Evaluation of Antibody Response to Heterologous Prime–Boost Vaccination with ChAdOx1 nCoV-19 and BNT162b2: An Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects Enrolment
2.2. Sample Collection and Storing
2.3. Serological Analysis
2.4. Statistical Analysis
2.5. Ethical Aspects
3. Results
3.1. Anti S-RBD Antibody Levels 4 Weeks after the Second Vaccine Dose
3.2. Anti S-RBD Antibody Levels 3 Weeks after the First Vaccine Dose
3.3. Anti S-RBD Antibody Levels 15 Weeks after the Vaccine Booster
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bohn, M.K.; Loh, T.P.; Wang, C.B.; Mueller, R.; Koch, D.; Sethi, S.; Rawlinson, W.D.; Clementi, M.; Erasmus, R.; Leportier, M.; et al. Ifcc interim guidelines on serological testing of antibodies against sars-cov-2. Clin. Chem. Lab. Med. 2020, 58, 2001–2008. [Google Scholar] [CrossRef] [PubMed]
- Teijaro, J.R.; Farber, D.L. COVID-19 vaccines: Modes of immune activation and future challenges. Nat. Rev. Immunol. 2021, 21, 195–197. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine bnt162b1 elicits human antibody and th1 t cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the chadox1 ncov-19 vaccine against sars-cov-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I.; et al. Interim results of a phase 1-2a trial of ad26.Cov2.S COVID-19 vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic thrombocytopenia after chadox1 ncov-19 vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef]
- FARMACO, A.I.D. Utilizzo dei medicinali comirnaty e vaccino COVID-19 moderna per vaccinazione eterologa. (determina n. Dg/699/2021). In Gazzetta Ufficiale; Istituto Poligrafico e Zecca dello Stato: Rome, Italy, 2021; Volume 141, del 15-6-2021. [Google Scholar]
- Borobia, A.M.; Carcas, A.J.; Perez-Olmeda, M.; Castano, L.; Bertran, M.J.; Garcia-Perez, J.; Campins, M.; Portoles, A.; Gonzalez-Perez, M.; Garcia Morales, M.T.; et al. Immunogenicity and reactogenicity of bnt162b2 booster in chadox1-s-primed participants (combivacs): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet 2021, 398, 121–130. [Google Scholar] [CrossRef]
- Liu, X.; Shaw, R.H.; Stuart, A.S.V.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mrna COVID-19 vaccine (com-cov): A single-blind, randomised, non-inferiority trial. Lancet 2021, 398, 856–869. [Google Scholar] [CrossRef]
- Deming, M.E.; Lyke, K.E. A ‘mix and match’ approach to sars-cov-2 vaccination. Nat. Med. 2021, 27, 1510–1511. [Google Scholar] [CrossRef]
- Schmidt, T.; Klemis, V.; Schub, D.; Mihm, J.; Hielscher, F.; Marx, S.; Abu-Omar, A.; Ziegler, L.; Guckelmus, C.; Urschel, R.; et al. Immunogenicity and reactogenicity of heterologous chadox1 ncov-19/mrna vaccination. Nat. Med. 2021, 27, 1530–1535. [Google Scholar] [CrossRef]
- Barros-Martins, J.; Hammerschmidt, S.I.; Cossmann, A.; Odak, I.; Stankov, M.V.; Morillas Ramos, G.; Dopfer-Jablonka, A.; Heidemann, A.; Ritter, C.; Friedrichsen, M.; et al. Immune responses against sars-cov-2 variants after heterologous and homologous chadox1 ncov-19/bnt162b2 vaccination. Nat. Med. 2021, 27, 1525–1529. [Google Scholar] [CrossRef]
- Hillus, D.; Schwarz, T.; Tober-Lau, P.; Vanshylla, K.; Hastor, H.; Thibeault, C.; Jentzsch, S.; Helbig, E.T.; Lippert, L.J.; Tscheak, P.; et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with chadox1 ncov-19 and bnt162b2: A prospective cohort study. Lancet Respir Med. 2021. [Google Scholar] [CrossRef]
- Tenbusch, M.; Schumacher, S.; Vogel, E.; Priller, A.; Held, J.; Steininger, P.; Beileke, S.; Irrgang, P.; Brockhoff, R.; Salmanton-Garcia, J.; et al. Heterologous prime-boost vaccination with chadox1 ncov-19 and bnt162b2. Lancet Infect. Dis. 2021, 21, 1212–1213. [Google Scholar] [CrossRef]
- Hammerschmidt, S.I.; Bosnjak, B.; Bernhardt, G.; Friedrichsen, M.; Ravens, I.; Dopfer-Jablonka, A.; Hoffmann, M.; Pohlmann, S.; Behrens, G.M.N.; Forster, R. Neutralization of the sars-cov-2 delta variant after heterologous and homologous bnt162b2 or chadox1 ncov-19 vaccination. Cell Mol. Immunol. 2021, 18, 2455–2456. [Google Scholar] [CrossRef]
- Hirotsu, Y.; Amemiya, K.; Sugiura, H.; Shinohara, M.; Takatori, M.; Mochizuki, H.; Omata, M. Robust antibody responses to the bnt162b2 mrna vaccine occur within a week after the first dose in previously infected individuals and after the second dose in uninfected individuals. Front. Immunol. 2021, 12, 722766. [Google Scholar] [CrossRef]
- Dehgani-Mobaraki, P.; Zaidi, A.K.; Yadav, N.; Floridi, A.; Floridi, E. Longitudinal observation of antibody responses for 14 months after sars-cov-2 infection. Clin. Immunol. 2021, 230, 108814. [Google Scholar] [CrossRef] [PubMed]
- Dehgani-Mobaraki, P.; Kamber Zaidi, A.; Porreca, A.; Floridi, A.; Floridi, E.; Monti, M.; Dehgani-Mobaraki, M. Antibody persistency and trend post-sars-cov-2 infection at eight months. Ann. Ig 2021. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic sars-cov-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Steensels, D.; Pierlet, N.; Penders, J.; Mesotten, D.; Heylen, L. Comparison of sars-cov-2 antibody response following vaccination with bnt162b2 and mrna-1273. JAMA 2021, 326, 1533–1535. [Google Scholar] [CrossRef]
- Gilbert, P.B.; Montefiori, D.C.; McDermott, A.; Fong, Y.; Benkeser, D.C.; Deng, W.; Zhou, H.; Houchens, C.R.; Martins, K.; Jayashankar, L.; et al. Immune correlates analysis of the mrna-1273 COVID-19 vaccine efficacy trial. medRxiv 2021. [Google Scholar] [CrossRef]
- Padoan, A.; Bonfante, F.; Cosma, C.; Di Chiara, C.; Sciacovelli, L.; Pagliari, M.; Bortolami, A.; Costenaro, P.; Musso, G.; Basso, D.; et al. Analytical and clinical performances of a sars-cov-2 s-rbd igg assay: Comparison with neutralization titers. Clin. Chem. Lab. Med. 2021, 59, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Laterza, R.; Schirinzi, A.; Bruno, R.; Genco, R.; Contino, R.; Ostuni, A.; Di Serio, F. Sars-cov-2 antibodies: Comparison of three high-throughput immunoassays versus the neutralization test. Eur. J. Clin. Investig. 2021, 51, e13573. [Google Scholar] [CrossRef] [PubMed]
- Naaber, P.; Tserel, L.; Kangro, K.; Sepp, E.; Jurjenson, V.; Adamson, A.; Haljasmagi, L.; Rumm, A.P.; Maruste, R.; Karner, J.; et al. Dynamics of antibody response to bnt162b2 vaccine after six months: A longitudinal prospective study. Lancet Reg. Health Eur. 2021, 10, 100208. [Google Scholar] [CrossRef]
- Favresse, J.; Bayart, J.L.; Mullier, F.; Elsen, M.; Eucher, C.; Van Eeckhoudt, S.; Roy, T.; Wieers, G.; Laurent, C.; Dogne, J.M.; et al. Antibody titres decline 3-month post-vaccination with bnt162b2. Emerg. Microbes Infect. 2021, 10, 1495–1498. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Henry, B.M.; Pighi, L.; De Nitto, S.; Gianfilippi, G.L.; Lippi, G. Three-month analysis of total humoral response to pfizer bnt162b2 mrna COVID-19 vaccination in healthcare workers. J. Infect. 2021, 83, e4–e5. [Google Scholar] [CrossRef] [PubMed]
- Zaffina, S.; Alteri, C.; Ruggiero, A.; Cotugno, N.; Vinci, M.R.; Camisa, V.; Santoro, A.P.; Brugaletta, R.; Deriu, G.; Piano Mortari, E.; et al. Induction of immune response after sars-cov-2 mrna bnt162b2 vaccination in healthcare workers. J. Virus Erad. 2021, 7, 100046. [Google Scholar] [CrossRef]
- Dittadi, R.; Seguso, M.; Bertoli, I.; Afshar, H.; Carraro, P. Antibodies against sars-cov-2 time course in patients and vaccinated subjects: An evaluation of the harmonization of two different methods. Diagnostics 2021, 11, 1709. [Google Scholar] [CrossRef]
- Prendecki, M.; Clarke, C.; Edwards, H.; McIntyre, S.; Mortimer, P.; Gleeson, S.; Martin, P.; Thomson, T.; Randell, P.; Shah, A.; et al. Humoral and t-cell responses to sars-cov-2 vaccination in patients receiving immunosuppression. Ann. Rheum. Dis. 2021, 80, 1322–1329. [Google Scholar] [CrossRef]
- Simpson, C.R.; Shi, T.; Vasileiou, E.; Katikireddi, S.V.; Kerr, S.; Moore, E.; McCowan, C.; Agrawal, U.; Shah, S.A.; Ritchie, L.D.; et al. First-dose chadox1 and bnt162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in scotland. Nat. Med. 2021, 27, 1290–1297. [Google Scholar] [CrossRef]
- Petersen, I.; Douglas, I.; Whitaker, H. Self controlled case series methods: An alternative to standard epidemiological study designs. BMJ 2016, 354, i4515. [Google Scholar] [CrossRef] [Green Version]
- Hippisley-Cox, J.; Patone, M.; Mei, X.W.; Saatci, D.; Dixon, S.; Khunti, K.; Zaccardi, F.; Watkinson, P.; Shankar-Hari, M.; Doidge, J.; et al. Risk of thrombocytopenia and thromboembolism after COVID-19 vaccination and sars-cov-2 positive testing: Self-controlled case series study. BMJ 2021, 374, n1931. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/who-response-in-countries (accessed on 15 October 2021).
- Torjesen, I. COVID-19 vaccine shortages: What is the cause and what are the implications? BMJ 2021, 372, n781. [Google Scholar] [CrossRef] [PubMed]
- D’Amelio, R.; Asero, R.; Cassatella, M.A.; Laganà, B.; Lunardi, C.; Migliorini, P.; Nisini, R.; Parronchi, P.; Quinti, I.; Racanelli, V.; et al. Anti-COVID-19 vaccination in patients with autoimmune-autoinflammatory disorders and primary/secondary immunodeficiencies: The position of the task force on behalf of the italian immunological societies. Biomedicines 2021, 9, 1163. [Google Scholar] [CrossRef]


| Subjects Characteristics | BNT/BNT n = 50 | ChAd/ChAd n = 36 | ChAd/BNT n = 49 | BNT1dose/CoV2 n = 15 | COVID-19 Convalescent n = 24 |
|---|---|---|---|---|---|
| Age (years), median (IQR) | 33 (5.2) | 34 (18) | 25.2 (18.8) | 34 (24) | 47 (19.9) |
| Male, % | 42 | 42.5 | 19.6 | 40 | 33 |
| BMI, median (IQR) | 21.75 (5.17) | 22.31 (3.88) | 20.795 (2.85) | 21.42 (4.28) | 23.14 (4.42) |
| Current smoker, % | 14 | 15 | 15.7 | 13 | 7 |
| Diabetes, % | 0 | 0 | 0 | 0 | 0 |
| Weeks between vaccine doses, median (IQR) | 3 (0) | 11.14 (0.37) | 11.5 (0.28) | n.a. | n.a. |
| Analysis time after vaccine booster (days), median (IQR) | 29 (2) | 30 (7) | 27.5 (7) | n.a. | n.a. |
| Anti-RBD IgG AU/mL at T1: 3 Weeks after 1st dose | 1st Quartile | Median | 3rd Quartile | Comparison vs. ChAd/BNT p-Value § | Comparison vs. BNT/BNT p-Value § |
|---|---|---|---|---|---|
| BNT/BNT | 18.30 | 46.32 | 101.92 | 0.015 | - |
| ChAd/ChAd | 8.72 | 16.10 | 40.38 | 0.534 | 0.021 |
| ChAd/BNT | 10.26 | 26.24 | 41.38 | - | 0.015 |
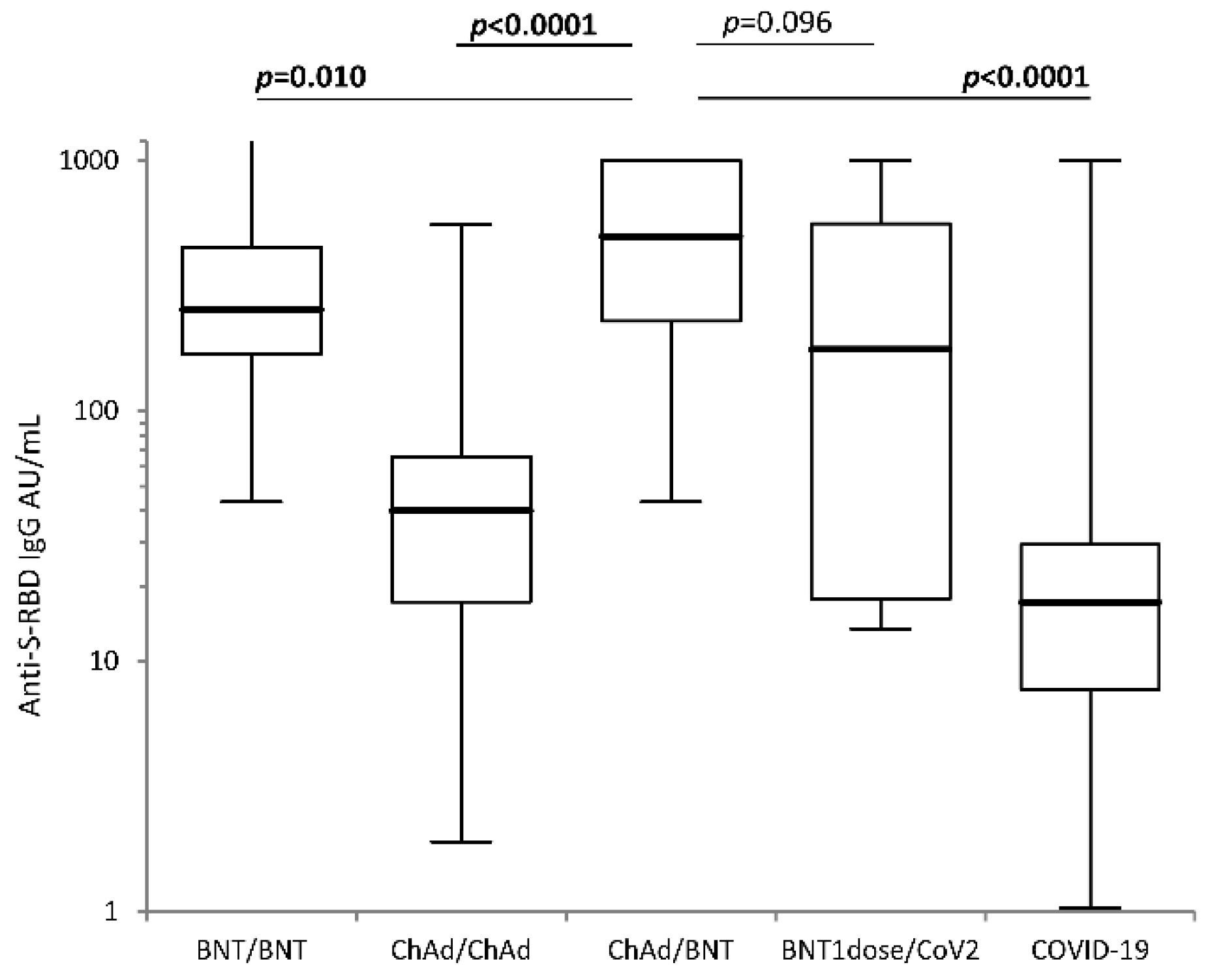
| anti-RBD IgG AU/mL at TFULL: 4 weeks after booster | 1st Quartile | Median | 3rd Quartile | Comparison vs. ChAd/BNT p-value § | Comparison vs. BNT/BNT p-value § |
| BNT/BNT | 169.24 | 253.75 | 452.15 | 0.010 | - |
| ChAd/ChAd | 17.27 | 40.015 | 65.45 | <0.0001 | <0.0001 |
| ChAd/BNT | 228.43 | 497.60 | 1000 | - | 0.010 |
| BNT1dose/CoV2 | 17.74 | 176.40 | 560.13 | 0.096 | 0.295 |
| COVID-19 convalescent | 7.73 | 17.19 | 29.23 | <0.0001 | <0.0001 |
| T15W: 15 weeks after booster | 1st Quartile | Median | 3rd Quartile | Comparison vs. ChAd/BNT p-value § | Comparison vs. BNT/BNT p-value § |
| BNT/BNT | 34.00 | 90.70 | 109.83 | p < 0.0001 | - |
| ChAd/ChAd | 6.13 | 19.74 | 29.26 | p < 0.0001 | 0.011 |
| ChAd/BNT | 123.00 | 318.20 | 400 | - | p < 0.0001 |
| BNT1dose/CoV2 | 6.41 | 13.44 | 57.97 | 0.025 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Firinu, D.; Perra, A.; Campagna, M.; Littera, R.; Meloni, F.; Sedda, F.; Conti, M.; Costanzo, G.; Erbi, M.; Usai, G.; et al. Evaluation of Antibody Response to Heterologous Prime–Boost Vaccination with ChAdOx1 nCoV-19 and BNT162b2: An Observational Study. Vaccines 2021, 9, 1478. https://doi.org/10.3390/vaccines9121478
Firinu D, Perra A, Campagna M, Littera R, Meloni F, Sedda F, Conti M, Costanzo G, Erbi M, Usai G, et al. Evaluation of Antibody Response to Heterologous Prime–Boost Vaccination with ChAdOx1 nCoV-19 and BNT162b2: An Observational Study. Vaccines. 2021; 9(12):1478. https://doi.org/10.3390/vaccines9121478
Chicago/Turabian StyleFirinu, Davide, Andrea Perra, Marcello Campagna, Roberto Littera, Federico Meloni, Francesca Sedda, Maria Conti, Giulia Costanzo, Monica Erbi, Gianmario Usai, and et al. 2021. "Evaluation of Antibody Response to Heterologous Prime–Boost Vaccination with ChAdOx1 nCoV-19 and BNT162b2: An Observational Study" Vaccines 9, no. 12: 1478. https://doi.org/10.3390/vaccines9121478
APA StyleFirinu, D., Perra, A., Campagna, M., Littera, R., Meloni, F., Sedda, F., Conti, M., Costanzo, G., Erbi, M., Usai, G., Locci, C., Carta, M. G., Cappai, R., Orrù, G., Del Giacco, S., Coghe, F., & Chessa, L. (2021). Evaluation of Antibody Response to Heterologous Prime–Boost Vaccination with ChAdOx1 nCoV-19 and BNT162b2: An Observational Study. Vaccines, 9(12), 1478. https://doi.org/10.3390/vaccines9121478









