Complicated Long Term Vaccine Induced Thrombotic Immune Thrombocytopenia—A Case Report
Abstract
:1. Introduction
2. Case Report
3. Brain Imaging
4. Laboratory Investigations
4.1. Anti-PF4/Polyanion Antibody Testing
4.2. SARS-CoV-2 Antibody Testing
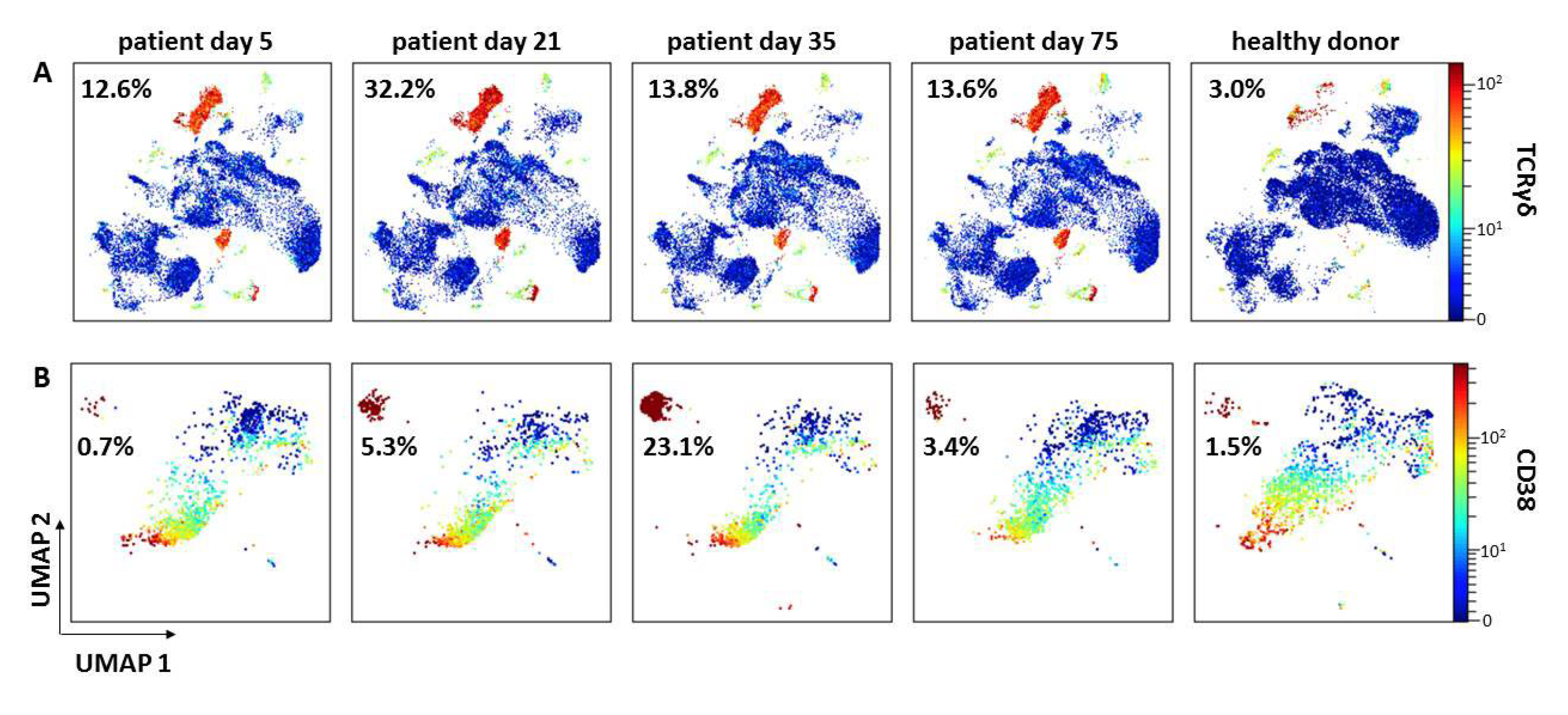
4.3. Immunophenotyping
5. Forensic Obduction
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef] [PubMed]
- Muir, K.L.; Kallam, A.; Koepsell, S.A.; Gundabolu, K. Thrombotic Thrombocytopenia after Ad26.COV2.S Vaccination. N. Engl. J. Med. 2021, 384, 1964–1965. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.T.; Black, S. Updated Proposed Brighton Collaboration Process for Developing a Standard case Definition for Study of New Clinical Syndrome X, as Applied to Thrombosis with Thrombocytopenia Syndrome (TTS). Available online: https://brightoncollaboration.us/wp-content/uploads/2021/05/TTS-Interim-Case-Definition-v10.16.3-May-23-2021.pdf (accessed on 1 August 2021).
- Scully, M.; Singh, D.; Lown, R.; Poles, A.; Solomon, T.; Levi, M.; Goldblatt, D.; Kotoucek, P.; Thomas, W.; Lester, W. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Schultz, N.H.; Sorvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.H.; Skattor, T.H.; Tjonnfjord, G.E.; et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2124–2130. [Google Scholar] [CrossRef]
- Perry, R.J.; Tamborska, A.; Singh, B.; Craven, B.; Marigold, R.; Arthur-Farraj, P.; Yeo, J.M.; Zhang, L.; Hassan-Smith, G.; Jones, M.; et al. Cerebral venous thrombosis after vaccination against COVID-19 in the UK: A multicentre cohort study. Lancet 2021, 398, 1147–1156. [Google Scholar] [CrossRef]
- Pavord, S.; Scully, M.; Hunt, B.J.; Lester, W.; Bagot, C.; Craven, B.; Rampotas, A.; Ambler, G.; Makris, M. Clinical Features of Vaccine-Induced Immune Thrombocytopenia and Thrombosis. N. Engl. J. Med. 2021, 385, 1680–1689. [Google Scholar] [CrossRef]
- Salih, F.; Schonborn, L.; Kohler, S.; Franke, C.; Mockel, M.; Dorner, T.; Bauknecht, H.C.; Pille, C.; Graw, J.A.; Alonso, A.; et al. Vaccine-Induced Thrombocytopenia with Severe Headache. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef]
- Oldenburg, J.; Klamroth, R.; Langer, F.; Albisetti, M.; von Auer, C.; Ay, C.; Korte, W.; Scharf, R.E.; Potzsch, B.; Greinacher, A. Diagnosis and Management of Vaccine-Related Thrombosis following AstraZeneca COVID-19 Vaccination: Guidance Statement from the GTH. Hamostaseologie 2021, 41, 184–189. [Google Scholar] [CrossRef]
- Patriquin, C.J.; Laroche, V.; Selby, R.; Pendergrast, J.; Barth, D.; Cote, B.; Gagnon, N.; Roberge, G.; Carrier, M.; Castellucci, L.A.; et al. Therapeutic Plasma Exchange in Vaccine-Induced Immune Thrombotic Thrombocytopenia. N. Engl. J. Med. 2021, 385, 857–859. [Google Scholar] [CrossRef]
- Greinacher, A.; Selleng, K.; Palankar, R.; Wesche, J.; Handtke, S.; Wolff, M.; Aurich, K.; Lalk, M.; Methling, K.; Volker, U.; et al. Insights in ChAdOx1 nCov-19 Vaccine-induced Immune Thrombotic Thrombocytopenia (VITT). Blood 2021. [Google Scholar] [CrossRef]
- Greinacher, A.; Selleng, K.; Mayerle, J.; Palankar, R.; Wesche, J.; Reiche, S.; Aebischer, A.; Warkentin, T.E.; Muenchhoff, M.; Hellmuth, J.C.; et al. Anti-platelet factor 4 antibodies causing VITT do not cross-react with SARS-CoV-2 spike protein. Blood 2021, 138, 1269–1277. [Google Scholar] [CrossRef]
- Schonborn, L.; Thiele, T.; Kaderali, L.; Greinacher, A. Decline in Pathogenic Antibodies over Time in VITT. N. Engl. J. Med. 2021, 385, 1815–1816. [Google Scholar] [CrossRef]
- Juhl, D.; Eichler, P.; Lubenow, N.; Strobel, U.; Wessel, A.; Greinacher, A. Incidence and clinical significance of anti-PF4/heparin antibodies of the IgG, IgM, and IgA class in 755 consecutive patient samples referred for diagnostic testing for heparin-induced thrombocytopenia. Eur. J. Haematol. 2006, 76, 420–426. [Google Scholar] [CrossRef]
- Kreimann, M.; Brandt, S.; Krauel, K.; Block, S.; Helm, C.A.; Weitschies, W.; Greinacher, A.; Delcea, M. Binding of anti-platelet factor 4/heparin antibodies depends on the thermodynamics of conformational changes in platelet factor 4. Blood 2014, 124, 2442–2449. [Google Scholar] [CrossRef] [Green Version]
- Handtke, S.; Wolff, M.; Zaninetti, C.; Wesche, J.; Schonborn, L.; Aurich, K.; Ulm, L.; Hubner, N.O.; Becker, K.; Thiele, T.; et al. A flow cytometric assay to detect platelet-activating antibodies in VITT after ChAdOx1 nCov-19 vaccination. Blood 2021, 137, 3656–3659. [Google Scholar] [CrossRef]
- Ainsworth, M.; Andersson, M.; Auckland, K.; Baillie, J.K.; Barnes, E.; Beer, S.; Beveridge, A.; Bibi, S.; Blackwell, L.; Borak, M.; et al. Performance characteristics of five immunoassays for SARS-CoV-2: A head-to-head benchmark comparison. Lancet Infect. Dis. 2020, 20, 1390–1400. [Google Scholar] [CrossRef]
- Bendall, S.C.; Simonds, E.F.; Qiu, P.; El-ad, D.A.; Krutzik, P.O.; Finck, R.; Bruggner, R.V.; Melamed, R.; Trejo, A.; Ornatsky, O.I.; et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 2011, 332, 687–696. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.E. Bilateral Adrenal Hemorrhage Secondary to Heparin-Induced Thrombocytopenia. Am. J. Med. 2020, 133, e376–e377. [Google Scholar] [CrossRef]
- Sanchez van Kammen, M.; Aguiar de Sousa, D.; Poli, S.; Cordonnier, C.; Heldner, M.R.; van de Munckhof, A.; Krzywicka, K.; van Haaps, T.; Ciccone, A.; Middeldorp, S.; et al. Characteristics and Outcomes of Patients With Cerebral Venous Sinus Thrombosis in SARS-CoV-2 Vaccine-Induced Immune Thrombotic Thrombocytopenia. JAMA Neurol. 2021, 78, 1314–1323. [Google Scholar] [CrossRef]
- Kowoll, C.M.; Kaminski, J.; Weiss, V.; Bosel, J.; Dietrich, W.; Juttler, E.; Flechsenhar, J.; Guenther, A.; Huttner, H.B.; Niesen, W.D.; et al. Severe Cerebral Venous and Sinus Thrombosis: Clinical Course, Imaging Correlates, and Prognosis. Neurocrit. Care 2016, 25, 392–399. [Google Scholar] [CrossRef]
- Greinacher, A.; Selleng, K.; Warkentin, T.E. Autoimmune heparin-induced thrombocytopenia. J. Thromb. Haemost. 2017, 15, 2099–2114. [Google Scholar] [CrossRef] [Green Version]
- Dugas, M.; Grote-Westrick, T.; Vollenberg, R.; Lorentzen, E.; Brix, T.; Schmidt, H.; Tepasse, P.R.; Kuhn, J. Less severe course of COVID-19 is associated with elevated levels of antibodies against seasonal human coronaviruses OC43 and HKU1 (HCoV OC43, HCoV HKU1). Int. J. Infect. Dis. 2021, 105, 304–306. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Shinagawa, T.; Kobata, H.; Nakagawa, H. Immunity against seasonal human coronavirus OC43 mitigates fatal deterioration of COVID-19. Int. J. Infect. Dis. 2021, 109, 261–268. [Google Scholar] [CrossRef]
- Passariello, M.; Vetrei, C.; Amato, F.; De Lorenzo, C. Interactions of Spike-RBD of SARS-CoV-2 and Platelet Factor 4: New Insights in the Etiopathogenesis of Thrombosis. Int. J. Mol. Sci. 2021, 22, 8562. [Google Scholar] [CrossRef] [PubMed]
- Wanke-Jellinek, L.; Keegan, J.W.; Dolan, J.W.; Lederer, J.A. Characterization of lung infection-induced TCRgammadelta T cell phenotypes by CyTOF mass cytometry. J. Leukoc. Biol. 2016, 99, 483–493. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Günther, A.; Brämer, D.; Pletz, M.W.; Kamradt, T.; Baumgart, S.; Mayer, T.E.; Baier, M.; Autsch, A.; Mawrin, C.; Schönborn, L.; et al. Complicated Long Term Vaccine Induced Thrombotic Immune Thrombocytopenia—A Case Report. Vaccines 2021, 9, 1344. https://doi.org/10.3390/vaccines9111344
Günther A, Brämer D, Pletz MW, Kamradt T, Baumgart S, Mayer TE, Baier M, Autsch A, Mawrin C, Schönborn L, et al. Complicated Long Term Vaccine Induced Thrombotic Immune Thrombocytopenia—A Case Report. Vaccines. 2021; 9(11):1344. https://doi.org/10.3390/vaccines9111344
Chicago/Turabian StyleGünther, Albrecht, Dirk Brämer, Mathias W. Pletz, Thomas Kamradt, Sabine Baumgart, Thomas E. Mayer, Michael Baier, Angelina Autsch, Christian Mawrin, Linda Schönborn, and et al. 2021. "Complicated Long Term Vaccine Induced Thrombotic Immune Thrombocytopenia—A Case Report" Vaccines 9, no. 11: 1344. https://doi.org/10.3390/vaccines9111344





