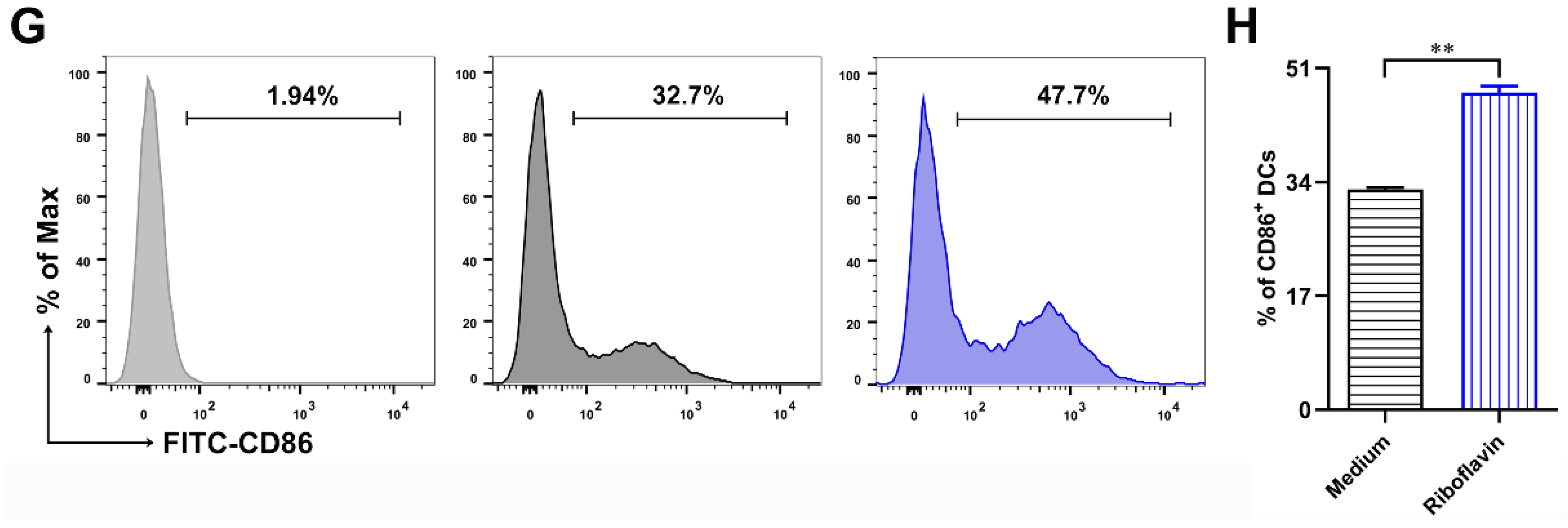
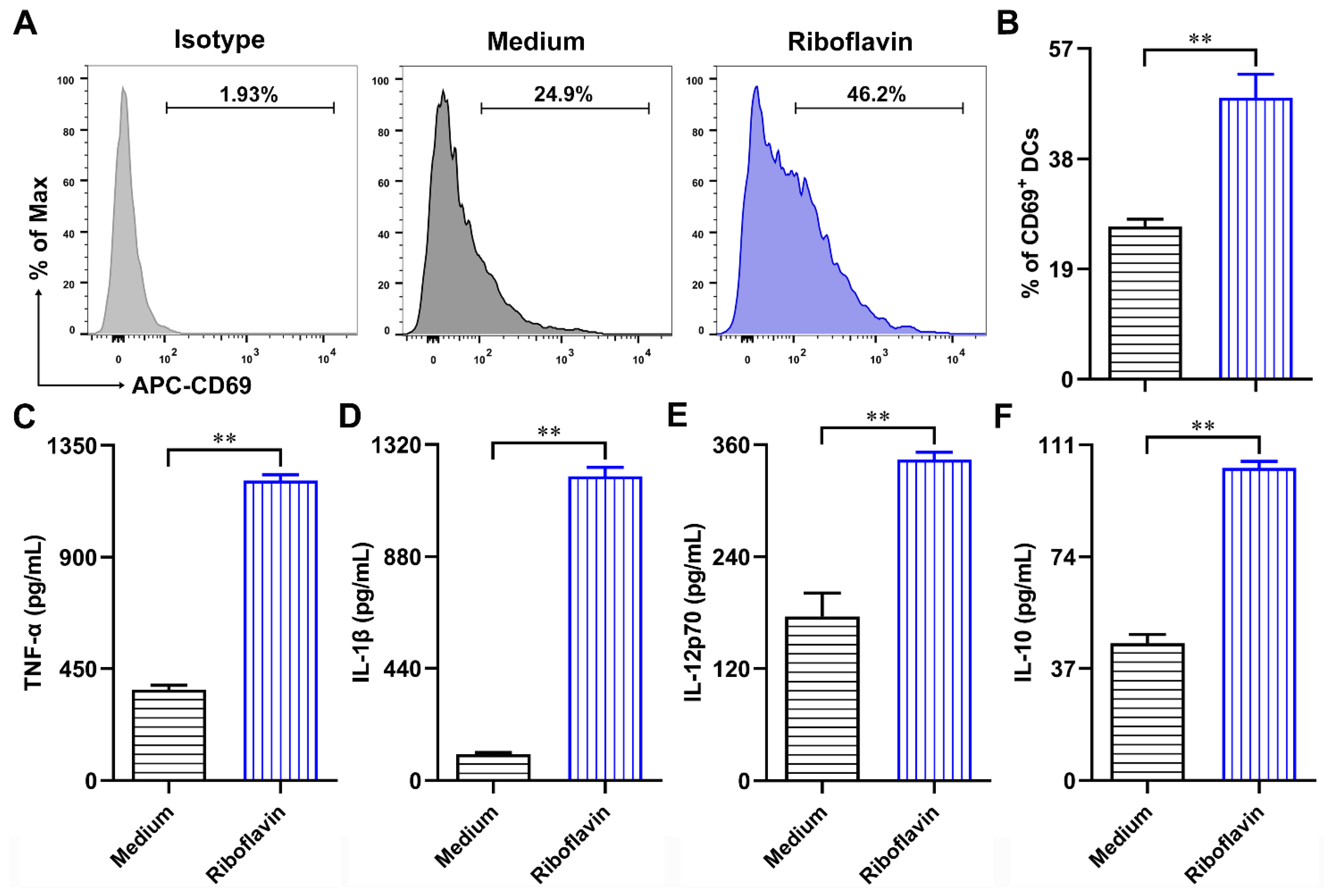
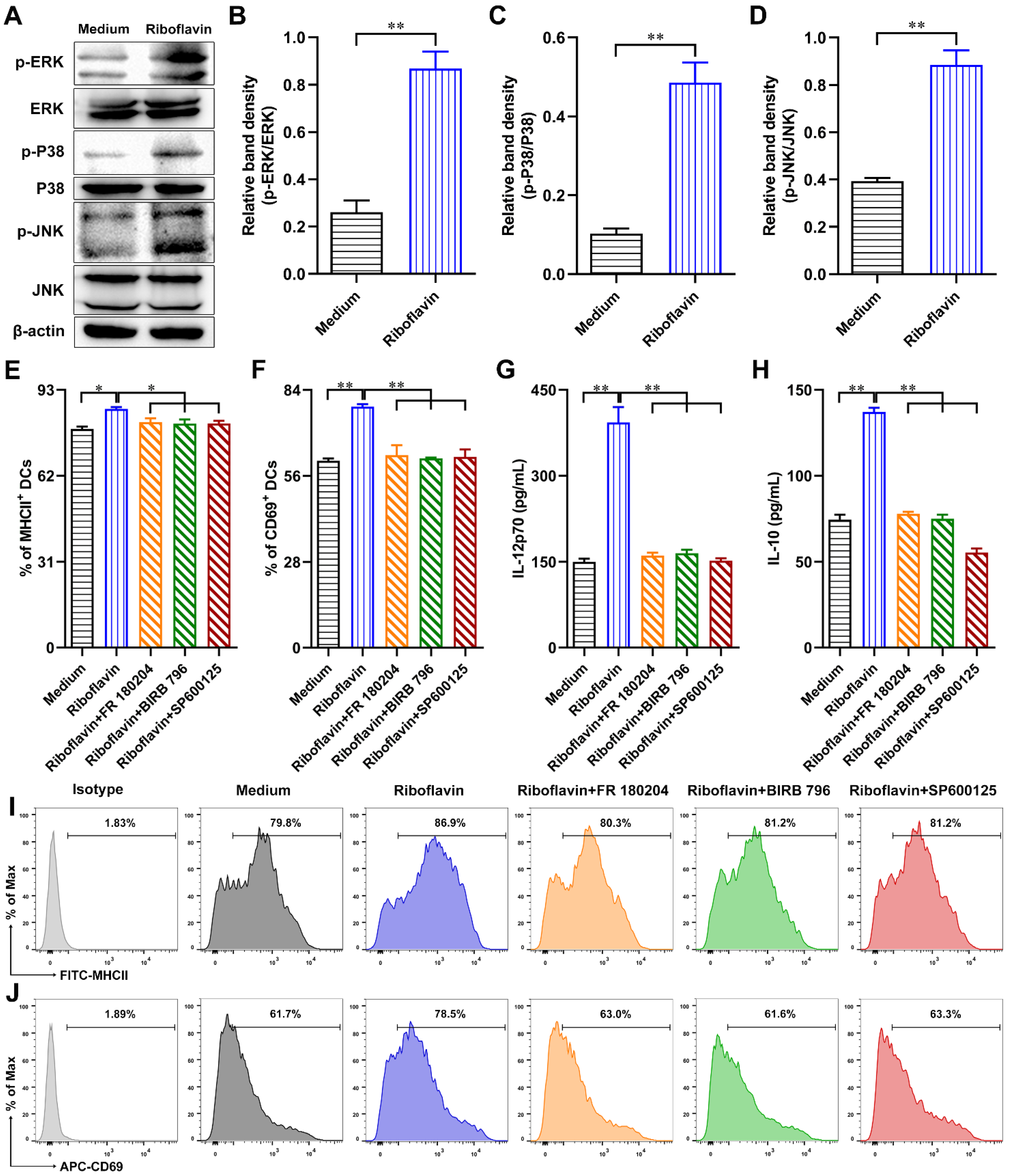
3.3. Riboflavin Promoted the Phenotypic Maturation of DCs
3.4. Riboflavin Enhanced the Cytokine Production of DCs
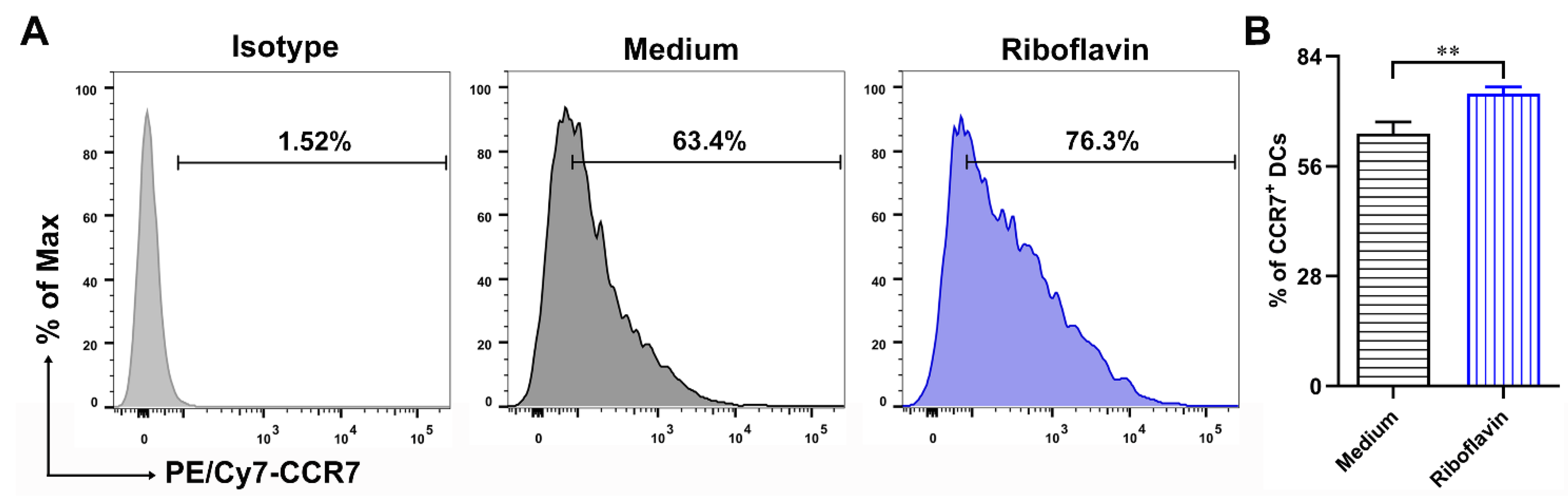
3.5. Riboflavin Improved the Migration Marker CCR7 Expression of DCs
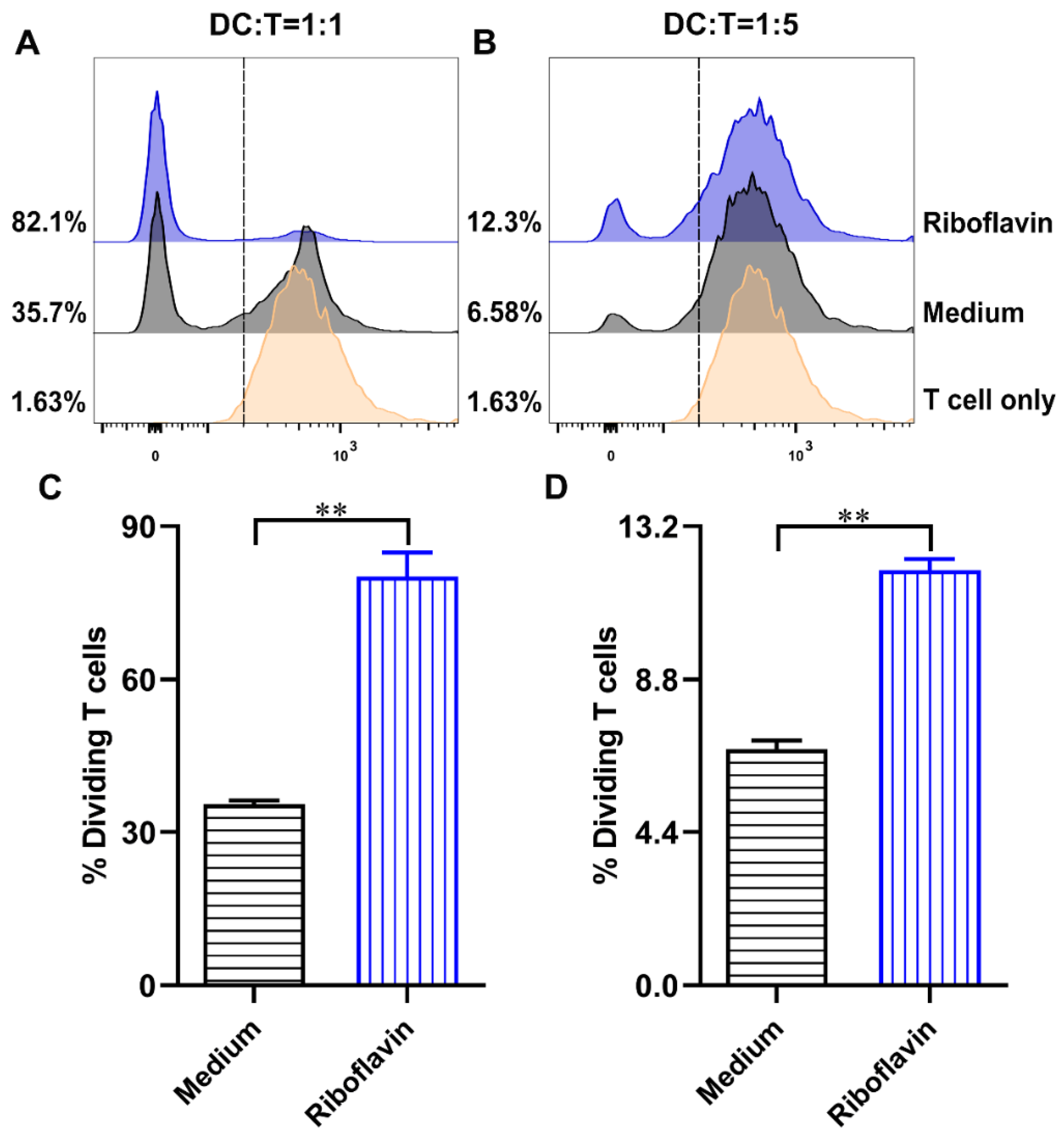
3.6. Riboflavin-Induced DCs Improved the Allostimulatory Capacity on CD4+ T Cells
3.7. Riboflavin Promoted DC Maturation via Activation of the MAPK Pathway
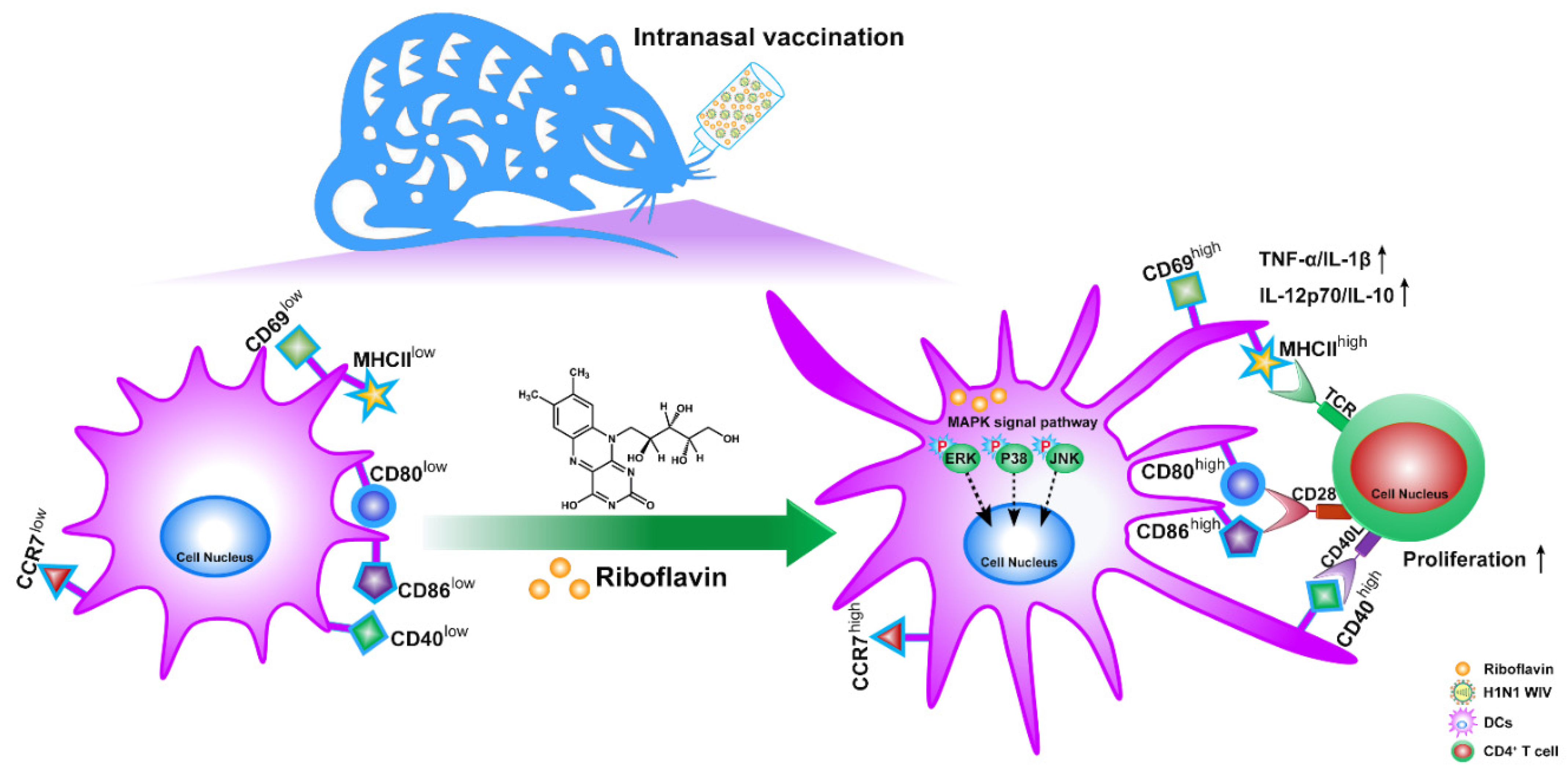
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peteranderl, C.; Herold, S.; Schmoldt, C. Human Influenza Virus Infections. Semin. Respir. Crit. Care Med. 2016, 37, 487–500. [Google Scholar] [CrossRef]
- Jester, B.; Uyeki, T.M.; Jernigan, D.B.; Tumpey, T.M. Historical and clinical aspects of the 1918 H1N1 pandemic in the United States. Virology 2019, 527, 32–37. [Google Scholar] [CrossRef]
- Wiley, S.K. The 2009 influenza pandemic: 10 years later. Nursing 2020, 50, 17–20. [Google Scholar] [CrossRef]
- Iwasaki, A.; Pillai, P.S. Innate immunity to influenza virus infection. Nat. Rev. Immunol. 2014, 14, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Ma, S.; Miao, X.; Tang, Y.; Huangfu, D.; Wang, J.; Jiang, J.; Xu, N.; Yin, Y.; Chen, S.; et al. Mucosal Vaccination for Influenza Protection Enhanced by Catalytic Immune-Adjuvant. Adv. Sci. 2020, 7, 2000771. [Google Scholar] [CrossRef]
- Kraehenbuhl, J.P.; Neutra, M.R. Mucosal vaccines: Where do we stand? Curr. Top. Med. Chem. 2013, 13, 2609–2628. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.B.; Czerkinsky, C.; Holmgren, J. Mucosally induced immunological tolerance, regulatory T cells and the adjuvant effect by cholera toxin B subunit. Scand. J. Immunol. 2010, 71, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Yin, Y.; Yu, Q.; Huang, L.; Wang, X.; Lin, J.; Yang, Q. CpG Oligodeoxynucleotides Facilitate Delivery of Whole Inactivated H9N2 Influenza Virus via Transepithelial Dendrites of Dendritic Cells in Nasal Mucosa. J. Virol. 2015, 89, 5904–5918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suwannasom, N.; Kao, I.; Pruss, A.; Georgieva, R.; Baumler, H. Riboflavin: The Health Benefits of a Forgotten Natural Vitamin. Int. J. Mol. Sci. 2020, 21, 950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashoori, M.; Saedisomeolia, A. Riboflavin (vitamin B(2)) and oxidative stress: A review. Br. J. Nutr. 2014, 111, 1985–1991. [Google Scholar] [CrossRef] [Green Version]
- Beztsinna, N.; Sole, M.; Taib, N.; Bestel, I. Bioengineered riboflavin in nanotechnology. Biomaterials 2016, 80, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Mosegaard, S.; Dipace, G.; Bross, P.; Carlsen, J.; Gregersen, N.; Olsen, R.K.J. Riboflavin Deficiency-Implications for General Human Health and Inborn Errors of Metabolism. Int. J. Mol. Sci. 2020, 21, 3847. [Google Scholar] [CrossRef] [PubMed]
- Casadey, R.; Challier, C.; Altamirano, M.; Spesia, M.B.; Criado, S. Antioxidant and antimicrobial properties of tyrosol and derivative-compounds in the presence of vitamin B2. Assays of synergistic antioxidant effect with commercial food additives. Food Chem. 2021, 335. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.T.; Zempleni, J. Riboflavin. Adv. Nutr. 2016, 7, 973–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, T.; Park, E.Y. Riboflavin production by Ashbya gossypii. Biotechnol. Lett. 2012, 34, 611–618. [Google Scholar] [CrossRef]
- Revuelta, J.L.; Ledesma-Amaro, R.; Lozano-Martinez, P.; Diaz-Fernandez, D.; Buey, R.M.; Jimenez, A. Bioproduction of riboflavin: A bright yellow history. J. Ind. Microbiol. Biotechnol. 2017, 44, 659–665. [Google Scholar] [CrossRef]
- Geeraedts, F.; Goutagny, N.; Hornung, V.; Severa, M.; de Haan, A.; Pool, J.; Wilschut, J.; Fitzgerald, K.A.; Huckriede, A. Superior immunogenicity of inactivated whole virus H5N1 influenza vaccine is primarily controlled by Toll-like receptor signalling. PLoS Pathog. 2008, 4, e1000138. [Google Scholar] [CrossRef]
- Qin, T.; Yin, Y.; Huang, L.; Yu, Q.; Yang, Q. H9N2 influenza whole inactivated virus combined with polyethyleneimine strongly enhances mucosal and systemic immunity after intranasal immunization in mice. Clin. Vaccine Immunol. 2015, 22, 421–429. [Google Scholar] [CrossRef]
- Gauger, P.C.; Vincent, A.L.; Loving, C.L.; Lager, K.M.; Janke, B.H.; Kehrli, M.E., Jr.; Roth, J.A. Enhanced pneumonia and disease in pigs vaccinated with an inactivated human-like (delta-cluster) H1N2 vaccine and challenged with pandemic 2009 H1N1 influenza virus. Vaccine 2011, 29, 2712–2719. [Google Scholar] [CrossRef]
- Yin, Y.; Qin, T.; Wang, X.; Lin, J.; Yu, Q.; Yang, Q. CpG DNA assists the whole inactivated H9N2 influenza virus in crossing the intestinal epithelial barriers via transepithelial uptake of dendritic cell dendrites. Mucosal. Immunol. 2015, 8, 799–814. [Google Scholar] [CrossRef] [Green Version]
- Worbs, T.; Hammerschmidt, S.I.; Forster, R. Dendritic cell migration in health and disease. Nat. Rev. Immunol. 2017, 17, 30–48. [Google Scholar] [CrossRef] [PubMed]
- Maassen, C.B.M.; Boersma, W.J.A.; van Holten-Neelen, C.; Claassen, E.; Laman, J.D. Growth phase of orally administered Lactobacillus strains differentially affects IgG1/IgG2a ratio for soluble antigens: Implications for vaccine development. Vaccine 2003, 21, 2751–2757. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Xu, N.; Shi, Y.; Zhou, B.; Sun, D.; Ma, B.; Xu, Z.; Yang, J.; Li, C. Astaxanthin Protects Dendritic Cells from Lipopolysaccharide-Induced Immune Dysfunction. Mar. Drugs 2021, 19, 346. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, J.; Adamsson, J.; Anjuere, F.; Clemens, J.; Czerkinsky, C.; Eriksson, K.; Flach, C.F.; George-Chandy, A.; Harandi, A.M.; Lebens, M.; et al. Mucosal adjuvants and anti-infection and anti-immunopathology vaccines based on cholera toxin, cholera toxin B subunit and CpG DNA. Immunol. Lett. 2005, 97, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Stahmann, K.P.; Revuelta, J.L.; Seulberger, H. Three biotechnical processes using Ashbya gossypii, Candida famata, or Bacillus subtilis compete with chemical riboflavin production. Appl. Microbiol. Biotechnol. 2000, 53, 509–516. [Google Scholar] [CrossRef]
- De Sousa-Pereira, P.; Woof, J.M. IgA: Structure, Function, and Developability. Antibodies 2019, 8, 57. [Google Scholar] [CrossRef] [Green Version]
- Nazeri, S.; Zakeri, S.; Mehrizi, A.A.; Sardari, S.; Djadid, N.D. Measuring of IgG2c isotype instead of IgG2a in immunized C57BL/6 mice with Plasmodium vivax TRAP as a subunit vaccine candidate in order to correct interpretation of Th1 versus Th2 immune response. Exp. Parasitol. 2020, 216, 107944. [Google Scholar] [CrossRef]
- Yang, Y.; Dong, M.; Hao, X.; Qin, A.; Shang, S. Revisiting cellular immune response to oncogenic Marek’s disease virus: The rising of avian T-cell immunity. Cell. Mol. Life Sci. 2020, 77, 3103–3116. [Google Scholar] [CrossRef] [Green Version]
- Tiberio, L.; Del Prete, A.; Schioppa, T.; Sozio, F.; Bosisio, D.; Sozzani, S. Chemokine and chemotactic signals in dendritic cell migration. Cell. Mol. Immunol. 2018, 15, 346–352. [Google Scholar] [CrossRef]
- Trzupek, D.; Dunstan, M.; Cutler, A.J.; Lee, M.; Godfrey, L.; Jarvis, L.; Rainbow, D.B.; Aschenbrenner, D.; Jones, J.L.; Uhlig, H.H.; et al. Discovery of CD80 and CD86 as recent activation markers on regulatory T cells by protein-RNA single-cell analysis. Genome Med. 2020, 12, 55. [Google Scholar] [CrossRef]
- Holling, T.M.; Schooten, E.; van Den Elsen, P.J. Function and regulation of MHC class II molecules in T-lymphocytes: Of mice and men. Hum. Immunol. 2004, 65, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Balan, S.; Saxena, M.; Bhardwaj, N. Dendritic cell subsets and locations. Int. Rev. Cell Mol. Biol. 2019, 348, 1–68. [Google Scholar] [CrossRef] [PubMed]
- Dudek, A.M.; Martin, S.; Garg, A.D.; Agostinis, P. Immature, Semi-Mature, and Fully Mature Dendritic Cells: Toward a DC-Cancer Cells Interface That Augments Anticancer Immunity. Front. Immunol. 2013, 4, 438. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhang, X.; Chen, K.; Cheng, Y.; Liu, S.; Xia, M.; Chen, Y.; Zhu, H.; Li, Z.; Cao, X. CCR7 Chemokine Receptor-Inducible lnc-Dpf3 Restrains Dendritic Cell Migration by Inhibiting HIF-1alpha-Mediated Glycolysis. Immunity 2019, 50, 600–615.e615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wehr, P.; Purvis, H.; Law, S.C.; Thomas, R. Dendritic cells, T cells and their interaction in rheumatoid arthritis. Clin. Exp. Immunol. 2019, 196, 12–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Himi, T.; Takano, K.; Ogasawara, N.; Go, M.; Kurose, M.; Koizumi, J.; Kamekura, R.; Kondo, A.; Ohkuni, T.; Masaki, T.; et al. Mucosal immune barrier and antigen-presenting system in human nasal epithelial cells. Adv. Otorhinolaryngol. 2011, 72, 28–30. [Google Scholar] [CrossRef] [Green Version]
- Qin, T.; Yin, Y.; Wang, X.; Liu, H.; Lin, J.; Yu, Q.; Yang, Q. Whole inactivated avian Influenza H9N2 viruses induce nasal submucosal dendritic cells to sample luminal viruses via transepithelial dendrites and trigger subsequent DC maturation. Vaccine 2015, 33, 1382–1392. [Google Scholar] [CrossRef]
- Barile, M.; Giancaspero, T.A.; Leone, P.; Galluccio, M.; Indiveri, C. Riboflavin transport and metabolism in humans. J. Inherit. Metab. Dis. 2016, 39, 545–557. [Google Scholar] [CrossRef]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [Green Version]
- Cuadrado, A.; Nebreda, A.R. Mechanisms and functions of p38 MAPK signalling. Biochem. J. 2010, 429, 403–417. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.Y.; Richardson, B.C. The MAPK signalling pathways and colorectal cancer. Lancet Oncol. 2005, 6, 322–327. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; Wang, J.; Xu, X.; Zhou, B.; Chen, S.; Qin, T.; Peng, D. Riboflavin as a Mucosal Adjuvant for Nasal Influenza Vaccine. Vaccines 2021, 9, 1296. https://doi.org/10.3390/vaccines9111296
Yin Y, Wang J, Xu X, Zhou B, Chen S, Qin T, Peng D. Riboflavin as a Mucosal Adjuvant for Nasal Influenza Vaccine. Vaccines. 2021; 9(11):1296. https://doi.org/10.3390/vaccines9111296
Chicago/Turabian StyleYin, Yinyan, Jinyuan Wang, Xing Xu, Bangyue Zhou, Sujuan Chen, Tao Qin, and Daxin Peng. 2021. "Riboflavin as a Mucosal Adjuvant for Nasal Influenza Vaccine" Vaccines 9, no. 11: 1296. https://doi.org/10.3390/vaccines9111296
APA StyleYin, Y., Wang, J., Xu, X., Zhou, B., Chen, S., Qin, T., & Peng, D. (2021). Riboflavin as a Mucosal Adjuvant for Nasal Influenza Vaccine. Vaccines, 9(11), 1296. https://doi.org/10.3390/vaccines9111296






