Race-ethnicity and COVID-19 Vaccination Beliefs and Intentions: A Cross-Sectional Study among the General Population in the San Francisco Bay Area
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Survey Instruments
2.3. Primary Outcome
2.4. Vaccine Beliefs: Motivators, Concerns and Worries
2.5. Sociodemographic Variables
2.6. Statistical Methods
2.6.1. Descriptive Analysis
2.6.2. Mediation Analysis
2.6.3. Least Absolute Shrinkage and Selection Operator (LASSO) Model
2.6.4. Sensitivity Analysis
3. Results
3.1. Vaccine Willingness
3.2. Association between Race–Ethnicity and Vaccine Beliefs
3.3. Beliefs as Mediators for the Association between Race–Ethnicity and Vaccine Willingness
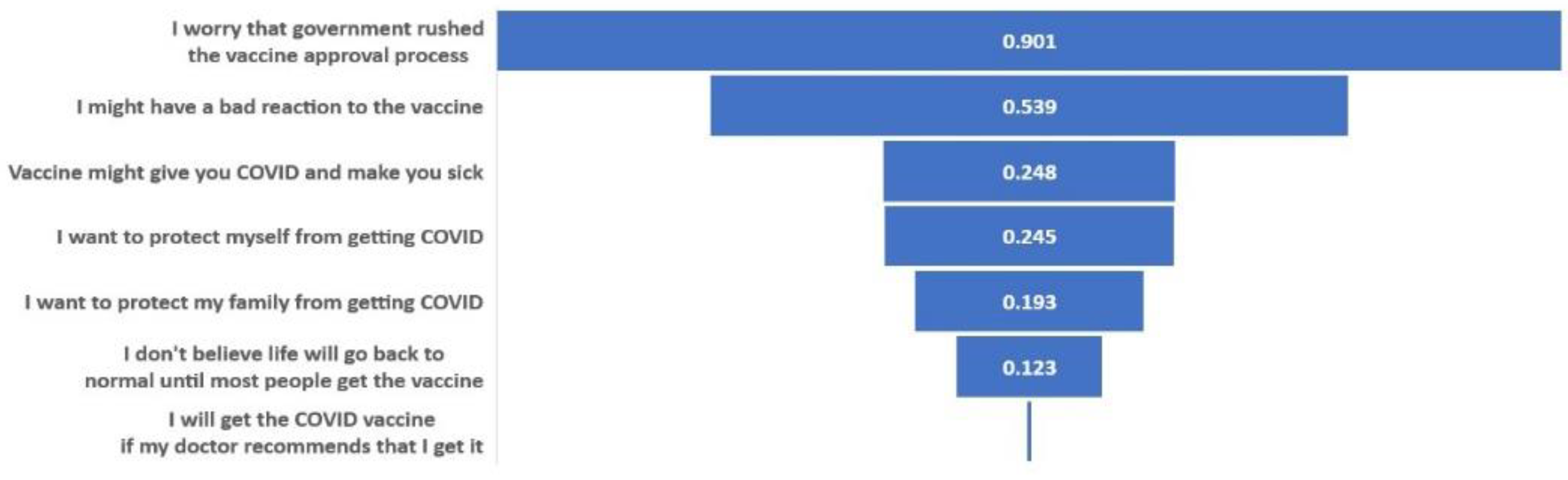
3.4. Top Sociodemographic and Vaccine Belief Predictors of Vaccination Willingness
3.5. Sensitivity Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix B
| Survey Instrument Questions | Available Choices | Dichotomized Answers |
|---|---|---|
| Reasons to get vaccinated [Motivators] | ||
| a. I want to protect myself from getting COVID | Not a reason to get the vaccine [1] Small reason to get the vaccine [2] Moderate reason to get the vaccine [3] Most important reason to get the vaccine [4] | 1: Most important reason to get the vaccine [4] 0: Not a reason to get the vaccine [1] or Small reason to get the vaccine [2] or Moderate reason to get the vaccine [3] |
| b. I want to protect my family from getting COVID | ||
| c. I want to protect my community from getting COVID | ||
| d. I don’t believe life will go back to normal until most people get the vaccine | ||
| e. I will get the COVID vaccine if my doctor recommends that I get it | ||
| f. My employer or school will require or expect me to get a vaccine | ||
| g. My family will want me to get a vaccine | ||
| Reasons to not get vaccinated [Concerns] | ||
| a. The vaccine may not stop me from getting COVID | Not a reason to not get the vaccine [1] Small reason to not get the vaccine [2] Moderate reason to not get the vaccine [3] Most important reason to not get the vaccine [4] | 1: Moderate reason to not get the vaccine [3] or Most important reason to not get the vaccine [4] 0: Not a reason to not get the vaccine [1] or Small reason to not get the vaccine [2] |
| b. I might have a bad reaction to the vaccine | ||
| c. I do not get vaccines in general | ||
| d. I do not think I will get COVID, even without getting a vaccine | ||
| e. The COVID-19 outbreak is not as serious as some people say it is | ||
| f. I do not trust the companies making COVID-19 vaccines | ||
| g. It is better to become immune to a disease by getting sick than by getting a shot | ||
| h. I worry that government rushed the vaccine approval process | ||
| Worries about vaccine [Worries] | ||
| Might not stop you from getting COVID | Not at all [1] Neutral [2] Somewhat worried [3] Very worried [4] | 1: Somewhat worried [3] or Very worried [4] 0: Not at all [1] or Neutral [2] |
| Demographic Characteristics | Non-Respondents (n = 774) | Respondents (n = 3161) | SMD * |
|---|---|---|---|
| Age in Years | 0.10 | ||
| 18–39 | 252 (32.6) | 885 (28.0) | |
| 40–64 | 355 (45.9) | 1534 (48.5) | |
| ≥65 | 167 (21.6) | 742 (23.5) | |
| mean/sd | 49.8 (16.9) | 51.1 (15.8) | 0.09 |
| Gender | 0.04 | ||
| Female | 421 (54.4) | 1702 (53.8) | |
| Male | 348 (45.0) | 1431 (45.3) | |
| Other | 5 (0.6) | 27 (0.9) | |
| Unknown | 0 (0.0) | 1 (0.0) | |
| Race–ethnicity | 0.45 | ||
| White | 308 (39.8) | 1928 (61.0) | |
| Black | 52 (6.7) | 116 (3.7) | |
| Asian | 195 (25.2) | 575 (18.2) | |
| Hispanic | 147 (19.0) | 312 (9.9) | |
| Multiple races | 46 (5.9) | 154 (4.9) | |
| Other/Unknown | 26 (3.4) | 76 (2.4) | |
| Education | 0.34 | ||
| Less than college | 161 (20.8) | 340 (10.8) | |
| College | 371 (47.9) | 1506 (47.6) | |
| Higher than college | 217 (28.0) | 1261 (39.9) | |
| Unknown | 25 (3.2) | 54 (1.7) |

References
- WHO. Sage Report: Report of Sage Working Group on Vaccine Hesitancy. 2014. Available online: https://www.who.int/immunization/sage/meetings/2014/october/1_report_working_group_vaccine_hesitancy_final.pdf (accessed on 15 April 2021).
- Press Ganey. Vaccine Hesitancy and Acceptance: Data Segmentation Helps Address Barriers. 2021. Available online: https://www.pressganey.com/resources/white-papers/vaccine-hesitancy-and-acceptance?s=white_paper-pr (accessed on 15 April 2021).
- Fisher, K.A.; Bloomstone, S.J.; Walder, J.; Crawford, S.; Fouayzi, H.; Mazor, K.M. Attitudes toward a potential SARS-CoV-2 vaccine: A survey of US adults. Ann. Intern. Med. 2020, 173, 964–973. [Google Scholar] [CrossRef]
- Malik, A.A.; McFadden, S.M.; Elharake, J.; Omer, S.B. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine 2020, 26, 100495. [Google Scholar] [CrossRef]
- Mercadante, A.R.; Law, A.V. Will they, or Won’t they? Examining patients’ vaccine intention for flu and COVID-19 using the Health Belief Model. Res. Soc. Adm. Pharm. 2020, 17, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.; Jones, A.; Daly, M. International Estimates of Intended Uptake and Refusal of COVID-19 Vaccines: A Rapid Systematic Review and Meta-Analysis of Large Nationally Representative Samples. Medrxiv 2020. [Google Scholar] [CrossRef]
- Walker, A.; Singhvi, A.; Holder, J.; Gebeloff, R.; Avila, Y. Pandemic’s Racial Disparities Persist in Vaccine Rollout. The New York Times. Available online: https://www.nytimes.com/interactive/2021/03/05/us/vaccine-racial-disparities.html (accessed on 21 April 2021).
- Corbie-Smith, G. Vaccine Hesitancy Is a Scapegoat for Structural Racism. JAMA Health Forum 2021, 2, e210434. [Google Scholar] [CrossRef]
- Grumbach, K.; Judson, T.; Desai, M.; Jain, V.; Lindan, C.; Doernberg, S.B.; Holubar, M. Association of Race/Ethnicity With Likeliness of COVID-19 Vaccine Uptake Among Health Workers and the General Population in the San Francisco Bay Area. JAMA Intern. Med. 2021, 181, 1008–1011. [Google Scholar] [CrossRef] [PubMed]
- Lindan, C.; Desai, M.; Boothroyd, D.; Judson, T.; Bollyky, J.; Sample, H.; Weng, Y.; Cheng, Y.; Dahlen, A.; Hedlin, H.; et al. Design of a Population-Based Longitudinal Cohort Study of Sars-Cov-2 Incidence and Prevalence among Adults in the San Francisco Bay Area. Ann. Epidemiol. 2021. [Google Scholar] [CrossRef]
- UCSF School of Medicine Dean’s Office of Population Health and Health Equityucsf. Ucsf Health Atlas. Available online: https://healthatlas.ucsf.edu/?active=covid_new_cases_percap (accessed on 16 February 2021).
- Phizer. Pfizer and Biontech Conclude Phase 3 Study of COVID-19 Vaccine Candidate, Meeting All Primary Efficacy Endpoints. Phizer. 2020. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine (accessed on 6 February 2021).
- Moderna. Moderna’s COVID-19 Vaccine Candidate Meets Its Primary Efficacy Endpoint in the First Interim Analysis of the Phase 3 Cove Study. Moderna. 2020. Available online: https://investors.modernatx.com/news-releases/news-release-details/modernas-covid-19-vaccine-candidate-meets-its-primary-efficacy (accessed on 6 February 2021).
- National Institutes of Health. Community Engagement Alliance. 2020. Available online: https://covid19community.nih.gov/about (accessed on 16 February 2021).
- MacDonald, N.E.; Sage Working Group on Vaccine Hesitancy. Vaccine hesitancy: Definition, scope and determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef] [PubMed]
- Larson, H.J.; Jarrett, C.; Eckersberger, E.; Smith, D.M.D.; Paterson, P. Understanding Vaccine Hesitancy around Vaccines and Vaccination from a Global Perspective: A Systematic Review of Published Literature, 2007–2012. Vaccine 2014, 32, 2150–2159. [Google Scholar] [CrossRef] [PubMed]
- Brewer, N.T.; Chapman, G.B.; Rothman, A.J.; Leask, J.; Kempe, A. Increasing Vaccination: Putting Psychological Science into Action. Psychol. Sci. Public Interes. 2017, 18, 149–207. [Google Scholar] [CrossRef] [Green Version]
- Fishbein, M.; Ajzen, I. Predicting and Changing Behavior: The Reasoned Action Approach; Psychology Press: East Sussex, UK, 2011. [Google Scholar] [CrossRef]
- Yang, Y.; Zou, H.; Bhatnagar, S. Gglasso: Group Lasso Penalized Learning Using a Unified Bmd Algorithm. R Package Version 1.5. Available online: https://cran.r-project.org/package=gglasso4 (accessed on 1 February 2021).
- Khubchandani, J.; Sharma, S.; Price, J.H.; Wiblishauser, M.J.; Sharma, M.; Webb, F.J. COVID-19 Vaccination Hesitancy in the United States: A Rapid National Assessment. J. Community Health 2021, 46, 270–277. [Google Scholar] [CrossRef]
- Kaiser Family Foundation. Poll: Most Americans Worry Political Pressure Will Lead to Premature Approval of a COVID-19 Vaccine; Half Say They Would Not Get a Free Vaccine Approved before Election Day. 2020. Available online: https://www.kff.org/coronavirus-covid-19/press-release/poll-most-americans-worry-political-pressure-will-lead-to-premature-approval-of-a-covid-19-vaccine-half-say-they-would-not-get-a-free-vaccine-approved-before-election-day/ (accessed on 16 February 2021).
- Hamel, L.; Kirzinger, A.; Munana, C.; Brodie, M. Kff COVID-19 Vaccine Monitor: December 2020. Kaiser Family Foundation. 2020. Available online: https://www.kff.org/coronavirus-covid-19/report/kff-covid-19-vaccine-monitor-december-2020/ (accessed on 16 February 2021).
- Gadoth, A.; Martin-Blais, R.; Tobin, N.H.; Ferbas, K.G.; Geffen, D.; Aldrovandi, G.M.; Rimoin, A.W. Assessment of COVID-19 vaccine acceptance among healthcare workers in Los Angeles. medRxiv 2020. [Google Scholar] [CrossRef]
- Shaw, J.; Stewart, T.; Anderson, K.B.; Hanley, S.; Thomas, S.J.; Salmon, D.A.; Morley, C. Assessment of US Healthcare Personnel Attitudes towards Coronavirus Disease 2019 (COVID-19) Vaccination in a Large University Healthcare System. Clin. Infect. Dis. 2021, 73, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, T.; Moghtaderi, A.; Lueck, J.A.; Hotez, P.J.; Strych, U.; Dor, A.; Franklin Fowler, E.; Motta, M. Correlates and Disparities of COVID-19 Vaccine Hesitancy. Ssrn Pre-Print. 2020. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3667971 (accessed on 16 February 2021).
- Carpiano, R.M. Demographic Differences in US Adult Intentions to Receive a Potential Coronavirus Vaccine and Implications for Ongoing Study. medRxiv 2020. [Google Scholar] [CrossRef]
- Daly, M.; Robinson, E. Willingness to vaccinate against COVID-19 in the US: Longitudinal evidence from a nationally representative sample of adults from April–October 2020. medRxiv 2020. [Google Scholar] [CrossRef]
- Murphy, J.; Vallières, F.; Bentall, R.P.; Shevlin, M.; Mcbride, O.; Hartman, T.K.; Levita, L. Preparing for a COVID-19 vaccine: Identifying and psychologically profiling those who are vaccine hesitant or resistant in two general population samples. PsyarXiv 2020. Available online: https://psyarxiv.com/pev2b/ (accessed on 16 February 2021).
- Neumann-Böhme, S.; Varghese, N.E.; Sabat, I.; Barros, P.P.; Brouwer, W.; Van Exel, J.; Schreyögg, J.; Stargardt, T. Once we have it, will we use it? A European survey on willingness to be vaccinated against COVID-19. Eur. J. Health Econ. 2020, 21, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Bendau, A.; Plag, J.; Petzold, M.B.; Ströhle, A. COVID-19 vaccine hesitancy and related fears and anxiety. Int. Immunopharmacol. 2021, 97, 107724. [Google Scholar] [CrossRef]
- Killgore, W.; Cloonan, S.; Taylor, E.; Dailey, N. The COVID-19 Vaccine Is Here—Now Who Is Willing to Get It? Vaccines 2021, 9, 339. [Google Scholar] [CrossRef]
- Wake, A.D. The Willingness to Receive COVID-19 Vaccine and Its Associated Factors:“Vaccination Refusal Could Prolong the War of This Pandemic”–A Systematic Review. Risk Manag. Healthc. Policy 2021, 14, 2609. [Google Scholar] [CrossRef]
- Napoli, P.E.; Nioi, M.; Fossarello, M. The “Quarantine Dry Eye”: The Lockdown for Coronavirus Disease 2019 and Its Implications for Ocular Surface Health. Health Policy 2021, 14, 1629–1636. [Google Scholar] [CrossRef]
- Pfefferbaum, B.; North, C.S. Mental Health and the COVID-19 Pandemic. N. Engl. J. Med. 2020, 383, 510–512. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Nayar, K.R. COVID 19 and its mental health consequences. J. Ment. Health 2020, 30, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C. COVID-19 and lockdown: Delayed effects on health. Indian J. Psychiatry 2020, 62, 247–249. [Google Scholar] [CrossRef]
- Nioi, M.; Napoli, P.E.; Finco, G.; Demontis, R.; Fossarello, M.; D’Aloja, E. Fear of the COVID-19 and medical liability. Insights from a series of 130 consecutives medico-legal claims evaluated in a single institution during SARS-COV-2-related pandemic. Signa Vitae. 2021, 17, 79–85. [Google Scholar]
- Le, T.T.; Cramer, J.P.; Chen, R.; Mayhew, S. Evolution of the COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020, 19, 667–668. [Google Scholar] [CrossRef]
- Le, T.T.; Andreadakis, Z.; Kumar, A.; Gómez Román, R.; Tollefsen, S.; Saville, M.; Mayhew, S. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020, 19, 305–306. [Google Scholar] [CrossRef] [PubMed]
- Bok, K.; Sitar, S.; Graham, B.S.; Mascola, J.R. Accelerated COVID-19 vaccine development: Milestones, lessons, and prospects. Immunity 2021, 54, 1636–1651. [Google Scholar] [CrossRef]
- Corey, B.L.; Mascola, J.R.; Fauci, A.S.; Collins, F.S. A strategic approach to COVID-19 vaccine R&D. Science 2020, 368, 948–950. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Gruber, W.C. Safety and Efficacy of the Bnt162b2 mRna COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Mahase, E. COVID-19: Moderna Vaccine Is Nearly 95% Effective, Trial Involving High Risk and Elderly People Shows. BMJ Br. Med. J. 2020, 371, m4471. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of The Mrna-1273 Sars-Cov-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I.; et al. Interim Results of a Phase 1–2a Trial of Ad26. Cov2. S COVID-19 Vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Hall, V.J.; Foulkes, S.; Saei, A.; Andrews, N.; Oguti, B.; Charlett, A.; Wellington, E.; Stowe, J.; Gillson, N.; Atti, A.; et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): A prospective, multicentre, cohort study. Lancet 2021, 397, 1725–1735. [Google Scholar] [CrossRef]
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.L.; Yoon, S.K.; Meece, J.; Olsho, L.E.W.; Caban-Martinez, A.J.; Fowlkes, A.; Lutrick, K.; et al. Interim estimates of vaccine effectiveness of BNT162B2 and MRNA-1273 COVID-19 vaccines in preventing SARS-COV-2 infection among health care personnel, first responders, and other essential and frontline workers—Eight US locations, December 2020–March 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 495. [Google Scholar] [CrossRef]
- Loomba, S.; de Figueiredo, A.; Piatek, S.J.; de Graaf, K.; Larson, H.J. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat. Hum. Behav. 2021, 5, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Lockyer, B.; Islam, S.; Rahman, A.; Dickerson, J.; Pickett, K.; Sheldon, T.; Wright, J.; McEachan, R.; Sheard, L.; Bradford Institute for Health Research COVID-19 Scientific Advisory Group. Understanding COVID-19 misinformation and vaccine hesitancy in context: Findings from a qualitative study involving citizens in Bradford, UK. Health Expect. 2021, 24, 1158–1167. [Google Scholar] [CrossRef]
- Kricorian, K.; Civen, R.; Equils, O. COVID-19 vaccine hesitancy: Misinformation and perceptions of vaccine safety. Hum. Vaccin. Immunother. 2021, 2021, 1–8. [Google Scholar] [CrossRef]
- Morgan, V.; Auskova, A.; Janoskova, K. Pervasive misinformation, COVID-19 vaccine hesitancy, and lack of trust in science. Rev. Contemp. Philos. 2021, 20, 128–138. [Google Scholar]
- Harrison, E.A.; Wu, J.W. Vaccine confidence in the time of COVID-19. Eur. J. Epidemiol. 2020, 35, 325–330. [Google Scholar] [CrossRef] [Green Version]
- Rzymski, P.; Borkowski, L.; Drąg, M.; Flisiak, R.; Jemielity, J.; Krajewski, J.; Mastalerz-Migas, A.; Matyja, A.; Pyrć, K.; Simon, K.; et al. The Strategies to Support the COVID-19 Vaccination with Evidence-Based Communication and Tackling Misinformation. Vaccines 2021, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Latkin, C.A.; Dayton, L.; Yi, G.; Konstantopoulos, A.; Boodram, B. Trust in a COVID-19 vaccine in the U.S.: A social-ecological perspective. Soc. Sci. Med. 2021, 270, 113684. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.A.; Crews, D.C. COVID-19, racism, and the pursuit of health care and research worthy of trust. J. Clin. Investig. 2020, 130, 5033–5035. [Google Scholar] [CrossRef]
- Wilkins, C.H. Effective Engagement Requires Trust and Being Trustworthy. Med. Care 2018, 56, S6–S8. [Google Scholar] [CrossRef] [PubMed]
- Boyd, R. Black People Need Better Vaccine Access, Not Better Vaccine Attitudes. Available online: https://www.nytimes.com/2021/03/05/opinion/us-covid-black-people.html?searchresultposition=1 (accessed on 16 February 2021).
| Demographic Characteristics | Survey Respondents (N = 3161) |
|---|---|
| n (%) | |
| Age, years | |
| 18–39 | 885 (28.0) |
| 40–64 | 1543 (48.5) |
| ≥65 | 742 (23.5) |
| Age, years (mean (SD)) | 51.1 (15.8) |
| Gender | |
| Female | 1702 (53.8) |
| Male | 1431 (45.3) |
| Other 1 | 27 (0.9) |
| Unknown | 1 (0) |
| Race–ethnicity Group | |
| White | 1928 (61.0) |
| Black | 116 (3.7) |
| Hispanic/Latinx | 312 (9.9) |
| Asian | 575 (18.2) |
| Multiple races | 154 (4.9) |
| Other | 73 (2.3) |
| Unknown | 3 (0.1) |
| Education | |
| Less than college | 340 (10.8) |
| College 2 | 1506 (47.6) |
| Higher than college | 1261 (39.9) |
| Unknown | 54 (1.7) |
| Occupation | |
| Employed in health sector | 258 (8.2) |
| Not employed in health sector | 2903 (91.8) |
| Race-Ethnicity Groups | Number of Respondents | Percentage Reporting High Vaccine Willingness (95% CI) |
|---|---|---|
| All Respondents | 3161 | 66% (64–67%) |
| White | 1928 | 72% (70–74%) |
| Black/African American | 116 | 41% (32–50%) |
| Latinx/Hispanic | 312 | 55% (49–60%) |
| Asian | 575 | 58% (54–62%) |
| Other Race | 73 | 58% (46–69%) |
| Multiple Races | 154 | 59% (51–67%) |
| Outcomes | White (n = 1928) | Black (n = 116) | Asian (n = 575) | Hispanic/Latinx (n = 312) | Multiple Races (n = 154) | Other Races (n = 73) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | aOR [95% CI] | n (%) | aOR [95% CI] | n (%) | aOR [95% CI] | n (%) | aOR [95% CI] | n (%) | aOR [95% CI] | |
| Reasons to get vaccinated (motivators), % indicating most important reason | |||||||||||
| a. I want to protect myself from getting COVID | 1422 (74.2) | 86 (74.1) | 1.01 (0.65, 1.57) | 418 (73.1) | 1.22 (0.98, 1.53) | 208 (67.3) | 0.93 (0.71, 1.23) | 99 (64.3) | 0.86 (0.60, 1.24) | 50 (68.5) | 0.90 (0.53, 1.53) |
| b. I want to protect my family from getting COVID | 1522 (79.6) | 89 (77.4) | 0.81 (0.52, 1.28) | 465 (81.0) | 1.12 (0.87, 1.42) | 256 (83.1) | 1.20 (0.86, 1.67) | 127 (82.5) | 1.19 (0.77, 1.86) | 55 (75.3) | 0.79 (0.45, 1.39) |
| c. I want to protect my community from getting COVID | 1317 (69.1) | 73 (64.0) | 0.75 (0.50, 1.12) | 367 (64.2) | 0.86 (0.70, 1.06) | 211 (68.3) | 0.96 (0.73, 1.26) | 103 (66.9) | 0.90 (0.63, 1.29) | 47 (64.4) | 0.81 (0.49, 1.34) |
| d. I don’t believe life will go back to normal until most people get the vaccine | 1221 (64.0) | 68 (59.1) | 0.81 (0.55, 1.20) | 356 (62.2) | 1.05 (0.86, 1.28) | 188 (61.0) | 0.95 (0.73, 1.24) | 87 (56.5) | 0.83 (0.59, 1.16) | 43 (58.9) | 0.93 (0.56, 1.52) |
| e. I will get the COVID vaccine if my doctor recommends that I get it | 617 (32.4) | 47 (41.2) | 1.42 (0.95, 2.12) | 221 (38.6) | 1.60 (1.31, 1.97) | 109 (35.9) | 1.26 (0.96, 1.66) | 52 (33.8) | 1.30 (0.90, 1.86) | 22 (30.1) | 1.01 (0.60, 1.69) |
| f. My employer or school will require or expect me to get a vaccine | 300 (15.9) | 17 (15.3) | 0.89 (0.52, 1.52) | 130 (23.1) | 1.57 (1.23, 1.99) | 67 (22.0) | 1.33 (0.97, 1.82) | 32 (21.2) | 1.26 (0.82, 1.93) | 15 (20.8) | 1.28 (0.70, 2.35) |
| g. My family will want me to get a vaccine | 653 (34.5) | 36 (32.1) | 0.81 (0.53, 1.25) | 208 (36.6) | 1.38 (1.12, 1.70) | 106 (34.9) | 1.16 (0.88, 1.53) | 49 (32.2) | 1.13 (0.78, 1.64) | 28 (38.4) | 1.20 (0.72, 1.99) |
| Reasons to not get vaccinated (concerns), % indicating most important or moderately important reason | |||||||||||
| a. The vaccine may not stop me from getting COVID | 224 (11.8) | 31 (27.7) | 2.62 (1.67, 4.10) | 104 (18.3) | 1.68 (1.29, 2.19) | 74 (24.3) | 2.16 (1.58, 2.96) | 26 (17.2) | 1.48 (0.93, 2.35) | 12 (16.9) | 1.38 (0.71, 2.69) |
| b. I might have a bad reaction to the vaccine | 466 (24.5) | 54 (49.1) | 2.56 (1.72, 3.81) | 250 (44.0) | 2.39 (1.95, 2.93) | 132 (43.1) | 2.05 (1.57, 2.66) | 54 (35.3) | 1.49 (1.04, 2.14) | 31 (42.5) | 2.07 (1.27, 3.38) |
| c. I do not get vaccines in general | 81 (4.3) | 18 (16.2) | 3.96 (2.26, 6.94) | 49 (8.7) | 2.19 (1.50, 3.19) | 34 (11.2) | 2.56 (1.65, 3.99) | 12 (7.8) | 1.69 (0.87, 3.28) | 4 (5.6) | 1.30 (0.46, 3.67) |
| d. I do not think I will get COVID, even without getting a vaccine | 39 (2.1) | 16 (14.5) | 8.05 (4.27, 15.2) | 38 (6.7) | 3.58 (2.23, 5.75) | 21 (6.9) | 3.45 (1.94, 6.14) | 4 (2.6) | 0.96 (0.29, 3.20) | 4 (5.6) | 2.15 (0.64, 7.19) |
| e. The COVID-19 outbreak is not as serious as some people say it is | 37 (1.9) | 14 (12.5) | 6.06 (3.08, 11.9) | 31 (5.5) | 2.74 (1.66, 4.53) | 23 (7.6) | 3.82 (2.17, 6.72) | 6 (3.9) | 1.70 (0.65, 4.47) | 2 (2.8) | 1.41 (0.33, 6.01) |
| f. I do not trust the companies making COVID-19 vaccines | 142 (7.5) | 28 (24.8) | 3.60 (2.24, 5.78) | 72 (12.8) | 1.70 (1.25, 2.32) | 54 (17.7) | 2.21 (1.54, 3.15) | 26 (17.1) | 1.96 (1.20, 3.19) | 10 (13.9) | 1.50 (0.70, 3.21) |
| g. It is better to become immune to a disease by getting sick than by getting a shot | 42 (2.2) | 10 (8.8) | 4.11 (1.99, 8.50) | 32 (5.7) | 2.86 (1.76, 4.64) | 25 (8.3) | 3.86 (2.25, 6.62) | 10 (6.6) | 3.00 (1.41, 6.40) | 4 (5.6) | 2.67 (0.92, 7.69) |
| h. I worry that government rushed the vaccine approval process | 344 (18.2) | 43 (37.7) | 2.39 (1.59, 3.61) | 190 (33.6) | 2.01 (1.61, 2.50) | 108 (35.4) | 1.87 (1.42, 2.47) | 49 (32.2) | 1.59 (1.09, 2.32) | 20 (28.6) | 1.35 (0.76, 2.39) |
| Worries about vaccine (worries), % strongly agreeing or agreeing | |||||||||||
| Might not stop you from getting COVID | 502 (26.2) | 46 (39.7) | 1.73 (1.16, 2.57) | 191 (33.2) | 1.42 (1.15, 1.75) | 121 (39.3) | 1.74 (1.34, 2.27) | 48 (31.2) | 1.21 (0.83, 1.74) | 26 (35.6) | 1.36 (0.81, 2.27) |
| Might give you COVID and make you sick | 247 (12.9) | 43 (38.1) | 3.69 (2.44, 5.59) | 135 (23.6) | 2.27 (1.78, 2.90) | 104 (33.8) | 3.01 (2.26, 4.03) | 38 (24.8) | 2.01 (1.33, 3.04) | 19 (26.4) | 2.45 (1.41, 4.27) |
| Predictors | Outcome: High Willingness to Get Vaccinated | |
|---|---|---|
| Demographic Only Model (aPR [95% CI]) | Full Model (aPR (95% CI)) | |
| Race–ethnicity (ref: White) | ||
| Asian | 0.82 (0.73, 0.93) | 0.87 (0.77, 1.00) |
| Black | 0.62 (0.46, 0.84) | 0.72 (0.52, 1.00) |
| Hispanic/Latinx | 0.83 (0.68, 1.01) | 0.93 (0.76, 1.14) |
| Multiple races | 0.80 (0.67, 0.96) | 0.86 (0.72, 1.04) |
| Other | 0.85 (0.63, 1.16) | 0.91 (0.65, 1.26) |
| Belief Mediators | ||
| Motivators | ||
| I want to protect myself from getting COVID | 1.22 (1.08, 1.38) | |
| I want to protect my family from getting COVID | 1.23 (1.07, 1.43) | |
| I want to protect community from getting COVID | 0.97 (0.86, 1.09) | |
| I don’t believe life will go back to normal until most people get the vaccine | 1.16 (1.04, 1.29) | |
| I will get the COVID vaccine if my doctor recommends that I get it | 1.03 (0.92, 1.15) | |
| My employer or school will require or expect me to get a vaccine | 1.09 (0.95, 1.25) | |
| My family will want me to get a vaccine | 1.02 (0.90, 1.14) | |
| Concerns | ||
| The vaccine may not stop me from getting COVID | 0.89 (0.74, 1.07) | |
| I might have a bad reaction to the vaccine | 0.76 (0.67, 0.87) | |
| I do not get vaccines in general | 0.92 (0.68, 1.22) | |
| I do not think I will get COVID, even without getting a vaccine | 0.97 (0.67, 1.40) | |
| The COVID-19 outbreak is not as serious as some people say it is | 1.06 (0.74, 1.53) | |
| I do not trust the companies making COVID-19 vaccines | 0.89 (0.69, 1.14) | |
| It is better to become immune to a disease by getting sick than by getting a shot | 1.13 (0.77, 1.64) | |
| I worry that government rushed the vaccine approval process | 0.63 (0.54, 0.75) | |
| Worries | ||
| COVID-19 vaccine might not stop you from getting COVID | 1.03 (0.92, 1.15) | |
| Might give you COVID and make you sick | 0.84 (0.71, 0.99) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, Y.; Lu, D.; Bollyky, J.; Jain, V.; Desai, M.; Lindan, C.; Boothroyd, D.; Judson, T.; Doernberg, S.B.; Holubar, M.; et al. Race-ethnicity and COVID-19 Vaccination Beliefs and Intentions: A Cross-Sectional Study among the General Population in the San Francisco Bay Area. Vaccines 2021, 9, 1406. https://doi.org/10.3390/vaccines9121406
Weng Y, Lu D, Bollyky J, Jain V, Desai M, Lindan C, Boothroyd D, Judson T, Doernberg SB, Holubar M, et al. Race-ethnicity and COVID-19 Vaccination Beliefs and Intentions: A Cross-Sectional Study among the General Population in the San Francisco Bay Area. Vaccines. 2021; 9(12):1406. https://doi.org/10.3390/vaccines9121406
Chicago/Turabian StyleWeng, Yingjie, Di Lu, Jenna Bollyky, Vivek Jain, Manisha Desai, Christina Lindan, Derek Boothroyd, Timothy Judson, Sarah B. Doernberg, Marisa Holubar, and et al. 2021. "Race-ethnicity and COVID-19 Vaccination Beliefs and Intentions: A Cross-Sectional Study among the General Population in the San Francisco Bay Area" Vaccines 9, no. 12: 1406. https://doi.org/10.3390/vaccines9121406






