Optimized Detoxification of a Live Attenuated Vaccine Strain (SG9R) to Improve Vaccine Strategy against Fowl Typhoid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria and Experimental Birds
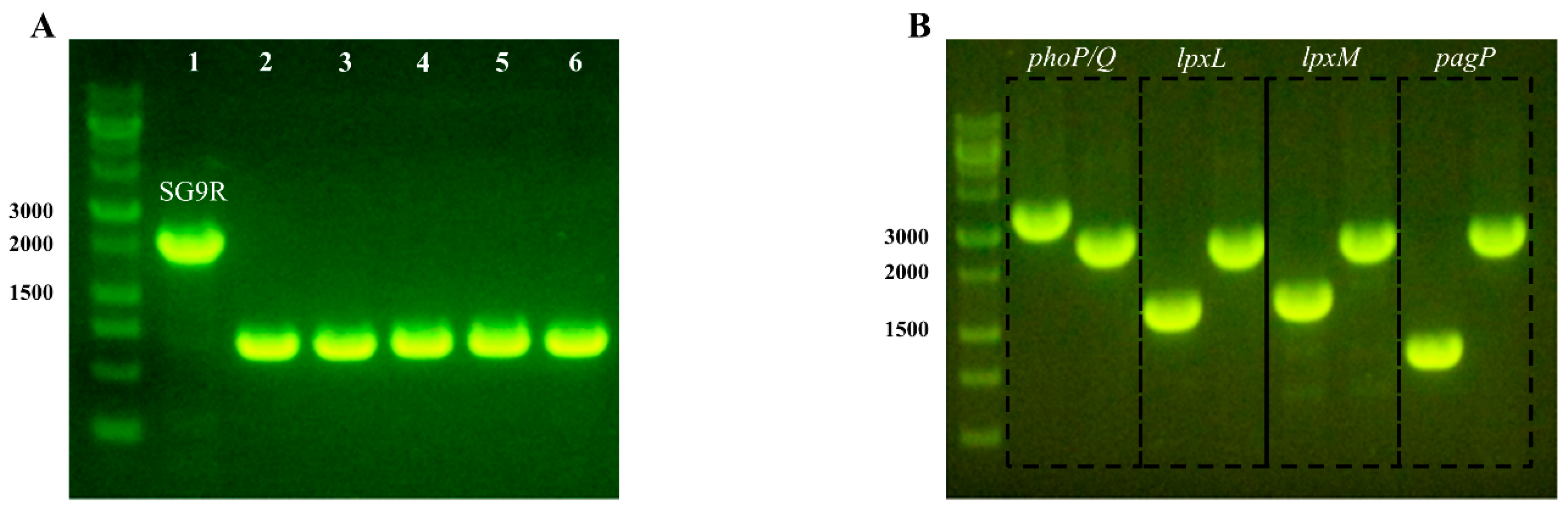
2.2. Generation of Knockout Mutant Strains
2.3. PCR and Sequencing
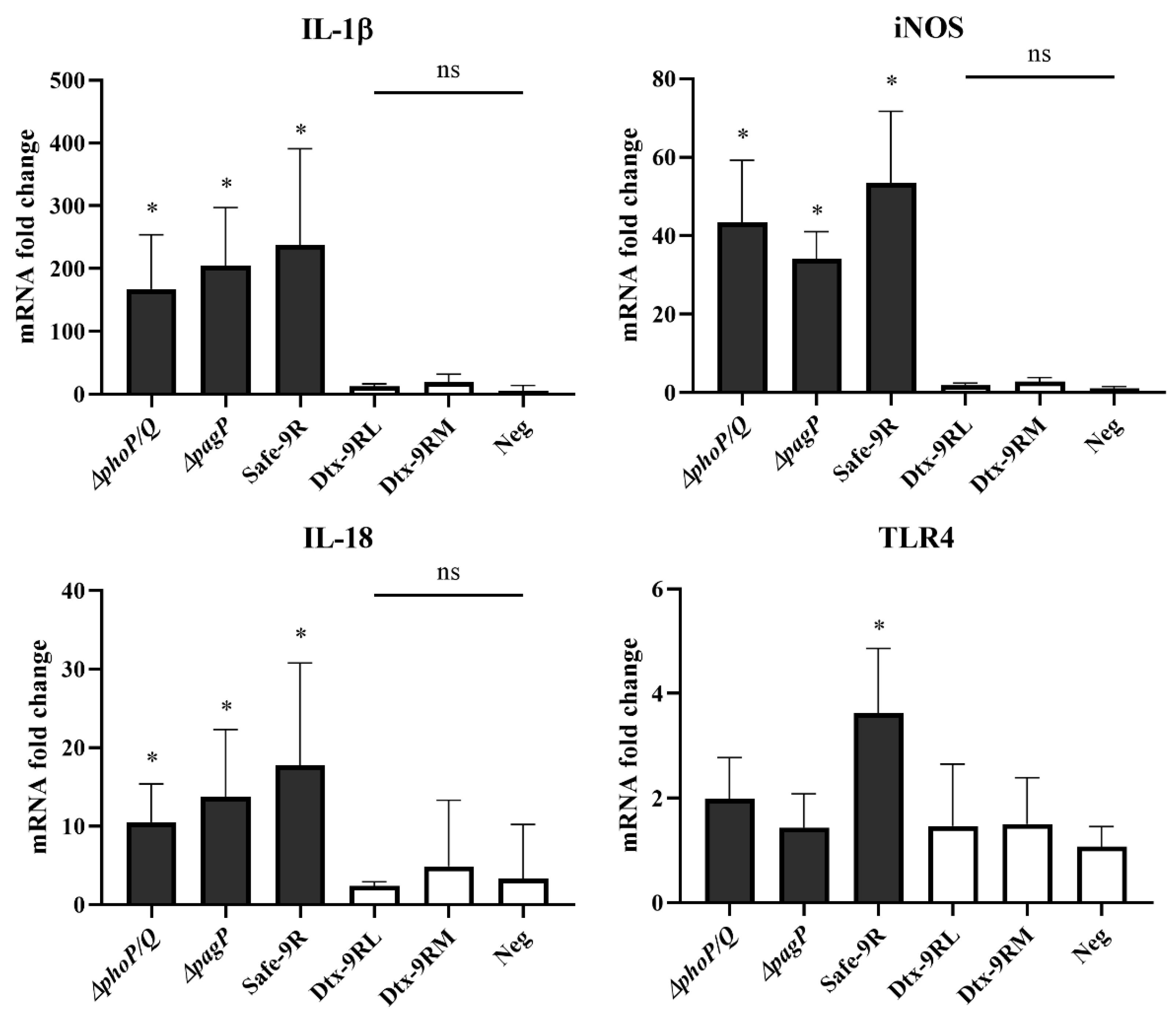
2.4. Detection of Pro-Inflammatory Cytokine Expression in HD11 Cells
2.5. Growth Temperature Sensitivity Test
2.6. Analysis of Protective Efficacy and Toxicity of Safe-9R
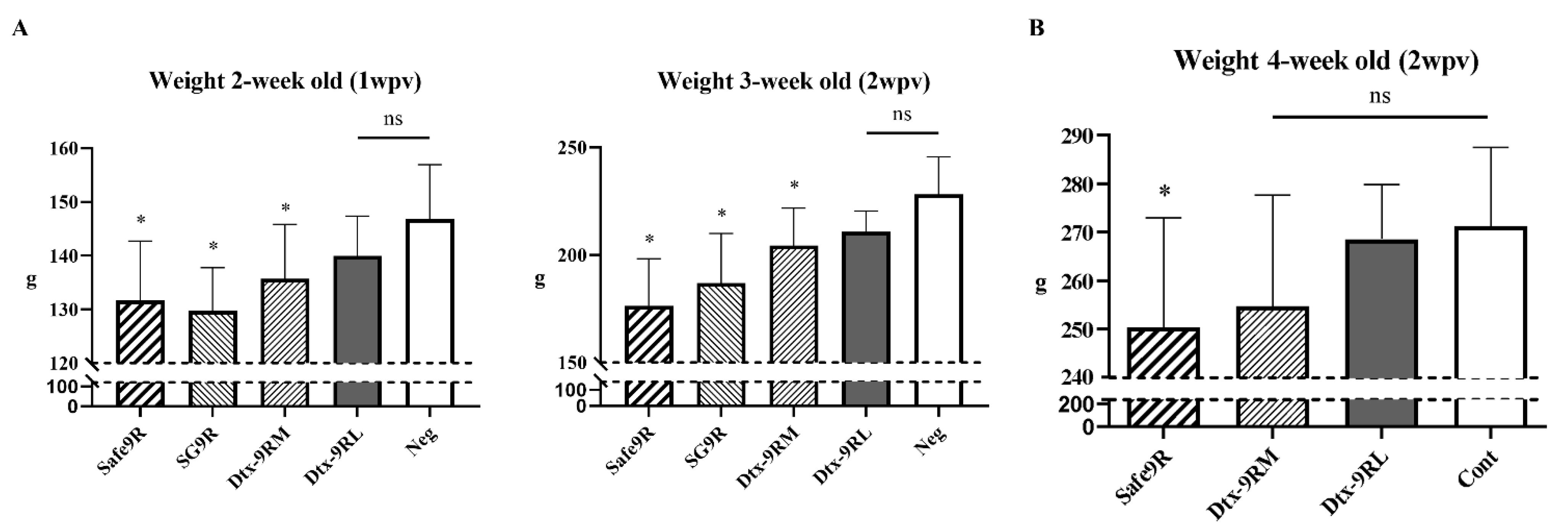
2.7. Assessment of Humoral Immunity and Weight Gain after Administration of OE Vaccines of Detoxified Strains
2.8. Evaluation of Protection Efficacy and Pathogenicity of Live Detoxified Vaccines
2.9. Assessment of T-Cell Stimulation by Fluorescence Activated Cell Sorting
2.10. Statistical Analysis
3. Results
3.1. Generation of Safe-9R
3.2. Generation and Characterization of Gene Knockout Strains for Detoxification
3.3. Protective Efficacy and Humoral Immunogenicity of Safe-9R
3.4. In Vivo Verification of Detoxification
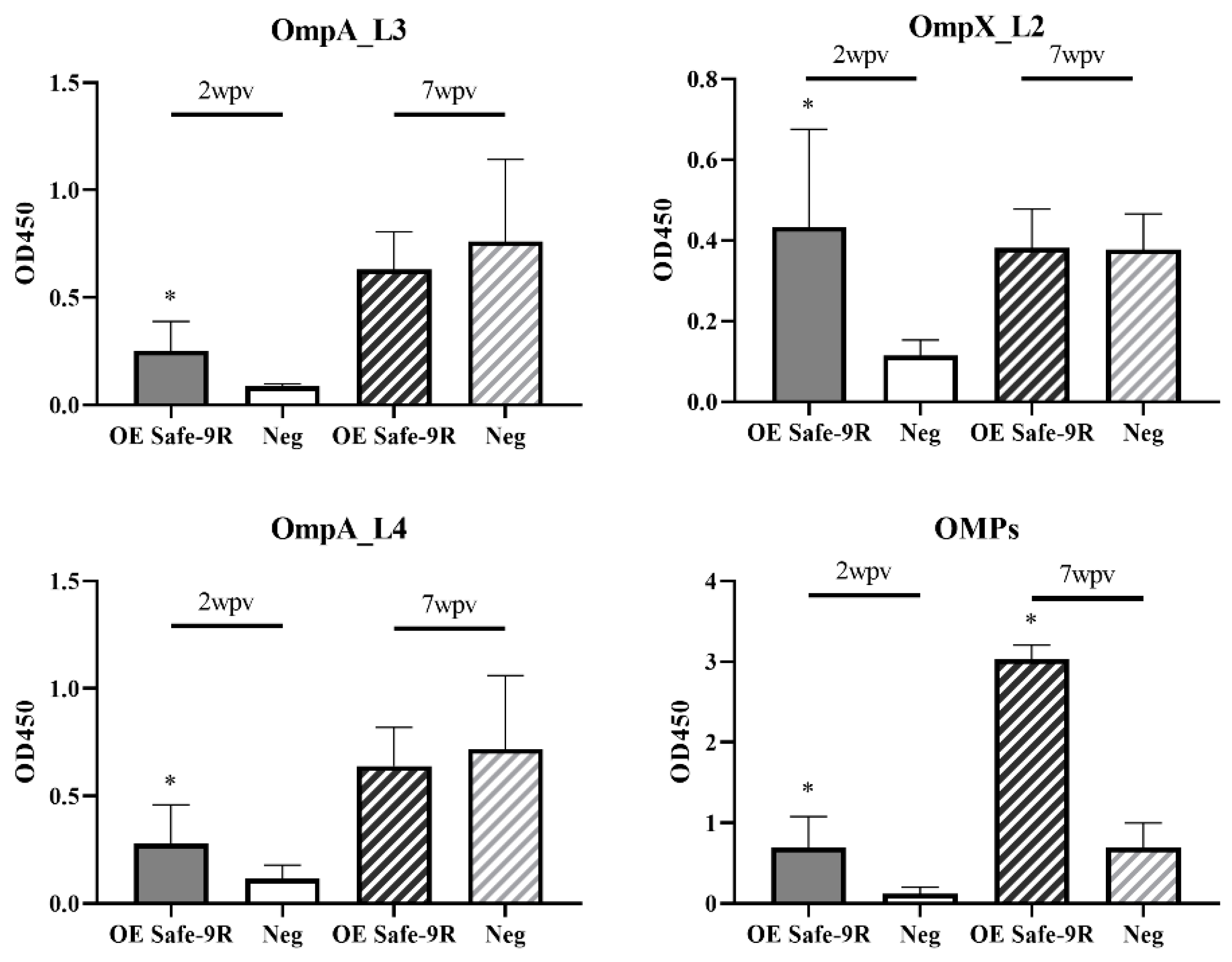
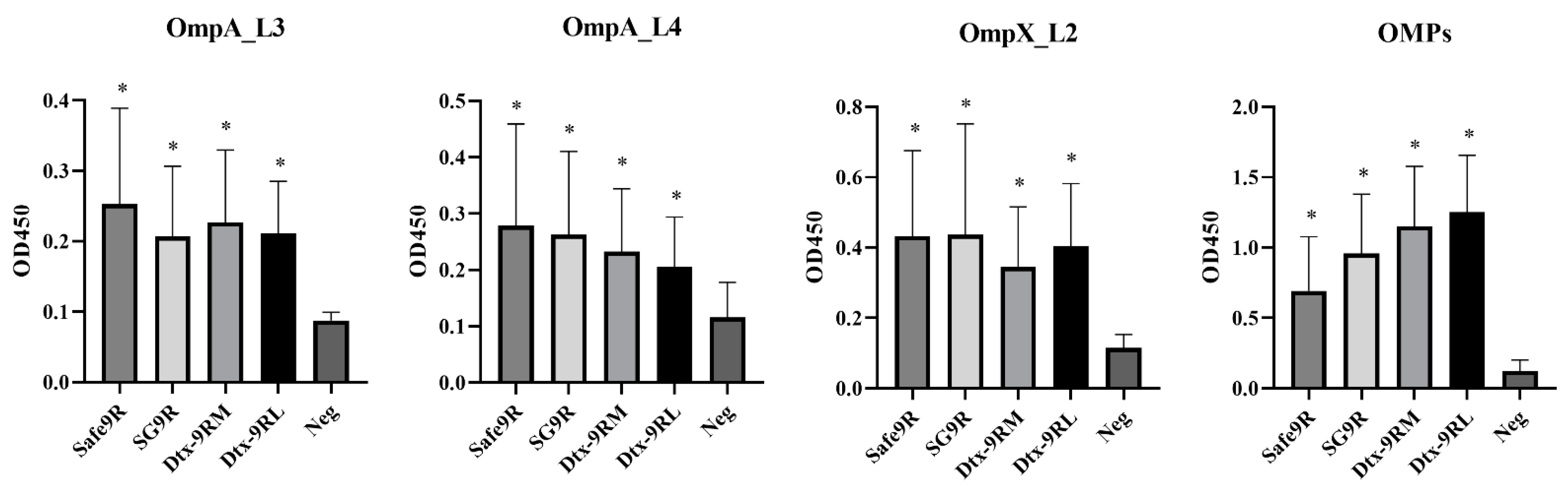
3.5. Humoral Immunogenicity of OE Vaccines of Detoxified Strains
3.6. Evaluation of Residual Pathogenicity and Protective Efficacy of Live Detoxified Strains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, H.W. The use of live vaccines in experimental Salmonella Gallinarum infection in chickens with observations on their interference effect. Epidemiol. Infect. 1956, 54, 419–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.-J.; Kim, K.-S.; Kwon, Y.-K.; Kang, M.-S.; Mo, I.-P.; Kim, J.-H.; Tak, R.-B. Prevalent characteristics of fowl typhoid in Korea. J. Vet. Clin. 2003, 20, 155–158. [Google Scholar]
- Kwon, H.-J.; Cho, S.-H. Pathogenicity of SG 9R, a rough vaccine strain against fowl typhoid. Vaccine 2011, 29, 1311–1318. [Google Scholar] [CrossRef]
- De Carli, S.; Gräf, T.; Kipper, D.; Lehmann, F.K.M.; Zanetti, N.; Siqueira, F.M.; Cibulski, S.; Fonseca, A.S.K.; Ikuta, N.; Lunge, V.R. Molecular and phylogenetic analyses of Salmonella Gallinarum trace the origin and diversification of recent outbreaks of fowl typhoid in poultry farms. Vet. Microbiol. 2017, 212, 80–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Immerseel, F.; Studholme, D.J.; Eeckhaut, V.; Heyndrickx, M.; Dewulf, J.; Dewaele, I.; Van Hoorebeke, S.; Haesebrouck, F.; Van Meirhaeghe, H.; Ducatelle, R. Salmonella Gallinarum field isolates from laying hens are related to the vaccine strain SG9R. Vaccine 2013, 31, 4940–4945. [Google Scholar] [CrossRef]
- Silva, E.; Snoeyenbos, G.; Weinack, O.M.; Smyser, C. Studies on the use of 9R strain of Salmonella Gallinarum as a vaccine in chickens. Avian Dis. 1981, 38–52. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kang, M.S. Safety and efficacy of Salmonella Gallinarum 9R vaccine in young laying chickens. Avian Pathol. 2005, 34, 362–366. [Google Scholar] [CrossRef]
- Kim, N.-H.; Ha, E.-J.; Ko, D.-S.; Choi, K.-S.; Kwon, H.-J. Comparison of humoral immune responses to different forms of Salmonella enterica serovar Gallinarum biovar Gallinarum. Front. Vet. Sci. 2020, 7, 938. [Google Scholar] [CrossRef]
- Cho, S.-H.; Ahn, Y.-J.; Kim, T.-E.; Kim, S.-J.; Huh, W.; Moon, Y.-S.; Lee, B.-H.; Kim, J.-H.; Kwon, H.J. Establishment of a live vaccine strain against fowl typhoid and paratyphoid. Korean J. Vet. Res. 2015, 55, 241–246. [Google Scholar] [CrossRef] [Green Version]
- Blatteis, C. Influence of body weight and temperature on the pyrogenic effect of endotoxin in guinea pigs. Toxicol. Appl. Pharmacol. 1974, 29, 249–258. [Google Scholar] [CrossRef]
- Xie, H.; Rath, N.; Huff, G.; Huff, W.; Balog, J. Effects of Salmonella Typhimurium lipopolysaccharide on broiler chickens. Poult. Sci. 2000, 79, 33–40. [Google Scholar] [CrossRef]
- Lee, S.-R.; Kim, S.-H.; Jeong, K.-J.; Kim, K.-S.; Kim, Y.-H.; Kim, S.-J.; Kim, E.; Kim, J.-W.; Chang, K.-T. Multi-immunogenic outer membrane vesicles derived from an MsbB-deficient Salmonella enterica serovar Typhimurium mutant. J. Microbiol. Biotechnol. 2009, 19, 1271–1279. [Google Scholar] [PubMed]
- Vaara, M.; Nurminen, M. Outer membrane permeability barrier in Escherichia coli mutants that are defective in the late acyltransferases of lipid a biosynthesis. Antimicrob. Agents Chemother. 1999, 43, 1459–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clementz, T.; Bednarski, J.J.; Raetz, C.R. Function of the htrB high temperature requirement gene of Escherichia coli in the acylation of lipid a HtrB catalyzed incorporation of laurate. J. Biol. Chem. 1996, 271, 12095–12102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clementz, T.; Zhou, Z.; Raetz, C.R. Function of the Escherichia coli msbB gene, a multicopy suppressor of htrB knockouts, in the acylation of lipid A acylation by MsbB follows laurate incorporation by HtrB. J. Biol. Chem. 1997, 272, 10353–10360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feodorova, V.; Pan’kina, L.; Savostina, E.; Sayapina, L.; Motin, V.; Dentovskaya, S.; Shaikhutdinova, R.; Ivanov, S.; Lindner, B.; Kondakova, A. A Yersinia pestis lpxM-mutant live vaccine induces enhanced immunity against bubonic plague in mice and guinea pigs. Vaccine 2007, 25, 7620–7628. [Google Scholar] [CrossRef] [PubMed]
- Fisseha, M.; Chen, P.; Brandt, B.; Kijek, T.; Moran, E.; Zollinger, W. Characterization of native outer membrane vesicles from lpxL mutant strains of Neisseria meningitidis for use in parenteral vaccination. Infect. Immun. 2005, 73, 4070–4080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Buchholz, F.; Muyrers, J.P.; Stewart, A.F. A new logic for DNA engineering using recombination in Escherichia coli. Nat. Genet. 1998, 20, 123–128. [Google Scholar] [CrossRef]
- Kim, N.H.; Ha, E.J.; Ko, D.S.; Lee, C.Y.; Kim, J.H.; Kwon, H.J. Molecular evolution of Salmonella enterica subsp. enterica serovar Gallinarum biovar Gallinarum in the field. Vet. Microbiol. 2019, 235, 63–70. [Google Scholar] [CrossRef]
- Han, D.; Lee, H.T.; Lee, J.B.; Kim, Y.; Lee, S.J.; Yoon, J.W. A bioprocessed polysaccharide from Lentinus edodes mycelia cultures with turmeric protects chicks from a lethal challenge of Salmonella Gallinarum. J. Food Prot. 2017, 80, 245–250. [Google Scholar] [CrossRef]
- Rychlik, I.; Karasova, D.; Sebkova, A.; Volf, J.; Sisak, F.; Havlickova, H.; Kummer, V.; Imre, A.; Szmolka, A.; Nagy, B. Virulence potential of five major pathogenicity islands (SPI-1 to SPI-5) of Salmonella enterica serovar Enteritidis for chickens. BMC Microbiol. 2009, 9, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajput, I.R.; Hussain, A.; Li, Y.L.; Zhang, X.; Xu, X.; Long, M.Y.; You, D.Y.; Li, W.F. Saccharomyces boulardii and Bacillus subtilis B10 modulate TLRs mediated signaling to induce immunity by chicken BMDCs. J. Cell. Biochem. 2014, 115, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Feberwee, A.; De Vries, T.; Hartman, E.; De Wit, J.; Elbers, A.; De Jong, W. Vaccination against Salmonella Enteritidis in Dutch commercial layer flocks with a vaccine based on a live Salmonella Gallinarum 9R strain: Evaluation of efficacy, safety, and performance of serologic Salmonella tests. Avian Dis. 2001, 45, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Matthews, T.D.; Schmieder, R.; Silva, G.G.; Busch, J.; Cassman, N.; Dutilh, B.E.; Green, D.; Matlock, B.; Heffernan, B.; Olsen, G.J. Genomic comparison of the closely-related Salmonella enterica serovars Enteritidis, Dublin and Gallinarum. PLoS ONE 2015, 10, e0126883. [Google Scholar] [CrossRef] [PubMed]
- Rabsch, W.; Hargis, B.M.; Tsolis, R.M.; Kingsley, R.A.; Hinz, K.H.; Tschape, H.; Baumler, A.J. Competitive exclusion of Salmonella enteritidis by Salmonella gallinarum in poultry. Emerg. Infect. Dis. 2000, 6, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Cheong, H.J.; Lee, Y.J.; Hwang, I.S.; Kee, S.Y.; Cheong, H.W.; Song, J.Y.; Kim, J.M.; Park, Y.H.; Jung, J.-H.; Kim, W.J. Characteristics of non-typhoidal Salmonella isolates from human and broiler-chickens in southwestern Seoul, Korea. J. Korean Med. Sci. 2007, 22, 773–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S. Salmonella serovars from foodborne and waterborne diseases in Korea, 1998–2007: Total isolates decreasing versus rare serovars emerging. J. Korean Med. Sci. 2010, 25, 1693–1699. [Google Scholar] [CrossRef] [Green Version]
- Wigley, P.; Hulme, S.; Powers, C.; Beal, R.; Smith, A.; Barrow, P. Oral infection with the Salmonella enterica serovar Gallinarum 9R attenuated live vaccine as a model to characterise immunity to fowl typhoid in the chicken. BMC Vet. Res. 2005, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Halstead, S.B.; Mahalingam, S.; Marovich, M.A.; Ubol, S.; Mosser, D.M. Intrinsic antibody-dependent enhancement of microbial infection in macrophages: Disease regulation by immune complexes. Lancet Infect. Dis. 2010, 10, 712–722. [Google Scholar] [CrossRef] [Green Version]
- Clements, A.; Tull, D.; Jenney, A.W.; Farn, J.L.; Kim, S.-H.; Bishop, R.E.; McPhee, J.B.; Hancock, R.E.; Hartland, E.L.; Pearse, M.J. Secondary acylation of Klebsiella pneumoniae lipopolysaccharide contributes to sensitivity to antibacterial peptides. J. Biol. Chem. 2007, 282, 15569–15577. [Google Scholar] [CrossRef] [Green Version]
- Mills, G.; Dumigan, A.; Kidd, T.; Hobley, L.; Bengoechea, J.A. Identification and characterization of two Klebsiella pneumoniae lpxL lipid A late acyltransferases and their role in virulence. Infect. Immun. 2017, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, B.D.; Nichols, W.A.; Gibson, B.W.; Sunshine, M.G.; Apicella, M.A. Study of the role of the htrB gene in Salmonella Typhimurium virulence. Infect. Immun. 1997, 65, 4778–4783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karow, M.; Georgopoulos, C. Isolation and characterization of the Escherichia coli msbB gene, a multicopy suppressor of null mutations in the high-temperature requirement gene htrB. J. Bacteriol. 1992, 174, 702–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunshine, M.G.; Gibson, B.W.; Engstrom, J.J.; Nichols, W.A.; Jones, B.D.; Apicella, M.A. Mutation of the htrB gene in a virulent Salmonella Typhimurium strain by intergeneric transduction: Strain construction and phenotypic characterization. J. Bacteriol. 1997, 179, 5521–5533. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Group | Vaccination | Survival Rate b (%) | ||
|---|---|---|---|---|
| Live Safe-9R | Vaccinated | 100 (10/10) * | ||
| Non-vaccinated | 0 (0/10) | |||
| OE Safe-9R a—2 wpv c | Vaccinated | 60 (6/10) * | 50 (5/10) | 80 (8/10) |
| Non-vaccinated | 0 (0/10) | 11.1 (1/9) | 50 (5/10) | |
| OE Safe-9R a—7 wpv c | Vaccinated | 87.5 (7/8) | 50 (5/10) | 70 (7/10) |
| Non-vaccinated | 80 (8/10) | 90 (9/10) | 90 (9/10) | |
| Groups | Dtx-9RL | Dtx-9RM | Safe-9R | SG9R | Negative |
|---|---|---|---|---|---|
| Lesion a | 0/5 | 5/5 | 5/5 | 5/5 | 0/5 |
| Re-isolation b | 0/5 | 0/5 | 4/5 | 3/5 | 0/5 |
| Group | Dtx-9RL | Dtx-9RM | Safe-9R | SG9R | Negative | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vaccinated Age | A 1 | B 2 | A | B | A | B | A | B | A | B |
| 0 a | 7 | 4 | 2 | 2 | 2 | 2 | 3 | 2 | 4 | 5 |
| 1 b | 1 | 0 | 8 | 4 | 0 | 1 | 1 | 1 | 0 | 1 |
| 2 c | 0 | 1 | 0 | 3 | 3 | 5 | 5 | 3 | 2 | 0 |
| 3 d | 1 | 1 | 0 | 1 | 5 | 2 | 1 | 4 | 2 | 1 |
| 4 e | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 |
| Severe liver lesions * | 20% | 60% | 0% | 40% | 80% | 70% | 60% | 70% | 60% | 40% |
| Number of chickens | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Group | Challenged at 1 wpv a | Challenged at 2 wpv | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Dtx-9RL | Dtx-9RM | Safe-9R | SG9R | Negative | Dtx-9RL | Dtx-9RM | Safe-9R | SG9R | Negative |
| Re-isolation | 2/9 b | 0/10 | 4/10 | 3/10 | 4/8 b | 0/6 b | 0/10 | 0/10 | 1/10 | 0/7 b |
| Smooth/Rough c | 10/0 | Nt e | 10/0 | 5/0 d | 10/0 | nt | nt | nt | 0/4 d | nt |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, N.-H.; Ko, D.-S.; Ha, E.-J.; Ahn, S.; Choi, K.-S.; Kwon, H.-J. Optimized Detoxification of a Live Attenuated Vaccine Strain (SG9R) to Improve Vaccine Strategy against Fowl Typhoid. Vaccines 2021, 9, 122. https://doi.org/10.3390/vaccines9020122
Kim N-H, Ko D-S, Ha E-J, Ahn S, Choi K-S, Kwon H-J. Optimized Detoxification of a Live Attenuated Vaccine Strain (SG9R) to Improve Vaccine Strategy against Fowl Typhoid. Vaccines. 2021; 9(2):122. https://doi.org/10.3390/vaccines9020122
Chicago/Turabian StyleKim, Nam-Hyung, Dae-Sung Ko, Eun-Jin Ha, Sunmin Ahn, Kang-Seuk Choi, and Hyuk-Joon Kwon. 2021. "Optimized Detoxification of a Live Attenuated Vaccine Strain (SG9R) to Improve Vaccine Strategy against Fowl Typhoid" Vaccines 9, no. 2: 122. https://doi.org/10.3390/vaccines9020122






