Cross-Reactivity Conferred by Homologous and Heterologous Prime-Boost A/H5 Influenza Vaccination Strategies in Humans: A Literature Review
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Homologous Prime-Boost Vaccination Regimens
3.1.1. Description of the Dataset
3.1.2. Discussion of the Data
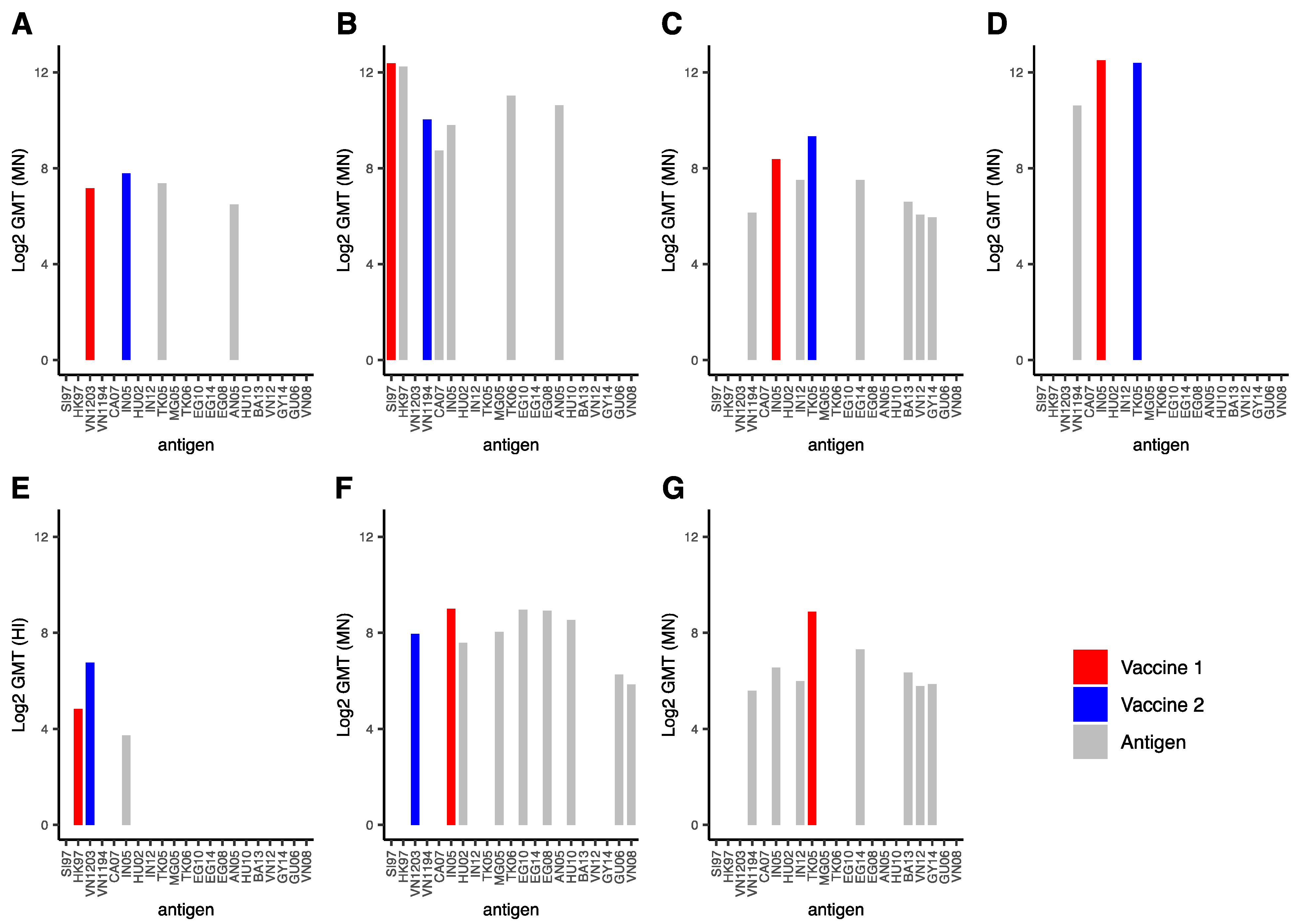
3.2. Heterologous Vaccination
3.2.1. Description of the Dataset
3.2.2. Discussion of the Data
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monto, A.S.; Fukuda, K. Lessons from Influenza Pandemics of the Last 100 Years. Clin. Infect. Dis. 2020, 70, 951–957. [Google Scholar] [CrossRef]
- Mostafa, A.; Abdelwhab, E.M.; Mettenleiter, T.C.; Pleschka, S. Zoonotic Potential of Influenza A Viruses: A Comprehensive Overview. Viruses 2018, 10, 497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Jong, J.C.; Claas, E.C.J.; Osterhaus, A.D.M.E.; Webster, R.G.; Lim, W.L. A Pandemic Warning? Nature 1997, 389, 554. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cumulative Number of Confirmed Human Cases for Avian Influenza A (H5N1) Reported to WHO, 2003–2021. 2021. Available online: https://cdn.who.int/media/docs/default-source/influenza/h5n1-human-case-cumulative-table/2021_may_tableh5n1.pdf?sfvrsn=79ac908a_8&download=true (accessed on 7 December 2021).
- Herfst, S.; Schrauwen, E.J.A.; Linster, M.; Chutinimitkul, S.; De Wit, E.; Munster, V.J.; Sorrell, E.M.; Bestebroer, T.M.; Burke, D.F.; Smith, D.J.; et al. Airborne Transmission of Influenza A/H5N1 Virus between Ferrets. Science 2012, 336, 1534–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, M.; Watanabe, T.; Hatta, M.; Das, S.C.; Ozawa, M.; Shinya, K.; Zhong, G.; Hanson, A.; Katsura, H.; Watanabe, S.; et al. Experimental Adaptation of an Influenza H5 HA Confers Respiratory Droplet Transmission to a Reassortant H5 HA/H1N1 Virus in Ferrets. Nature 2012, 486, 420–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linster, M.; Van Boheemen, S.; De Graaf, M.; Schrauwen, E.J.A.; Lexmond, P.; Mänz, B.; Bestebroer, T.M.; Baumann, J.; Van Riel, D.; Rimmelzwaan, G.F.; et al. Identification, Characterization, and Natural Selection of Mutations Driving Airborne Transmission of A/H5N1 Virus. Cell 2014, 157, 329–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO/OIE/FAO H5N1 Evolution Working Group. Toward a Unified Nomenclature System for Highly Pathogenic Avian Influenza Virus (H5N1). Emerg. Infect. Dis. 2008, 14, e1. [Google Scholar] [CrossRef]
- Smith, G.J.D.; Donis, R.O. Nomenclature Updates Resulting from the Evolution of Avian Influenza A(H5) Virus Clades 2.1.3.2a, 2.2.1, and 2.3.4 during 2013–2014. Influenza Other Respir. Viruses 2015, 9, 271–276. [Google Scholar] [CrossRef]
- WHO. Antigenic and Genetic Characteristics of Zoonotic Influenza Viruses and Development of Candidate Vaccine Viruses for Pandemic Preparedness, March 2021. 2021. Available online: https://www.who.int/influenza/vaccines/virus/202103_zoonotic_vaccinevirusupdate.pdf (accessed on 7 December 2021).
- Rockman, S.; Laurie, K.; Barr, I. Pandemic Influenza Vaccines: What Did We Learn from the 2009 Pandemic and Are We Better Prepared Now? Vaccines 2020, 8, 211. [Google Scholar] [CrossRef]
- Couch, R.B.; Decker, W.K.; Utama, B.; Atmar, R.L.; Niño, D.; Feng, J.Q.; Halpert, M.M.; Air, G.M. Evaluations for In Vitro Correlates of Immunogenicity of Inactivated Influenza A H5, H7 and H9 Vaccines in Humans. PLoS ONE 2012, 7, e50830. [Google Scholar] [CrossRef] [Green Version]
- SAGE Working Group on Influenza Vaccines and Immunizations: Influenza A (H5N1) Vaccine Stockpile and Inter-Pandemic Vaccine Use. Available online: https://www.who.int/immunization/sage/meetings/2013/november/SAGE_WG_H5vaccine_background_paper_16Oct2013_v4.pdf?ua=1 (accessed on 7 December 2021).
- Lofano, G.; Kumar, A.; Finco, O.; Del Giudice, G.; Bertholet, S. B Cells and Functional Antibody Responses to Combat Influenza. Front. Immunol. 2015, 6, 336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Tables on Clinical Evaluation of Influenza Vaccines-Pandemic/Potentially Pandemic Influenza Vaccines. Available online: https://www.who.int/immunization/diseases/influenza/clinical_evaluation_tables/en/ (accessed on 28 March 2021).
- Levie, K.; Leroux-Roels, I.; Hoppenbrouwers, K.; Kervyn, A.D.; Vandermeulen, C.; Forgus, S.; Leroux-Roels, G.; Pichon, S.; Kusters, I. An Adjuvanted, Low-Dose, Pandemic Influenza A (H5N1) Vaccine Candidate Is Safe, Immunogenic, and Induces Cross-Reactive Immune Responses in Healthy Adults. J. Infect. Dis. 2008, 198, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.W.S.; Hwang, S.J.; Lim, F.S.; Oh, H.M.L.; Thongcharoen, P.; Yang, P.C.; Bock, H.L.; Dramé, M.; Gillard, P.; Hutagalung, Y.; et al. Immunogenicity and Tolerability of an AS03A-Adjuvanted Prepandemic Influenza Vaccine: A Phase III Study in a Large Population of Asian Adults. Vaccine 2009, 27, 7428–7435. [Google Scholar] [CrossRef] [PubMed]
- Langley, J.M.; Frenette, L.; Ferguson, L.; Riff, D.; Sheldon, E.; Risi, G.; Johnson, C.; Li, P.; Kenney, R.; Innis, B.; et al. Safety and Cross-Reactive Immunogenicity of Candidate AS03-Adjuvanted Prepandemic H5N1 Influenza Vaccines: A Randomized Controlled Phase 1/2 Trial in Adults. J. Infect. Dis. 2010, 201, 1644–1653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, H.; Ikematsu, H.; Tenjinbaru, K.; Maeda, A.; Dramé, M.; Roman, F.P. A Phase II, Open-Label, Multicentre Study to Evaluate the Immunogenicity and Safety of an Adjuvanted Prepandemic (H5N1) Influenza Vaccine in Healthy Japanese Adults. BMC Infect. Dis. 2010, 10, 338. [Google Scholar] [CrossRef] [Green Version]
- Chu, D.W.S.; Kwong, A.S.K.; Tsui, W.W.S.; Wang, J.H.L.; Ngai, C.K.H.; Wan, P.K.T.; Ong, G.; Tang, H.W.; Roman, F.; Dramé, M.; et al. Cross-Clade Immunogenicity and Safety of an AS03A Adjuvanted Prepandemic H5N1 Influenza Vaccine in Hong Kong. Hong Kong Med. J. 2011, 17, 39–46. [Google Scholar] [PubMed]
- Lasko, B.; Reich, D.; Madan, A.; Roman, F.; Li, P.; Vaughn, D. Rapid Immunization against H5N1: A Randomized Trial Evaluating Homologous and Cross-Reactive Immune Responses to AS03A-Adjuvanted Vaccination in Adults. J. Infect. Dis. 2011, 204, 574–581. [Google Scholar] [CrossRef]
- Thongcharoen, P.; Auewarakul, P.; Hutagalung, Y.; Ong, G.; Gillard, P.; Drame, M.; Bock, H.L. Cross-Clade Immunogenicity and Antigen-Sparing with an AS03A-Adjuvanted Prepandemic Influenza Vaccine in a Thai Population. J. Med. Assoc. Thail. 2011, 94, 916–926. [Google Scholar]
- Yang, P.C.; Yu, C.J.; Chang, S.C.; Hsieh, S.M.; Drame, M.; Walravens, K.; Roman, F.; Gillard, P. Safety and Immunogenicity of a Split-Virion AS03 A-Adjuvanted A/Indonesia/05/2005 (H5N1) Vaccine in Taiwanese Adults. J. Formos. Med. Assoc. 2012, 111, 333–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izurieta, P.; Kim, W.J.; Wie, S.H.; Lee, J.; Lee, J.S.; Dramé, M.; Vaughn, D.W.; Schuind, A. Immunogenicity and Safety of an AS03-Adjuvanted H5N1 Pandemic Influenza Vaccine in Korean Adults: A Phase IV, Randomized, Open-Label, Controlled Study. Vaccine 2015, 33, 2800–2807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naruse, T.; Fukuda, T.; Tanabe, T.; Ichikawa, M.; Oda, Y.; Tochihara, S.; Kimachi, K.; Kino, Y.; Ueda, K. A Clinical Phase I Study of an EB66 Cell-Derived H5N1 Pandemic Vaccine Adjuvanted with AS03. Vaccine 2015, 33, 6078–6084. [Google Scholar] [CrossRef]
- Schuind, A.; Segall, N.; Drame, M.; Innis, B.L. Immunogenicity and Safety of an EB66 Cell-Culture-Derived Influenza A/Indonesia/5/2005(H5N1) AS03-Adjuvanted Vaccine: A Phase 1 Randomized Trial. J. Infect. Dis. 2015, 212, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, I.; Bugarini, R.; Nicholson, K.G.; Podda, A.; Wood, J.M.; Zambon, M.C.; Katz, J.M. Cross-Reactivity to Highly Pathogenic Avian Influenza H5N1 Viruses after Vaccination with Nonadjuvanted and MF59-Adjuvanted Influenza A/Duck/Singapore/97 (H5N3) Vaccine: A Potential Priming Strategy. J. Infect. Dis. 2005, 191, 1210–1215. [Google Scholar] [CrossRef]
- Banzhoff, A.; Gasparini, R.; Laghi-Pasini, F.; Staniscia, T.; Durando, P.; Montomoli, E.; Capecchi, P.L.; Capecchi, P.; di Giovanni, P.; Sticchi, L.; et al. MF59-Adjuvanted H5N1 Vaccine Induces Immunologic Memory and Heterotypic Antibody Responses in Non-Elderly and Elderly Adults. PLoS ONE 2009, 4, e4384. [Google Scholar] [CrossRef]
- Beran, J.; Abdel-Messih, I.A.; Raupachova, J.; Hobzova, L.; Fragapane, E. A Phase III, Randomized, Open-Label Study to Assess the Tolerability and Immunogenicity of an H5N1 Influenza Vaccine Administered to Healthy Adults with a 1-, 2-, 3-, or 6-Week Interval between First and Second Doses. Clin. Ther. 2010, 32, 2186–2197. [Google Scholar] [CrossRef]
- Bihari, I.; Pánczél, G.; Kovacs, J.; Beygo, J.; Fragapanec, E. Assessment of Antigen-Specific and Cross-Reactive Antibody Responses to an MF59-Adjuvanted A/H5N1 Prepandemic Influenza Vaccine in Adult and Elderly Subjects. Clin. Vaccine Immunol. 2012, 19, 1943–1948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vesikari, T.; Forstén, A.; Herbinger, K.-H.; Cioppa, G.D.; Beygo, J.; Borkowski, A.; Groth, N.; Bennati, M.; von Sonnenburg, F. Safety and Immunogenicity of an MF59®-Adjuvanted A/H5N1 Pre-Pandemic Influenza Vaccine in Adults and the Elderly. Vaccine 2012, 30, 1388–1396. [Google Scholar] [CrossRef]
- Ehrlich, H.J.; Müller, M.; Oh, H.M.L.; Tambyah, P.A.; Joukhadar, C.; Montomoli, E.; Fisher, D.; Berezuk, G.; Fritsch, S.; Löw-Baselli, A.; et al. A Clinical Trial of a Whole-Virus H5N1 Vaccine Derived from Cell Culture. N. Engl. J. Med. 2008, 358, 2573–2584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Fang, H.H.; Chen, J.T.; Zhou, J.C.; Feng, Z.J.; Li, C.G.; Qiu, Y.Z.; Liu, Y.; Lu, M.; Liu, L.Y.; et al. Immunogenicity, Safety, and Cross-Reactivity of an Inactivated, Adjuvanted, Prototype Pandemic Influenza (H5N1) Vaccine: A Phase II, Double-Blind, Randomized Trial. Clin. Infect. Dis. 2009, 48, 1087–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tambyah, P.A.; Wilder-Smith, A.; Pavlova, B.G.; Barrett, P.N.; Oh, H.M.L.; Hui, D.S.; Yuen, K.Y.; Fritsch, S.; Aichinger, G.; Loew-Baselli, A.; et al. Safety and Immunogenicity of Two Different Doses of a Vero Cell-Derived, Whole Virus Clade 2 H5N1 (A/Indonesia/05/2005) Influenza Vaccine. Vaccine 2012, 30, 329–335. [Google Scholar] [CrossRef]
- Sansyzbay, A.R.; Erofeeva, M.K.; Khairullin, B.M.; Sandybayev, N.T.; Kydyrbayev, Z.K.; Mamadaliyev, S.M.; Kassenov, M.M.; Sergeeva, M.V.; Romanova, J.R.; Krivitskaya, V.Z.; et al. An Inactivated, Adjuvanted Whole Virion Clade 2.2 H5N1 (A/Chicken/Astana/6/ 05) Influenza Vaccine Is Safe and Immunogenic in a Single Dose in Humans. Clin. Vaccine Immunol. 2013, 20, 1314–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landry, N.; Ward, B.J.; Trépanier, S.; Montomoli, E.; Le Dargis, M.; Lapini, G.; Vézina, L.P. Preclinical and Clinical Development of Plant-Made Virus-like Particle Vaccine against Avian H5N1 Influenza. PLoS ONE 2010, 5, e15559. [Google Scholar] [CrossRef] [Green Version]
- Rudenko, L.; Desheva, J.; Korovkin, S.; Mironov, A.; Rekstin, A.; Grigorieva, E.; Donina, S.; Gambaryan, A.; Katlinsky, A. Safety and Immunogenicity of Live Attenuated Influenza Reassortant H5 Vaccine (Phase I-II Clinical Trials). Influenza Other Respir. Viruses 2008, 2, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Pitisuttithum, P.; Boonnak, K.; Chamnanchanunt, S.; Puthavathana, P.; Luvira, V.; Lerdsamran, H.; Kaewkungwal, J.; Lawpoolsri, S.; Thanachartwet, V.; Silachamroon, U.; et al. Safety and Immunogenicity of a Live Attenuated Influenza H5 Candidate Vaccine Strain A/17/Turkey/Turkey/05/133 H5N2 and Its Priming Effects for Potential Pre-Pandemic Use: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Infect. Dis. 2017, 17, 833–842. [Google Scholar] [CrossRef] [Green Version]
- Kreijtz, J.H.C.M.; Goeijenbier, M.; Moesker, F.M.; van den Dries, L.; Goeijenbier, S.; De Gruyter, H.L.M.; Lehmann, M.H.; de Mutsert, G.D.; van de Vijver, D.A.M.C.; Volz, A.; et al. Safety and Immunogenicity of a Modified-Vaccinia-Virus-Ankara-Based Influenza A H5N1 Vaccine: A Randomised, Double-Blind Phase 1/2a Clinical Trial. Lancet Infect. Dis. 2014, 14, 1196–1207. [Google Scholar] [CrossRef]
- Khurana, S.; Coyle, E.M.; Manischewitz, J.; King, L.R.; Ishioka, G.; Alexander, J.; Smith, J.; Gurwith, M.; Golding, H. Oral Priming with Replicating Adenovirus Serotype 4 Followed by Subunit H5N1 Vaccine Boost Promotes Antibody Affinity Maturation and Expands H5n1 Cross-Clade Neutralization. PLoS ONE 2015, 10, e0115476. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, T.F.; Horacek, T.; Knuf, M.; Damman, H.G.; Roman, F.; Dramé, M.; Gillard, P.; Jilg, W. Single Dose Vaccination with AS03-Adjuvanted H5N1 Vaccines in a Randomized Trial Induces Strong and Broad Immune Responsiveness to Booster Vaccination in Adults. Vaccine 2009, 27, 6284–6290. [Google Scholar] [CrossRef]
- Leroux-Roels, I.; Roman, F.; Forgus, S.; Maes, C.; De Boever, F.; Dramé, M.; Gillard, P.; van der Most, R.; Van Mechelen, M.; Hanon, E.; et al. Priming with AS03A-Adjuvanted H5N1 Influenza Vaccine Improves the Kinetics, Magnitude and Durability of the Immune Response after a Heterologous Booster Vaccination: An Open Non-Randomised Extension of a Double-Blind Randomised Primary Study. Vaccine 2010, 28, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Risi, G.; Frenette, L.; Langley, J.M.; Li, P.; Riff, D.; Sheldon, E.; Vaughn, D.W.; Fries, L. Immunological Priming Induced by a Two-Dose Series of H5N1 Influenza Antigen, Administered Alone or in Combination with Two Different Formulations of AS03 Adjuvant in Adults: Results of a Randomised Single Heterologous Booster Dose Study at 15 Months. Vaccine 2011, 29, 6408–6418. [Google Scholar] [CrossRef] [PubMed]
- Gillard, P.; Caplanusi, A.; Knuf, M.; Roman, F.; Walravens, K.; Moris, P.; Dramé, M.; Schwarz, T.F. An Assessment of Prime-Boost Vaccination Schedules with AS03A-Adjuvanted Prepandemic H5N1 Vaccines: A Randomized Study in European Adults. Influenza Other Respir. Viruses 2013, 7, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Gillard, P.; Chu, D.W.S.; Hwang, S.-J.; Yang, P.-C.; Thongcharoen, P.; Lim, F.S.; Dramé, M.; Walravens, K.; Roman, F. Long-Term Booster Schedules with AS03A-Adjuvanted Heterologous H5N1 Vaccines Induces Rapid and Broad Immune Responses in Asian Adults. BMC Infect. Dis. 2014, 14, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winokur, P.L.; Patel, S.M.; Brady, R.; Chen, W.H.; El-Kamary, S.S.; Edwards, K.; Creech, C.B.; Frey, S.; Keitel, W.A.; Belshe, R.; et al. Safety and Immunogenicity of a Single Low Dose or High Dose of Clade 2 Influenza A(H5N1) Inactivated Vaccine in Adults Previously Primed with Clade 1 Influenza A(H5N1) Vaccine. J. Infect. Dis. 2015, 212, 525–530. [Google Scholar] [CrossRef] [Green Version]
- Levine, M.Z.; Holiday, C.; Jefferson, S.; Gross, F.L.; Liu, F.; Li, S.; Friel, D.; Boutet, P.; Innis, B.L.; Mallett, C.P.; et al. Heterologous Prime-Boost with A(H5N1) Pandemic Influenza Vaccines Induces Broader Cross-Clade Antibody Responses than Homologous Prime-Boost. npj Vaccines 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Lopez, P.; Caicedo, Y.; Sierra, A.; Tilman, S.; Clemens, R.; Banzhoff, A. Combined Administration of MF59-Adjuvanted A/H5N1 Prepandemic and Seasonal Influenza Vaccines: Long-Term Antibody Persistence and Robust Booster Responses 1 Year after a One-Dose Priming Schedule. Clin. Vaccine Immunol. 2013, 20, 753–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belshe, R.B.; Frey, S.E.; Graham, I.L.; Anderson, E.L.; Jackson, L.A.; Spearman, P.; Edupuganti, S.; Mulligan, M.J.; Rouphael, N.; Winokur, P.; et al. Immunogenicity of Avian Influenza A/Anhui/01/2005(H5N1) Vaccine with MF59 Adjuvant a Randomized Clinical Trial. JAMA-J. Am. Med. Assoc. 2014, 312, 1420–1428. [Google Scholar] [CrossRef] [Green Version]
- Galli, G.; Hancock, K.; Hoschler, K.; DeVos, J.; Praus, M.; Bardelli, M.; Malzone, C.; Castellino, F.; Gentile, C.; McNally, T.; et al. Fast Rise of Broadly Cross-Reactive Antibodies after Boosting Long-Lived Human Memory B Cells Primed by an MF59 Adjuvanted Prepandemic Vaccine. Proc. Natl. Acad. Sci. USA 2009, 106, 7962–7967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khurana, S.; Coyle, E.M.; Dimitrova, M.; Castellino, F.; Nicholson, K.; Del Giudice, G.; Golding, H. Heterologous Prime-Boost Vaccination with MF59-Adjuvanted H5 Vaccines Promotes Antibody Affinity Maturation towards the Hemagglutinin HA1 Domain and Broad H5N1 Cross-Clade Neutralization. PLoS ONE 2014, 9, e95496. [Google Scholar] [CrossRef]
- Ehrlich, H.J.; Müller, M.; Fritsch, S.; Zeitlinger, M.; Berezuk, G.; Löw-Baselli, A.; Van Der Velden, M.V.W.; Pöllabauer, E.M.; Maritsch, F.; Pavlova, B.G.; et al. A Cell Culture (Vero)-Derived H5N1 Whole-Virus Vaccine Induces Cross-Reactive Memory Responses. J. Infect. Dis. 2009, 200, 1113–1118. [Google Scholar] [CrossRef] [Green Version]
- Goji, N.A.; Nolan, C.; Hill, H.; Wolff, M.; Noah, D.L.; Williams, T.B.; Rowe, T.; Treanor, J.J. Immune Responses of Healthy Subjects to a Single Dose of Intramuscular Inactivated Influenza A/Vietnam/1203/2004 (H5N1) Vaccine after Priming with an Antigenic Variant. J. Infect. Dis. 2008, 198, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Haveri, A.; Ikonen, N.; Savolainen-Kopra, C.; Julkunen, I. Long-Lasting Heterologous Antibody Responses after Sequential Vaccination with A/Indonesia/5/2005 and A/Vietnam/1203/2004 Pre-Pandemic Influenza A(H5N1) Virus Vaccines. Vaccine 2021, 39, 402–411. [Google Scholar] [CrossRef]
- Trombetta, C.M.; Perini, D.; Mather, S.; Temperton, N.; Montomoli, E. Overview of Serological Techniques for Influenza Vaccine Evaluation: Past, Present and Future. Vaccines 2014, 2, 707–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coudeville, L.; Bailleux, F.; Riche, B.; Megas, F.; Andre, P.; Ecochard, R. Relationship between Haemagglutination-Inhibiting Antibody Titres and Clinical Protection against Influenza: Development and Application of a Bayesian Random-Effects Model. BMC Med. Res. Methodol. 2010, 10, 18. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.J.; Shih, Y.J.; Chen, C.H.; Fang, C.T. Aluminum Salts as an Adjuvant for Pre-Pandemic Influenza Vaccines: A Meta-Analysis. Sci. Rep. 2018, 8, 11460. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Gao, F.; Zhao, C.; Ding, Y.; Cao, Y.; Yang, T.; Xu, X.; Chen, Z. Comparative Effectiveness of H7N9 Vaccines in Healthy Individuals. Hum. Vaccines Immunother. 2019, 15, 80–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vries, R.D.; Altenburg, A.F.; Nieuwkoop, N.J.; De Bruin, E.; Van Trierum, S.E.; Pronk, M.R.; Lamers, M.M.; Richard, M.; Nieuwenhuijse, D.F.; Koopmans, M.P.G.; et al. Induction of Cross-Clade Antibody and T-Cell Responses by a Modified Vaccinia Virus Ankara-Based Influenza A(H5N1) Vaccine in a Randomized Phase 1/2a Clinical Trial. J. Infect. Dis. 2018, 218, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, D.; Perry, S.; Hilchey, S.P.; Wiltse, A.; Treanor, J.J.; Sangster, M.Y.; Zand, M.S. Broadly Reactive IgG Responses to Heterologous H5 Prime-Boost Influenza Vaccination Are Shaped by Antigenic Relatedness to Priming Strains. mBio 2021, 12, e0044921. [Google Scholar] [CrossRef] [PubMed]
- Fonville, J.M.; Wilks, S.H.; James, S.L.; Fox, A.; Ventresca, M.; Aban, M.; Xue, L.; Jones, T.C.; Wong, Y.; Mosterin, A.; et al. Antibody Landscapes after Influenza Virus Infection or Vaccination. Science 2014, 346, 7–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.H.; Kim, E.-H.; Kaith, K.; Lloren, S.; Kwon, J.J.; Kwon, H.-I.; Ahn, S.J.; Kim, Y.-I.; Choi, W.-S.; Si, Y.-J.; et al. Preclinical Evaluation of the Efficacy of an H5N8 Vaccine Candidate (IDCDC-RG43A) in Mouse and Ferret Models for Pandemic Preparedness. Vaccine 2019, 37, 484–493. [Google Scholar] [CrossRef]
- Layton, R.C.; Gigliotti, A.; Armijo, P.; Myers, L.; Knight, J.; Donart, N.; Pyles, J.; Vaughan, S.; Plourde, J.; Fomukong, N.; et al. Enhanced Immunogenicity, Mortality Protection, and Reduced Viral Brain Invasion by Alum Adjuvant with an H5N1 Split-Virion Vaccine in the Ferret. PLoS ONE 2011, 6, e20641. [Google Scholar] [CrossRef] [Green Version]
- Govorkova, E.A.; Webby, R.J.; Humberd, J.; Seiler, J.P.; Webster, R.G. Immunization with Reverse-Genetics–Produced H5N1 Influenza Vaccine Protects Ferrets against Homologous and Heterologous Challenge. J. Infect. Dis. 2006, 194, 159–167. [Google Scholar] [CrossRef]
- Park, S.-J.; Si, Y.-J.; Kim, J.; Song, M.-S.; Kim, S.-M.; Kim, E.-H.; Kwon, H.-I.; Kim, Y.-I.; Lee, O.-J.; Shin, O.S.; et al. Cross-Protective Efficacies of Highly-Pathogenic Avian Influenza H5N1 Vaccines against a Recent H5N8 Virus. Virology 2016, 498, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Belser, J.A.; Pulit-Penaloza, J.A.; Creager, H.M.; Guo, Z.; Jefferson, S.N.; Liu, F.; York, I.A.; Stevens, J.; Maines, T.R.; et al. Stockpiled Pre-Pandemic H5N1 Influenza Virus Vaccines with AS03 Adjuvant Provide Cross-Protection from H5N2 Clade 2.3.4.4 Virus Challenge in Ferrets. Virology 2017, 508, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Vela, E.M.; Buccellato, M.A.; Tordoff, K.; Stark, G.; Bigger, J.E. Efficacy of a Heterologous Vaccine and Adjuvant in Ferrets Challenged with Influenza Virus H5N1. Influenza Other Respir. Viruses 2012, 6, 328–340. [Google Scholar] [CrossRef]
- Hoffmann, E.; Lipatov, A.S.; Webby, R.J.; Govorkova, E.A.; Webster, R.G. Role of Specific Hemagglutinin Amino Acids in the Immunogenicity and Protection of H5N1 Influenza Virus Vaccines. Proc. Natl. Acad. Sci. USA 2015, 102, 12915–12920. [Google Scholar] [CrossRef] [Green Version]
- Lipatov, A.S.; Hoffmann, E.; Salomon, R.; Yen, H.; Webster, R.G. Cross-Protectiveness and Immunogenicity of Influenza A/Duck/Singapore/3/97(H5) Vaccines against Infection with A/Vietnam/1203/04(H5N1) Virus in Ferrets. J. Infect. Dis. 2006, 194, 1040–1043. [Google Scholar] [CrossRef] [PubMed]
- Beyer, W.E.P.; Palache, A.M.; Osterhaus, A.D.M.E. Comparison of Serology and Reactogenicity between Influenza Subunit Vaccines and Whole Virus or Split Vaccines. A Review and Meta-Analysis of the Literature. Clin. Drug Investig. 1998, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.M.; Richmond, P.C.; Skeljo, M.V.; Pearce, G.; Hartel, G.; Formica, N.T.; Höschler, K.; Bennet, J.; Ryan, D.; Papanaoum, K.; et al. Phase I and II Randomised Trials of the Safety and Immunogenicity of a Prototype Adjuvanted Inactivated Split-Virus Influenza A (H5N1) Vaccine in Healthy Adults. Vaccine 2008, 26, 4160–4167. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, G.; Martosne-Mendi, R.; Jankovics, I.; Szilvasy, I.; Vajo, Z. Cross-Reactive Immunity to Clade 2 Strains of Influenza Virus A Subtype H5N1 Induced in Adults and Elderly Patients by Fluval, a Prototype Pandemic Influenza Virus Vaccine Derived by Reverse Genetics, Formulated with a Phosphate Adjuvant, and Directed to Clade 1 Strains. Clin. Vaccine Immunol. 2009, 16, 437–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oshansky, C.M.; Zhou, J.; Gao, Y.; Schweinle, J.E.; Biscardi, K.; DeBeauchamp, J.; Pavetto, C.; Wollish, A.; Webby, R.J.; Cioce, V.; et al. Safety and Immunogenicity of Influenza A(H5N1) Vaccine Stored up to Twelve Years in the National Pre-Pandemic Influenza Vaccine Stockpile (NPIVS). Vaccine 2019, 37, 435–443. [Google Scholar] [CrossRef]
- He, X.-S.; Holmes, T.H.; Zhang, C.; Mahmood, K.; Kemble, G.W.; Lewis, D.B.; Dekker, C.L.; Greenberg, H.B.; Arvin, A.M. Cellular Immune Responses in Children and Adults Receiving Inactivated or Live Attenuated Influenza Vaccines. J. Virol. 2006, 80, 11756–11766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyriakidis, N.C.; López-Cortés, A.; González, E.V.; Grimaldos, A.B.; Prado, E.O. SARS-CoV-2 Vaccines Strategies: A Comprehensive Review of Phase 3 Candidates. npj Vaccines 2021, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Luke, C.J.; Subbarao, K. Improving Pandemic H5N1 Influenza Vaccines by Combining Different Vaccine Platforms. Expert Rev. Vaccines 2014, 13, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Ainai, A.; van Riet, E.; Ito, R.; Ikeda, K.; Senchi, K.; Suzuki, T.; Tamura, S.I.; Asanuma, H.; Odagiri, T.; Tashiro, M.; et al. Human Immune Responses Elicited by an Intranasal Inactivated H5 Influenza Vaccine. Microbiol. Immunol. 2020, 64, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Weir, J.P.; Engelhardt, O.; Katz, J.M.; Cox, R.J. Meeting Report and Review: Immunological Assays and Correlates of Protection for next-Generation Influenza Vaccines. Influenza Other Respir. Viruses 2020, 14, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Feldman, R.A.; Fuhr, R.; Smolenov, I.; (Mick)Ribeiro, A.; Panther, L.; Watson, M.; Senn, J.J.; Smith, M.; Almarsson, Ӧ.; Pujar, H.S.; et al. MRNA Vaccines against H10N8 and H7N9 Influenza Viruses of Pandemic Potential Are Immunogenic and Well Tolerated in Healthy Adults in Phase 1 Randomized Clinical Trials. Vaccine 2019, 37, 3326–3334. [Google Scholar] [CrossRef] [PubMed]
- Petsch, B.; Schnee, M.; Vogel, A.B.; Lange, E.; Hoffmann, B.; Voss, D.; Schlake, T.; Thess, A.; Kallen, K.J.; Stitz, L.; et al. Protective Efficacy of in Vitro Synthesized, Specific MRNA Vaccines against Influenza A Virus Infection. Nat. Biotechnol. 2012, 30, 1210–1216. [Google Scholar] [CrossRef]
- Cobey, S.; Hensley, S.E. Immune History and Influenza Virus Susceptibility. Curr. Opin. Virol. 2017, 22, 105–111. [Google Scholar] [CrossRef] [Green Version]




| Study | Vaccine Type | Adjuvant | Vaccine Strain (Clade) | Dose (μg HA) 1 | Number of Doses | Interval between Doses (Days) 1 | Heterologous Strains (Clade) Used for Immunological Endpoint(s) |
|---|---|---|---|---|---|---|---|
| Levie et al., 2008 [16] | Inactivated split virus | AF03 | VN1194 (1) | 1.9, 3.75, 7.5, 15 | 2 | 21 | IN05 (2.1) |
| Chu et al., 2009 [17] | Inactivated split virus | AS03, none | VN1194 (1) | 3.75 | 2 | 21 | IN05 (2.1) |
| Langley et al., 2010 [18] | Inactivated split virus | AS03A, AS03B, none | IN05 (2.1) | 3.75 | 2 | 21 | VN1203 (1) |
| Nagai et al., 2010 [19] | Inactivated split virus | AS03 | IN05 (2.1) | 3.75 | 2 | 21 | VN1203 (1), TK05 (2.2) |
| Chu et al., 2011 [20] | Inactivated split virus | AS03, none | VN1194 (1) | 3.75 | 2 | 21 | IN05 (2.1) |
| Lasko et al., 2011 [21] | Inactivated split virus | AS03 | IN05 (2.1) | 3.75, 7.5 | 2 | 7, 14 or 28 | VN1203 (1), TK05 (2.2) |
| Thongcharoen et al., 2011 [22] | Inactivated split virus | AS03 | VN1194 (1) | 3.75 | 2 | 21 | IN05 (2.1) |
| Yang et al., 2012 [23] | Inactivated split virus | AS03 | IN05 (2.1) | 3.75 | 2 | 21 | VN1203 (1) |
| Izurieta et al., 2015 [24] | Inactivated split virus | AS03 | IN05 (2.1) | 3.75 | 2 | 21 | VN1203 (1) |
| Naruse et al., 2015 [25] | Inactivated split virus | AS03 | IN05 (2.1) | 1.9, 3.75, 7.5 | 2 | 21 | VN1194 (1), AN05 (2.3.4), QI05 (2.2) |
| Schuind et al., 2015 [26] | Inactivated split virus | AS03A, AS03B, none | IN05 (2.1) | 1.9, 3.75, 15 | 2 | 21 | VN1203 (1) |
| Stephenson et al., 2005 [27] | Inactivated subunit | MF59, none | SI97 (C) 2 | 7.5, 15, 30 3 | 2 + 1 | 21, + 16 months | HK97 (0) |
| Banzhof et al., 2009 [28] | Inactivated subunit | MF59 | VN1194 (1) | 7.5, 15 | 2 + 1 | 21, + 6 months | TK05 (2.2) |
| Beran et al., 2010 [29] | Inactivated subunit | MF59 | VN1194 (1) | 7.5 | 2 | 7, 14, 21 or 42 4 | TK05 (2.2) |
| Bihari et al., 2012 [30] | Inactivated subunit | MF59 | TK05 (2.2) | 7.5 | 2 | 21 | VN1194 (1), IN05 (2.1) |
| Vesikari et al., 2012 [31] | Inactivated subunit | MF59 | VN1194 (1) | 7.5 | 2 | 21 | VN1194 (1), TK05 (2.2) |
| Ehrlich et al., 2008 [32] | Whole inactivated | Aluminum salt, none | VN1203 (1) | 3.75, 7.5, 15, 30 | 2 | 21 | HK97 (0), IN05 (2.1) |
| Wu et al., 2009 [33] | Whole inactivated | Aluminum salt | VN1194 (1) | 5, 10, 15 | 2 | 14 or 28 | IN05 (2.1), TK05 (2.2), AN05 (2.3.4) |
| Tambyah et al., 2012 [34] | Whole inactivated | None | IN05 (2.1) | 3.75, 7.5 | 2 | 21 | VN1203 (1) |
| Sansyzbay et al., 2013 [35] | Whole inactivated | Aluminum salt | AS05 (2) | 7.5, 15 | 1 | NA | VN1203 (1) |
| Landry et al., 2010 [36] | VLP | Aluminium salt | IN05 (2.1) | 5, 10, 20 | 2 | 21 | VN1194 (1), TK05 (2.2), AN05 (2.3.4) |
| Rudenko et al., 2008 [37] | Live attenuated | NA | PD86 (C) | 106.9, 108.3 TCID50 | 2 | 21 | IN05 (2.1) |
| Pitisuttithum et al., 2017 [38] | Live attenuated + inactivated subunit | NA + Aluminum salt | TK05 (2.2) | 8 logEID + 7.5 µg HA | 2 | 21 | TH04 (1), IN05 (2.1), LA07 (2.3.4) |
| Kreijtz et al., 2014 [39] | MVA-vectored | NA | VN1194 (1) | 107 PFU, 108 PFU | 1/2 + 1 | 21 + 12 months | IN05 (2.1), NL14 (2.3.4.4) |
| Khurana et al., 2015 [40] | Ad4-vectored vaccine + inactivated subunit | NA | VN1194 (1) | 107, 1011 particles + 90 µg HA | 3 + 1 | 56 + 3–12 months | IN05 (2.1), TK05 (2.2), EG10 (2.2.1), AN05 (2.3.4) |
| Study | Primary Vaccine Type | Secondary Vaccine Type | Primary Vaccine Adjuvant | Secondary Vaccine Adjuvant | Primary Vaccine Strain (Clade) | Secondary Vaccine Strain (Clade) | Number of Doses | Interval (Months) 1 | Heterologous Strains (Clade) Used for Immunological Endpoint(s) |
|---|---|---|---|---|---|---|---|---|---|
| Schwarz et al., 2009 [41] 2 | Inactivated split virus | Inactivated split virus | AS03A | AS03A | VN1194 (1) | IN05 (2.1) | 1/2 + 1 | 6 | |
| Leroux-Roels et al., 2010 [42] | Inactivated split virus | Inactivated split virus | AS03A, none | AS03A | VN1194 (1) | IN05 (2.1) | 2 + 1/2 | 14 | |
| Risi et al., 2011 [43] | Inactivated split virus | Inactivated split virus | AS03A, AS03B, none | AS03A, none | IN05 (2.1) | TK05 (2.2) | 2 + 1 | 15 | VN1194 (1) |
| Gillard et al., 2013 [44] 2 | Inactivated split virus | Inactivated split virus | AS03A | AS03A | VN1203 (1) | IN05 (2.1) | 1/2 + 1 | 12 | |
| Gillard et al., 2014 [45] | Inactivated split virus | Inactivated split virus | AS03A, none | AS03A | VN1203 (1) | IN05 (2.1) | 2 + 1 | 6, 12 or 36 | |
| Winokur et al., 2015 [46] 2 | Inactivated split virus | Inactivated split virus | Aluminum salt, MF59, none 3 | None | VN1203 (1) | IN05 (2.1) | 2 + 1 | 1.4–3.7 years | |
| Levine et al., 2019 [47] 2 | Inactivated split virus | Inactivated split virus | AS03A | AS03A | IN05 (2.1) | TK05 (2.2) | 1 + 1 | 6, 18 | VN1194 (1), IN12 (2.1.3.2), EG14 (2.2.1), BA13 (2.3.2.1a), VN12 (2.3.2.1c), GY14 (2.3.4.4) |
| Lopez et al., 2013 [48] 2 | Inactivated subunit | Inactivated subunit | MF59 | MF59 | VN1194 (1) | TK05 (2.2) | 1 + 1 | 12 | |
| Belshe et al., 2014 [49] 2 | Inactivated subunit | Inactivated subunit | None | MF59, none | VN1194 (1) | AN05 (2.3.4) | 1/2 + 1 | 24 | |
| Galli et al., 2014 [50] 2 | Inactivated subunit | Inactivated subunit | MF59, none | MF59 | SI97 (C) | VN1194 (1) | 1 + 2 | 6 years | HK97 (0), CA07 (1), IN05 (2.1), TK06 (2.2), AN05 (2.3.4) |
| Khurana et al., 2014 [51] 2 | Inactivated subunit | Inactivated subunit | MF59, none | MF59 | SI97 (C) | VN1194 (1) | 1 + 2 4 | 6 years | HK97 (0), CA07 (1), IN05 (2.1), TK06 (2.2), AN05 (2.3.4) |
| Ehrlich et al., 2009 [52] | Whole inactivated | Whole inactivated | Aluminum salt, none | None | VN1203 (1) | IN05 (2.1) | 2 + 1 | 12–17 | TK05 (2.2), AN05 (2.3.4) |
| Goji et al., 2008 [53] 2 | Recombinant protein | Inactivated split virus | None | None | HK97 (0) | VN1203 (1) | 2 + 1 | 7 years | IN05 (2.1) |
| Haveri et al., 2021 [54] | Inactivated split virus | Whole inactivated | AS03A | None | IN05 (2.1) | VN1194 (1) | 2 + 2 | 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kok, A.; Fouchier, R.A.M.; Richard, M. Cross-Reactivity Conferred by Homologous and Heterologous Prime-Boost A/H5 Influenza Vaccination Strategies in Humans: A Literature Review. Vaccines 2021, 9, 1465. https://doi.org/10.3390/vaccines9121465
Kok A, Fouchier RAM, Richard M. Cross-Reactivity Conferred by Homologous and Heterologous Prime-Boost A/H5 Influenza Vaccination Strategies in Humans: A Literature Review. Vaccines. 2021; 9(12):1465. https://doi.org/10.3390/vaccines9121465
Chicago/Turabian StyleKok, Adinda, Ron A. M. Fouchier, and Mathilde Richard. 2021. "Cross-Reactivity Conferred by Homologous and Heterologous Prime-Boost A/H5 Influenza Vaccination Strategies in Humans: A Literature Review" Vaccines 9, no. 12: 1465. https://doi.org/10.3390/vaccines9121465
APA StyleKok, A., Fouchier, R. A. M., & Richard, M. (2021). Cross-Reactivity Conferred by Homologous and Heterologous Prime-Boost A/H5 Influenza Vaccination Strategies in Humans: A Literature Review. Vaccines, 9(12), 1465. https://doi.org/10.3390/vaccines9121465






