Safety and Immunogenicity of M2-Deficient, Single Replication, Live Influenza Vaccine (M2SR) in Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Vaccine and Placebo
2.2. Study Population, Design and Objectives
2.3. Safety Evaluation
2.4. Influenza Antibody Assays
2.5. Cellular Immune Response Assays
2.6. Statistical Methods
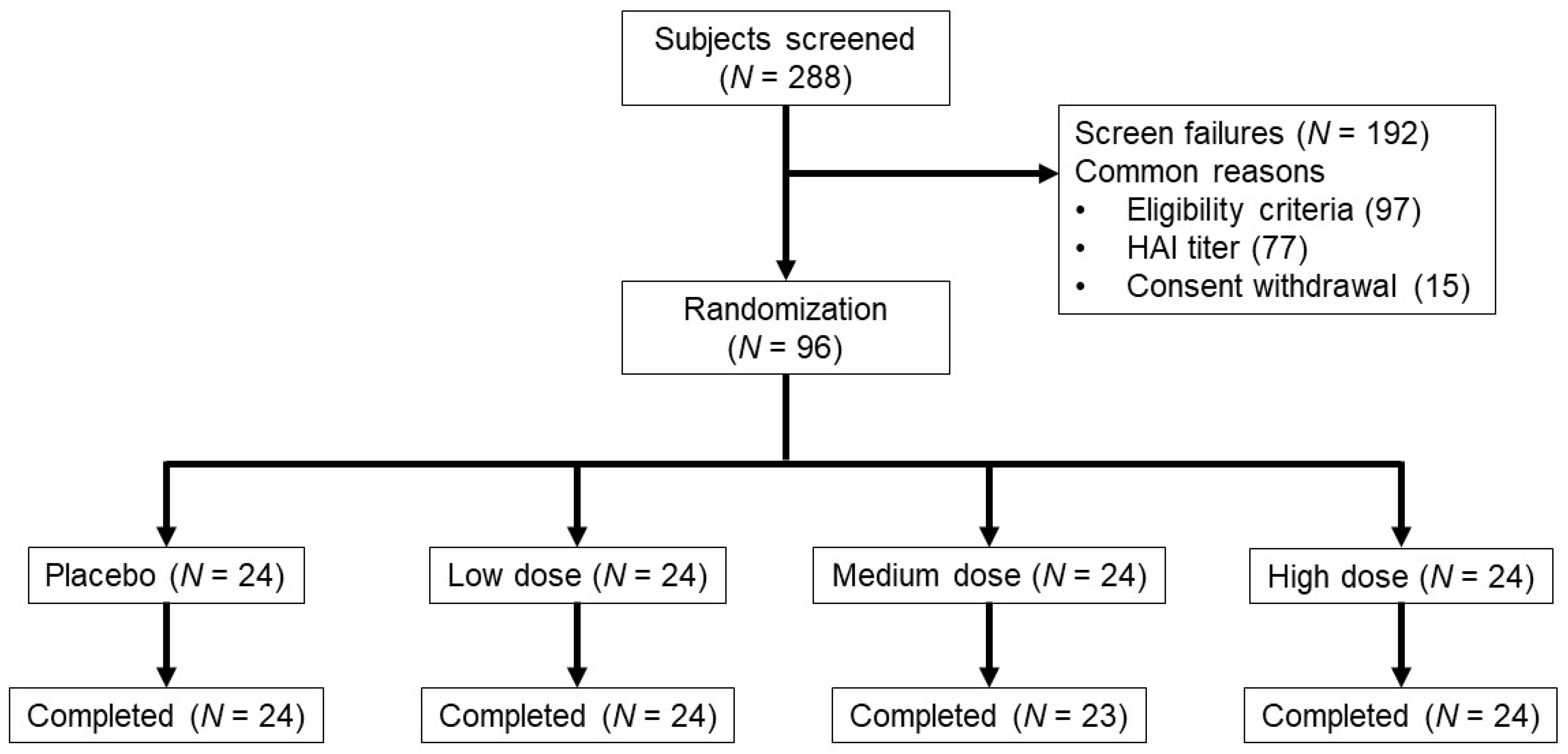
3. Results
3.1. Demographics
3.2. Vaccine Safety
3.3. Vaccine Virus Shedding
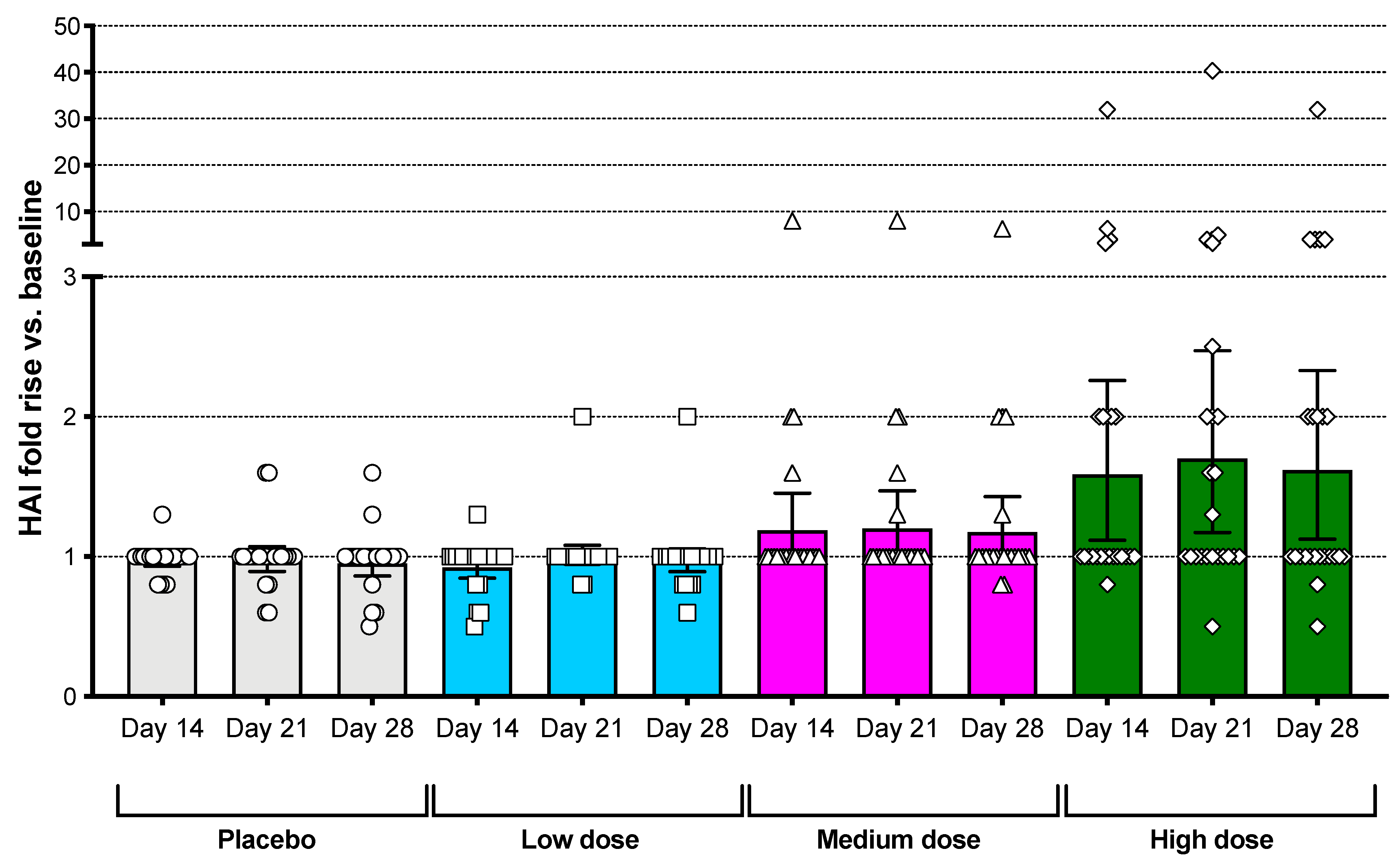
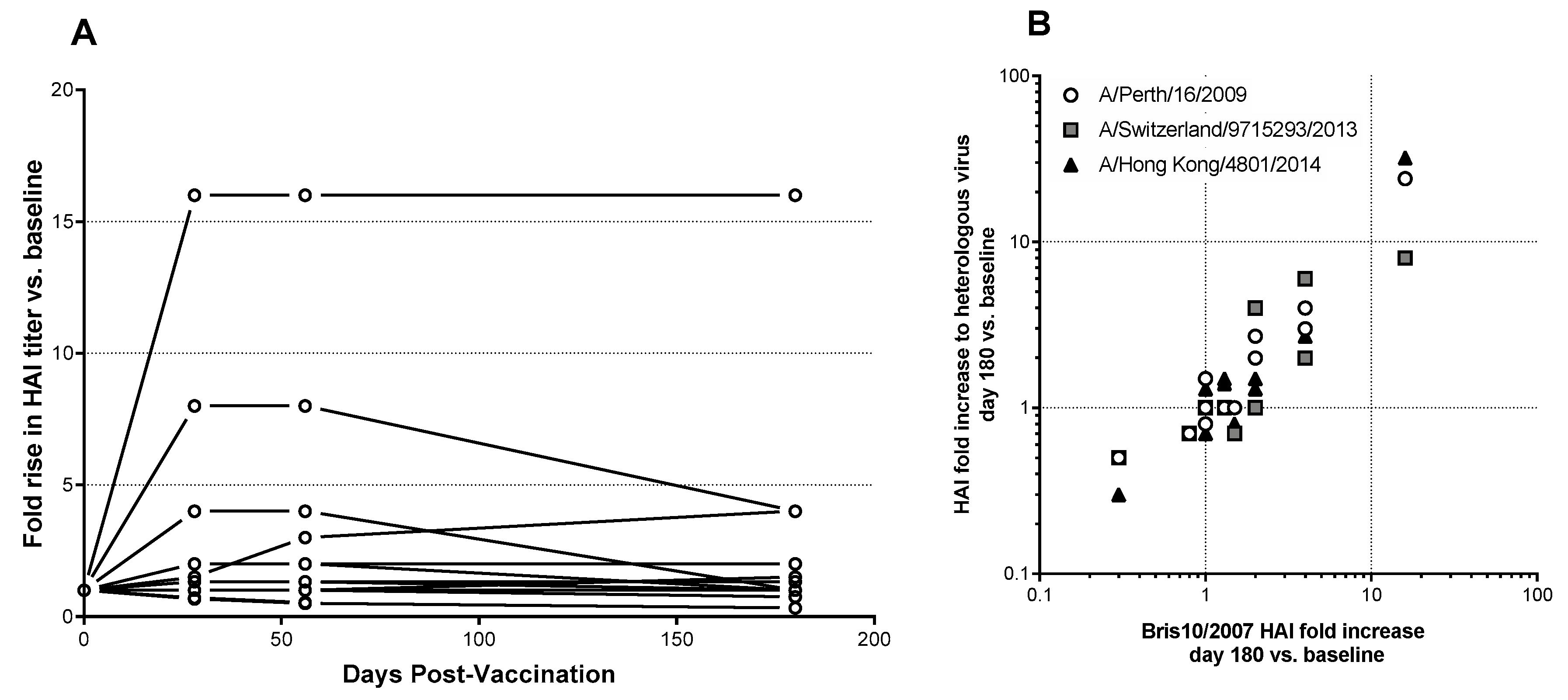
3.4. Serum Antibody Responses
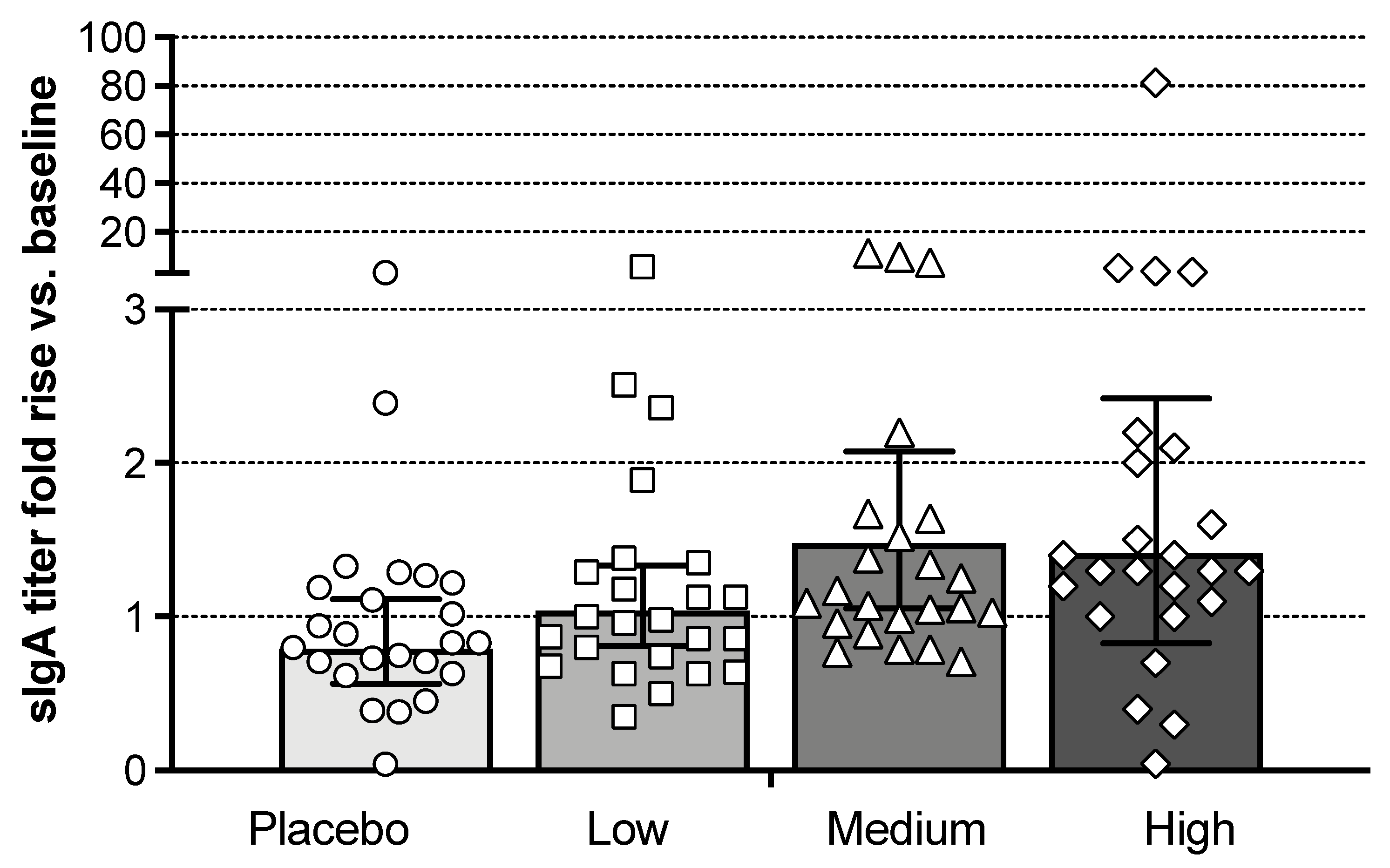
3.5. Mucosal Antibody Responses
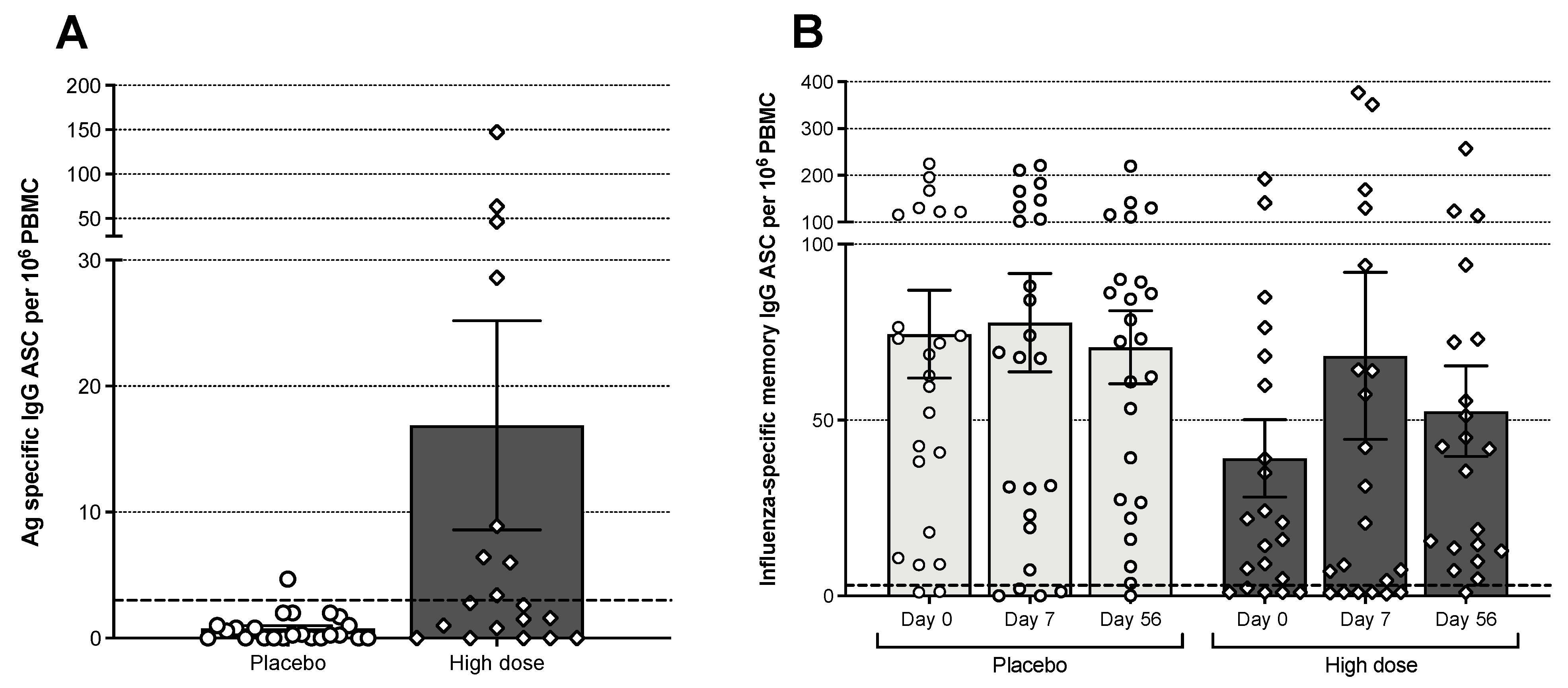
3.6. B Cell Responses
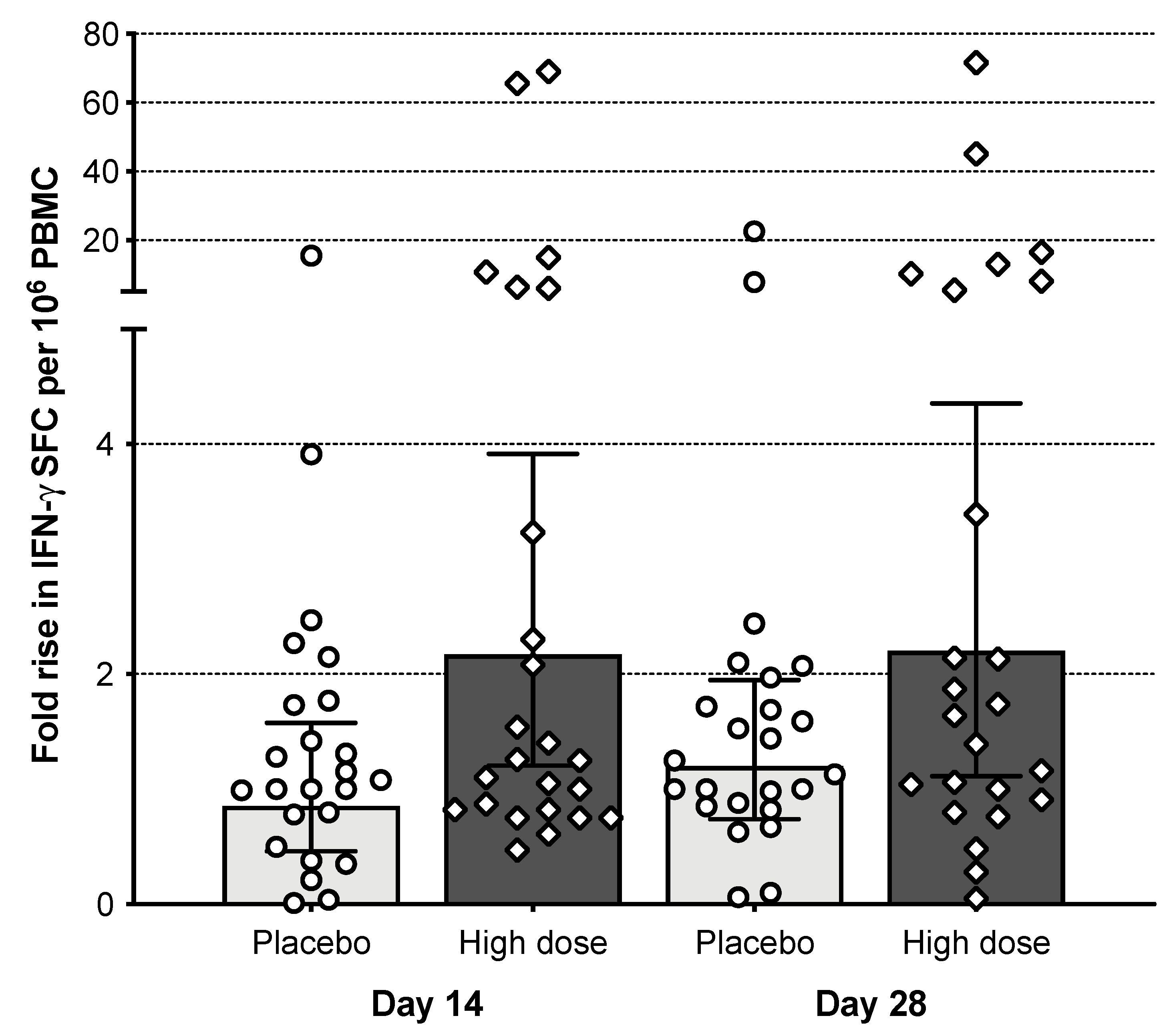
3.7. T Cell Responses
3.8. Cumulative Immune Response of M2SR (108 TCID50)
| SERUM | NASAL SWAB | PBMC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Subject No. | Baseline HAI | HAI | MNT | IgG | IgA | sIgA | IgG Plasmablast | IgG ASC (Day 7, 56) | IFN-γ |
| 1 | 5 | + | - | - | - | - | - | + | + |
| 2 | 5 | - | + | - | - | - | + | - | - |
| 3 | 5 | - | - | - | - | - | - | - | - |
| 4 | 5 | - | - | - | - | - | - | - | + |
| 5 | 5 | - | - | - | - | - | NT | - | + |
| 6 | 5 | + | + | + | + | + | + | + | + |
| 7 | 5 | + | + | - | + | - | - | + | + |
| 8 | 5 | - | - | + | - | + | - | - | + |
| 9 | 10 | + | + | + | + | + | - | + | - |
| 10 | 20 | + | + | - | + | - | + | - | - |
| 11 | 20 | + | + | - | + | - | NT | - | + |
| 12 | 20 | + | + | - | - | - | - | + | - |
| 13 | 20 | + | - | - | + | - | NT | NT | + |
| 14 | 40 | + | - | - | + | + | + | - | - |
| 15 | 40 | + | - | - | - | - | NT | NT | + |
| 16 | 40 | - | + | - | - | - | NT | - | - |
| 17 | 80 | - | - | - | - | - | - | - | + |
| 18 | 80 | - | - | - | - | - | + | - | - |
| 19 | 80 | - | - | - | - | - | - | + | - |
| 20 | 80 | - | - | - | - | - | - | + | + |
| 21 | 80 | - | - | - | - | - | + | - | - |
| 22 | 101 | - | - | - | - | + | + | + | - |
| 23 | 160 | - | + | - | - | + | + | + | - |
| 24 | 1280 | - | - | - | - | + | - | - | - |
| Total (% Response) | M2SR | 41.7 | 37.5 | 12.5 | 29.2 | 29.2 | 47.1 | 40.9 | 45.8 |
| Placebo | 0.0 | 4.2 | 0.0 | 0.0 | 8.3 | 4.3 | 29.2 | 33.3 | |
| p value | 0.0006 | 0.0102 | 0.234 | 0.0094 | 0.1365 | 0.0059 | 0.5379 | 0.5556 | |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Disease Burden of Influenza. Available online: https://www.cdc.gov/flu/about/burden/index.html (accessed on 23 June 2021).
- Centers for Disease Control and Prevention. Past Seasons Vaccine Effectiveness Estimates. Available online: https://www.cdc.gov/flu/vaccines-work/past-seasons-estimates.html (accessed on 23 February 2021).
- Belongia, E.A.; McLean, H.Q. Influenza Vaccine Effectiveness: Defining the H3N2 Problem. Clin. Infect. Dis. 2019, 69, 1817–1823. [Google Scholar] [CrossRef]
- Pebody, R.; Andrews, N.; McMenamin, J.; Durnall, H.; Ellis, J.; Thompson, C.I.; Robertson, C.; Cottrell, S.; Smyth, B.; Zambon, M.; et al. Vaccine effectiveness of 2011/12 trivalent seasonal influenza vaccine in preventing laboratory-confirmed influenza in primary care in the United Kingdom: Evidence of waning intra-seasonal protection. Euro. Surveill. 2013, 18, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Puig-Barbera, J.; Mira-Iglesias, A.; Tortajada-Girbes, M.; Lopez-Labrador, F.X.; Librero-Lopez, J.; Diez-Domingo, J.; Carballido-Fernandez, M.; Carratala-Munuera, C.; Correcher-Medina, P.; Gil-Guillen, V.; et al. Waning protection of influenza vaccination during four influenza seasons, 2011/2012 to 2014/2015. Vaccine 2017, 35, 5799–5807. [Google Scholar] [CrossRef]
- Ferdinands, J.M.; Fry, A.M.; Reynolds, S.; Petrie, J.; Flannery, B.; Jackson, M.L.; Belongia, E.A. Intraseason waning of influenza vaccine protection: Evidence from the US Influenza Vaccine Effectiveness Network, 2011-12 through 2014-15. Clin. Infect. Dis. 2017, 64, 544–550. [Google Scholar] [CrossRef] [Green Version]
- Ray, G.T.; Lewis, N.; Klein, N.P.; Daley, M.F.; Wang, S.V.; Kulldorff, M.; Fireman, B. Intraseason Waning of Influenza Vaccine Effectiveness. Clin. Infect. Dis. 2019, 68, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F. The human antibody response to influenza A virus infection and vaccination. Nat. Rev. Immunol. 2019, 19, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Wohlbold, T.J.; Zheng, N.Y.; Huang, M.; Huang, Y.; Neu, K.E.; Lee, J.; Wan, H.; Rojas, K.T.; Kirkpatrick, E.; et al. Influenza Infection in Humans Induces Broadly Cross-Reactive and Protective Neuraminidase-Reactive Antibodies. Cell 2018, 173, 417–429 e410. [Google Scholar] [CrossRef] [Green Version]
- Kreijtz, J.H.; Fouchier, R.A.; Rimmelzwaan, G.F. Immune responses to influenza virus infection. Virus Res. 2011, 162, 19–30. [Google Scholar] [CrossRef]
- Sridhar, S. Heterosubtypic T-Cell Immunity to Influenza in Humans: Challenges for Universal T-Cell Influenza Vaccines. Front. Immunol. 2016, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayward, A.C.; Wang, L.; Goonetilleke, N.; Fragaszy, E.B.; Bermingham, A.; Copas, A.; Dukes, O.; Millett, E.R.; Nazareth, I.; Nguyen-Van-Tam, J.S.; et al. Natural T Cell-mediated Protection against Seasonal and Pandemic Influenza. Results of the Flu Watch Cohort Study. Am. J. Respir. Crit. Care. Med. 2015, 191, 1422–1431. [Google Scholar] [CrossRef]
- Belshe, R.B.; Gruber, W.C.; Mendelman, P.M.; Mehta, H.B.; Mahmood, K.; Reisinger, K.; Treanor, J.; Zangwill, K.; Hayden, F.G.; Bernstein, D.I.; et al. Correlates of immune protection induced by live, attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine. J. Infect. Dis. 2000, 181, 1133–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, T.M.; Li, C.K.; Chui, C.S.; Huang, A.K.; Perkins, M.; Liebner, J.C.; Lambkin-Williams, R.; Gilbert, A.; Oxford, J.; Nicholas, B.; et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat. Med. 2012, 18, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Gould, V.M.W.; Francis, J.N.; Anderson, K.J.; Georges, B.; Cope, A.V.; Tregoning, J.S. Nasal IgA Provides Protection against Human Influenza Challenge in Volunteers with Low Serum Influenza Antibody Titre. Front. Microbiol. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clements, M.L.; Betts, R.F.; Tierney, E.L.; Murphy, B.R. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J. Clin. Microbiol. 1986, 24, 157–160. [Google Scholar] [CrossRef] [Green Version]
- Sridhar, S.; Brokstad, K.A.; Cox, R.J. Influenza Vaccination Strategies: Comparing Inactivated and Live Attenuated Influenza Vaccines. Vaccines 2015, 3, 373–389. [Google Scholar] [CrossRef]
- Hatta, Y.; Boltz, D.; Sarawar, S.; Kawaoka, Y.; Neumann, G.; Bilsel, P. M2SR, a novel live influenza vaccine, protects mice and ferrets against highly pathogenic avian influenza. Vaccine 2017, 35, 4177–4183. [Google Scholar] [CrossRef]
- Sarawar, S.; Hatta, Y.; Watanabe, S.; Dias, P.; Neumann, G.; Kawaoka, Y.; Bilsel, P. M2SR, a novel live single replication influenza virus vaccine, provides effective heterosubtypic protection in mice. Vaccine 2016, 34, 5090–5098. [Google Scholar] [CrossRef] [Green Version]
- Hatta, Y.; Boltz, D.; Sarawar, S.; Kawaoka, Y.; Neumann, G.; Bilsel, P. Novel influenza vaccine M2SR protects against drifted H1N1 and H3N2 influenza virus challenge in ferrets with pre-existing immunity. Vaccine 2018, 36, 5097–5103. [Google Scholar] [CrossRef]
- Moser, M.J.; Hatta, Y.; Gabaglia, C.; Sanchez, A.; Dias, P.; Sarawar, S.; Kawaoka, Y.; Hatta, M.; Neumann, G.; Bilsel, P. Single-replication BM2SR vaccine provides sterilizing immunity and cross-lineage influenza B virus protection in mice. Vaccine 2019, 37, 4533–4542. [Google Scholar] [CrossRef]
- Hatta, Y.; Hatta, M.; Bilsel, P.; Neumann, G.; Kawaoka, Y. An M2 cytoplasmic tail mutant as a live attenuated influenza vaccine against pandemic (H1N1) 2009 influenza virus. Vaccine 2011, 29, 2308–2312. [Google Scholar] [CrossRef] [Green Version]
- WHO Global Influenza Surveillance Network. Manual for the Laboratory Diagnosis and Virological Surveillance Of Influenza. 2011. Available online: https://apps.who.int/iris/bitstream/handle/10665/44518/9789241548090_eng.pdf (accessed on 25 June 2021).
- Hobson, D.; Curry, R.L.; Beare, A.S.; Ward-Gardner, A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J. Hyg. 1972, 70, 767–777. [Google Scholar] [CrossRef] [Green Version]
- Hoft, D.F.; Lottenbach, K.R.; Blazevic, A.; Turan, A.; Blevins, T.P.; Pacatte, T.P.; Yu, Y.; Mitchell, M.C.; Hoft, S.G.; Belshe, R.B. Comparisons of the Humoral and Cellular Immune Responses Induced by Live Attenuated Influenza Vaccine and Inactivated Influenza Vaccine in Adults. Clin. Vaccine Immunol. 2017, 24, e00414-16. [Google Scholar] [CrossRef] [Green Version]
- Jahnmatz, M.; Kesa, G.; Netterlid, E.; Buisman, A.M.; Thorstensson, R.; Ahlborg, N. Optimization of a human IgG B-cell ELISpot assay for the analysis of vaccine-induced B-cell responses. J. Immunol. Methods 2013, 391, 50–59. [Google Scholar] [CrossRef] [Green Version]
- Pinna, D.; Corti, D.; Jarrossay, D.; Sallusto, F.; Lanzavecchia, A. Clonal dissection of the human memory B-cell repertoire following infection and vaccination. Eur. J. Immunol. 2009, 39, 1260–1270. [Google Scholar] [CrossRef]
- Schlingmann, T.R.; Shive, C.L.; Targoni, O.S.; Tary-Lehmann, M.; Lehmann, P.V. Increased per cell IFN-gamma productivity indicates recent in vivo activation of T cells. Cell Immunol. 2009, 258, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, S.; Jaimes, M.C.; Holmes, T.H.; Dekker, C.L.; Mahmood, K.; Kemble, G.W.; Arvin, A.M.; Greenberg, H.B. Comparison of the influenza virus-specific effector and memory B-cell responses to immunization of children and adults with live attenuated or inactivated influenza virus vaccines. J. Virol. 2007, 81, 215–228. [Google Scholar] [CrossRef] [Green Version]
- MedImmune. FLUMIST QUADRIVALENT Highlights of Prescribing Information. Available online: https://www.azpicentral.com/flumistquadrivalent/flumistquadrivalent.pdf (accessed on 13 March 2021).
- Islam, S.; Zhou, F.; Lartey, S.; Mohn, K.G.I.; Krammer, F.; Cox, R.J.; Brokstad, K.A. Functional immune response to influenza H1N1 in children and adults after live attenuated influenza virus vaccination. Scand. J. Immunol. 2019, 90, e12801. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Williams, C.M.; Wijesundara, D.K.; Furuya, Y. Impact of Pre-Existing Immunity to Influenza on Live-Attenuated Influenza Vaccine (LAIV) Immunogenicity. Vaccines 2020, 8, 683. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Montinaro, V.; Groppali, E.; Tenconi, R.; Semino, M.; Principi, N. Live attenuated intranasal influenza vaccine. Hum. Vaccin. Immunother. 2012, 8, 76–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrose, C.S.; Levin, M.J.; Belshe, R.B. The relative efficacy of trivalent live attenuated and inactivated influenza vaccines in children and adults. Influenza Other Respir. Viruses 2011, 5, 67–75. [Google Scholar] [CrossRef] [PubMed]
- McElhaney, J.E.; Xie, D.; Hager, W.D.; Barry, M.B.; Wang, Y.; Kleppinger, A.; Ewen, C.; Kane, K.P.; Bleackley, R.C. T cell responses are better correlates of vaccine protection in the elderly. J. Immunol. 2006, 176, 6333–6339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eiden, J.; Volckaert, B.; Rudenko, O.; Aitchison, R.; Herber, R.; Belshe, R.; Greenberg, H.; Coelingh, K.; Marshall, D.; Kawaoka, Y.; et al. Single Replication M2SR Influenza Vaccine Induced Immune Responses Associated with Protection Against Human Challenge with Highly Drifted H3N2 Influenza Strain. J. Infect. Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Eiden, J.; Volckaert, B.; Rudenko, O.; Ellis, R.; Aitchison, R.; Herber, R.; Belshe, R.; Greenberg, H.; Coelingh, K.; Kawaoka, Y.; et al. Single Intranasal (IN) Dose of M2SR (M2-Deficient Single Replication) Live Influenza Vaccine Protects Adults Against Subsequent Challenge with a Substantially Drifted H3N2 Strain. Open Forum Infect. Diseases 2019, 6 (Suppl. 2), S967–S968. [Google Scholar] [CrossRef] [Green Version]
- Gostic, K.M.; Bridge, R.; Brady, S.; Viboud, C.; Worobey, M.; Lloyd-Smith, J.O. Childhood immune imprinting to influenza A shapes birth year-specific risk during seasonal H1N1 and H3N2 epidemics. PLoS Pathog. 2019, 15, e1008109. [Google Scholar] [CrossRef] [Green Version]
- Bodewes, R.; Kreijtz, J.H.; Rimmelzwaan, G.F. Yearly influenza vaccinations: A double-edged sword? Lancet. Infect. Dis. 2009, 9, 784–788. [Google Scholar] [CrossRef]
- Hoft, D.F.; Babusis, E.; Worku, S.; Spencer, C.T.; Lottenbach, K.; Truscott, S.M.; Abate, G.; Sakala, I.G.; Edwards, K.M.; Creech, C.B.; et al. Live and inactivated influenza vaccines induce similar humoral responses, but only live vaccines induce diverse T-cell responses in young children. J. Infect. Dis. 2011, 204, 845–853. [Google Scholar] [CrossRef]
- Belshe, R.B.; Ambrose, C.S.; Yi, T. Safety and efficacy of live attenuated influenza vaccine in children 2-7 years of age. Vaccine 2008, 26 (Suppl. 4), D10–D16. [Google Scholar] [CrossRef]
- Belshe, R.B.; Edwards, K.M.; Vesikari, T.; Black, S.V.; Walker, R.E.; Hultquist, M.; Kemble, G.; Connor, E.M.; Group, C.-T.C.E.S. Live attenuated versus inactivated influenza vaccine in infants and young children. N. Engl. J. Med. 2007, 356, 685–696. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Liepkalns, J.; Reber, A.J.; Lu, X.; Music, N.; Jacob, J.; Sambhara, S. Prior infection with influenza virus but not vaccination leaves a long-term immunological imprint that intensifies the protective efficacy of antigenically drifted vaccine strains. Vaccine 2016, 34, 495–502. [Google Scholar] [CrossRef] [Green Version]
- Guthmiller, J.J.; Wilson, P.C. Harnessing immune history to combat influenza viruses. Curr. Opin. Immunol. 2018, 53, 187–195. [Google Scholar] [CrossRef]
- Zhang, A.; Stacey, H.D.; Mullarkey, C.E.; Miller, M.S. Original Antigenic Sin: How First Exposure Shapes Lifelong Anti-Influenza Virus Immune Responses. J. Immunol. 2019, 202, 335–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, C.; Palm, A.E.; Krammer, F.; Wilson, P.C. From Original Antigenic Sin to the Universal Influenza Virus Vaccine. Trends. Immunol. 2018, 39, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Gostic, K.M.; Ambrose, M.; Worobey, M.; Lloyd-Smith, J.O. Potent protection against H5N1 and H7N9 influenza via childhood hemagglutinin imprinting. Science 2016, 354, 722–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristic | Placebo | Low Dose | Medium Dose | High Dose | All Treated |
|---|---|---|---|---|---|
| (n = 24) | (n = 24) | (n = 24) | (n = 24) | (n = 72) | |
| Gender (n, %) | |||||
| Female | 13 (54%) | 10 (42%) | 12 (50%) | 14 (58%) | 36 (50%) |
| Male | 11 (46%) | 14 (58%) | 12 (50%) | 10 (42%) | 36 (50%) |
| Race (n, %) | |||||
| Asian | 0 (0%) | 0 (0%) | 1 (4%) | 0 (0%) | 1 (1%) |
| Black or African American | 8 (33%) | 7 (29%) | 7 (29%) | 4 (17%) | 18 (25%) |
| White | 16 (67%) | 17 (71%) | 16 (67%) | 20 (83%) | 53 (74%) |
| Ethnicity (n, %) | |||||
| Hispanic | 3 (12%) | 3 (12%) | 4 (17%) | 2 (8%) | 9 (12%) |
| Not Hispanic | 21 (88%) | 21 (88%) | 20 (83%) | 2 (92%) | 63 (88%) |
| Age (years) | |||||
| Mean (SD) | 38.5 (7.3) | 38.1 (7.1) | 35.7 (6.8) | 34.2 (8.2) | 36.0 (7.5) |
| Weight (kg) | |||||
| Mean (SD) | 84.1 (15.3) | 84.7 (17.5) | 80.4 (19.6) | 84.4 (23.3) | 83.2 (20.1) |
| BMI (kg/m2) | |||||
| Mean (SD) | 28.2 (4.9) | 28.8 (5.0) | 27.4 (5.2) | 29.1 (6.3) | 28.4 (5.5) |
| Baseline HAI (n, %) | |||||
| <10 | 6 (25%) | 5 (21%) | 5 (21%) | 8 (33%) | 18 (25%) |
| ≥10 and <40 | 12 (50%) | 14 (58%) | 11 (46%) | 5 (21%) | 30 (42%) |
| ≥40 | 6 (25%) | 5 (21%) | 8 (33%) | 11 (46%) | 24 (33%) |
| Placebo | High Dose M2SR | |||||
|---|---|---|---|---|---|---|
| Baseline HAI | Seroconversion a | ≥4-Fold Increase b | ≥2-Fold Increase b | Seroconversion | ≥4-Fold Increase | ≥2-Fold Increase |
| <40 | 0% (0/18) | 0% (0/18) | 0% (0/18) | 23.1% (3/13) | 39% (5/13) c | 62% (8/13) c |
| ≥40 | 0% (0/6) | 0% (0/6) | 0% (0/6) | 0% (0/11) | 0% (0/11) | 18% (2/11) |
| All | 0% (0/24) | 0% (0/24) | 0% (0/24) | 12.5% (3/24) | 21% (5/24) b | 42% (10/24) d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eiden, J.; Gordon, G.; Fierro, C.; Herber, R.; Aitchison, R.; Belshe, R.; Greenberg, H.; Hoft, D.; Hatta, Y.; Moser, M.J.; et al. Safety and Immunogenicity of M2-Deficient, Single Replication, Live Influenza Vaccine (M2SR) in Adults. Vaccines 2021, 9, 1388. https://doi.org/10.3390/vaccines9121388
Eiden J, Gordon G, Fierro C, Herber R, Aitchison R, Belshe R, Greenberg H, Hoft D, Hatta Y, Moser MJ, et al. Safety and Immunogenicity of M2-Deficient, Single Replication, Live Influenza Vaccine (M2SR) in Adults. Vaccines. 2021; 9(12):1388. https://doi.org/10.3390/vaccines9121388
Chicago/Turabian StyleEiden, Joseph, Gilad Gordon, Carlos Fierro, Renee Herber, Roger Aitchison, Robert Belshe, Harry Greenberg, Daniel Hoft, Yasuko Hatta, Michael J. Moser, and et al. 2021. "Safety and Immunogenicity of M2-Deficient, Single Replication, Live Influenza Vaccine (M2SR) in Adults" Vaccines 9, no. 12: 1388. https://doi.org/10.3390/vaccines9121388






