Immunogenicity of Calvenza-03 EIV/EHV® Vaccine in Horses: Comparative In Vivo Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval/Animal Welfare
2.2. Vaccine
2.3. Experimental Animals
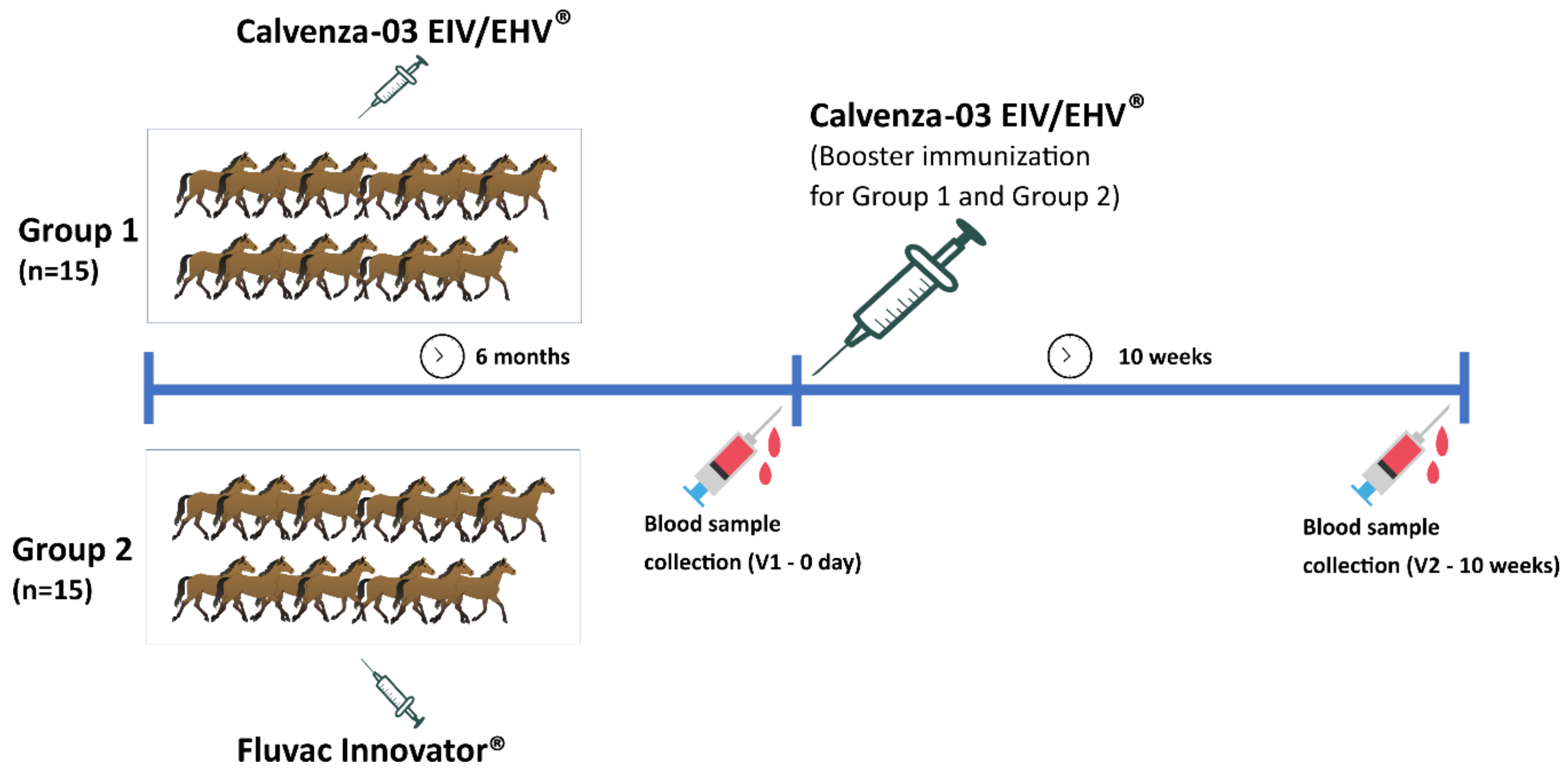
2.4. Vaccination Protocol
2.5. Sampling and Shipping
2.6. Serology
2.6.1. Single Radial Hemolysis
2.6.2. Virus Neutralization Assay
2.7. Interferon Gamma Assay
2.8. Statistical Data Analysis
3. Results
3.1. Clinical Signs
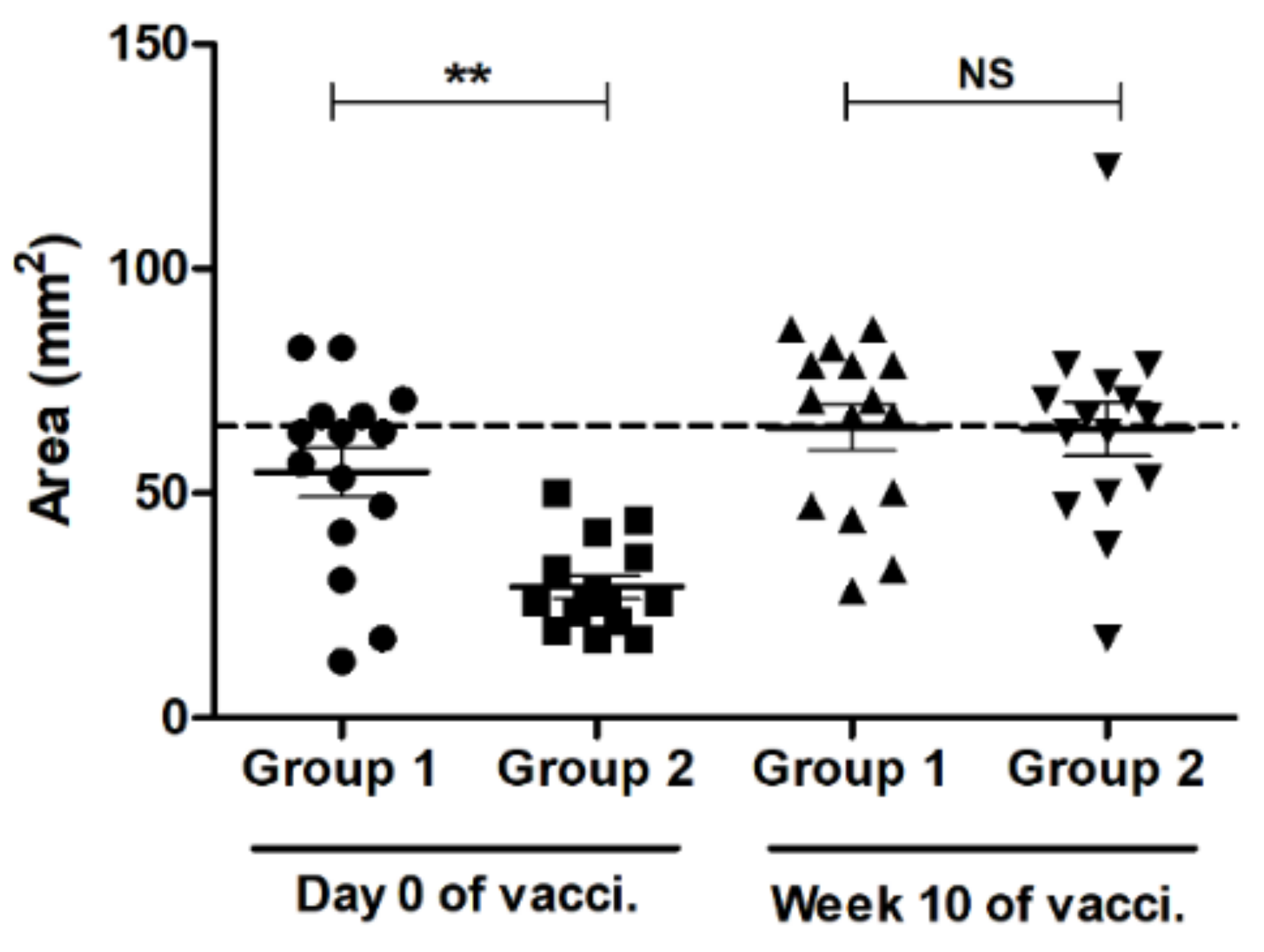
3.2. SRH Antibody Response to EIV
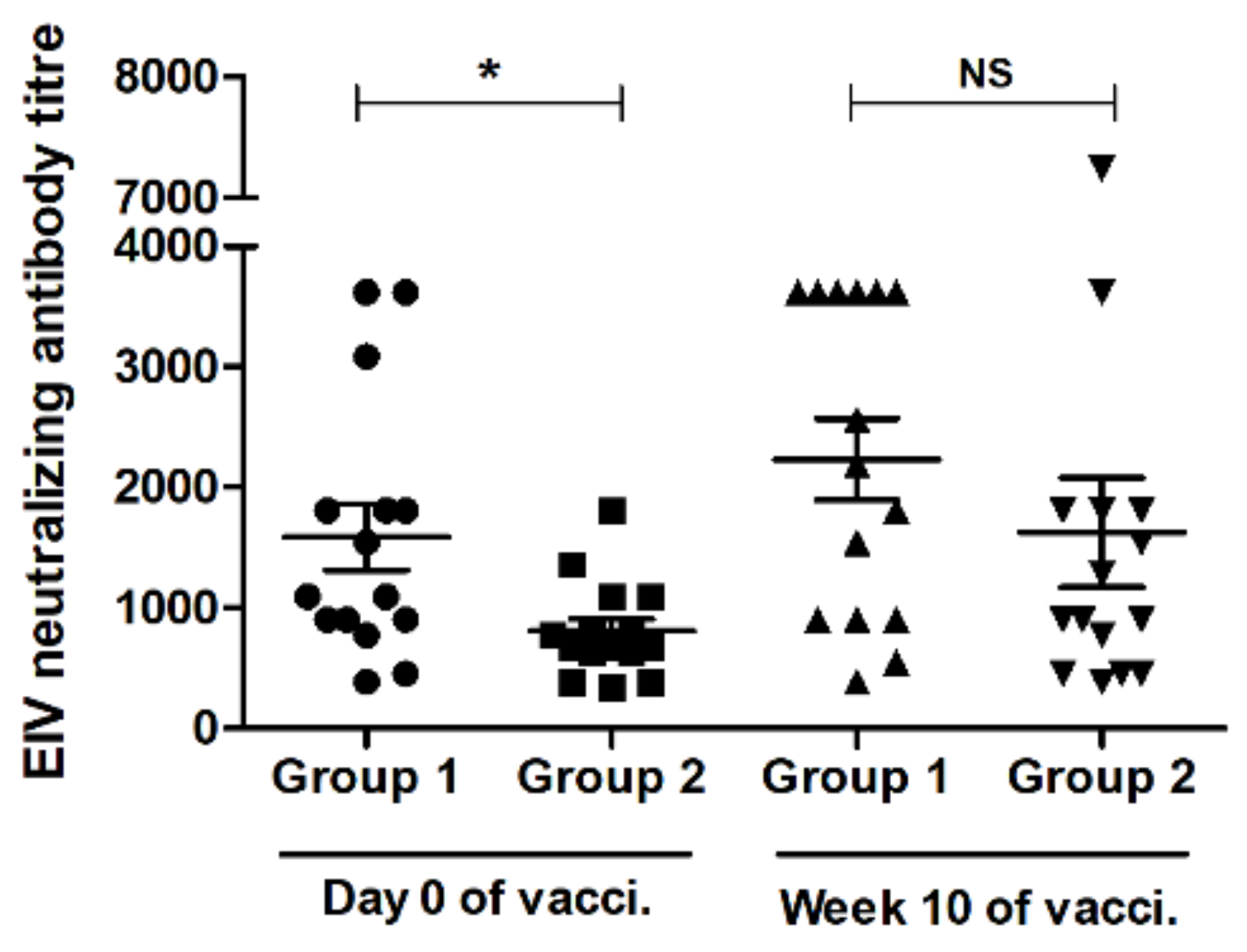
3.3. VN Assay Result
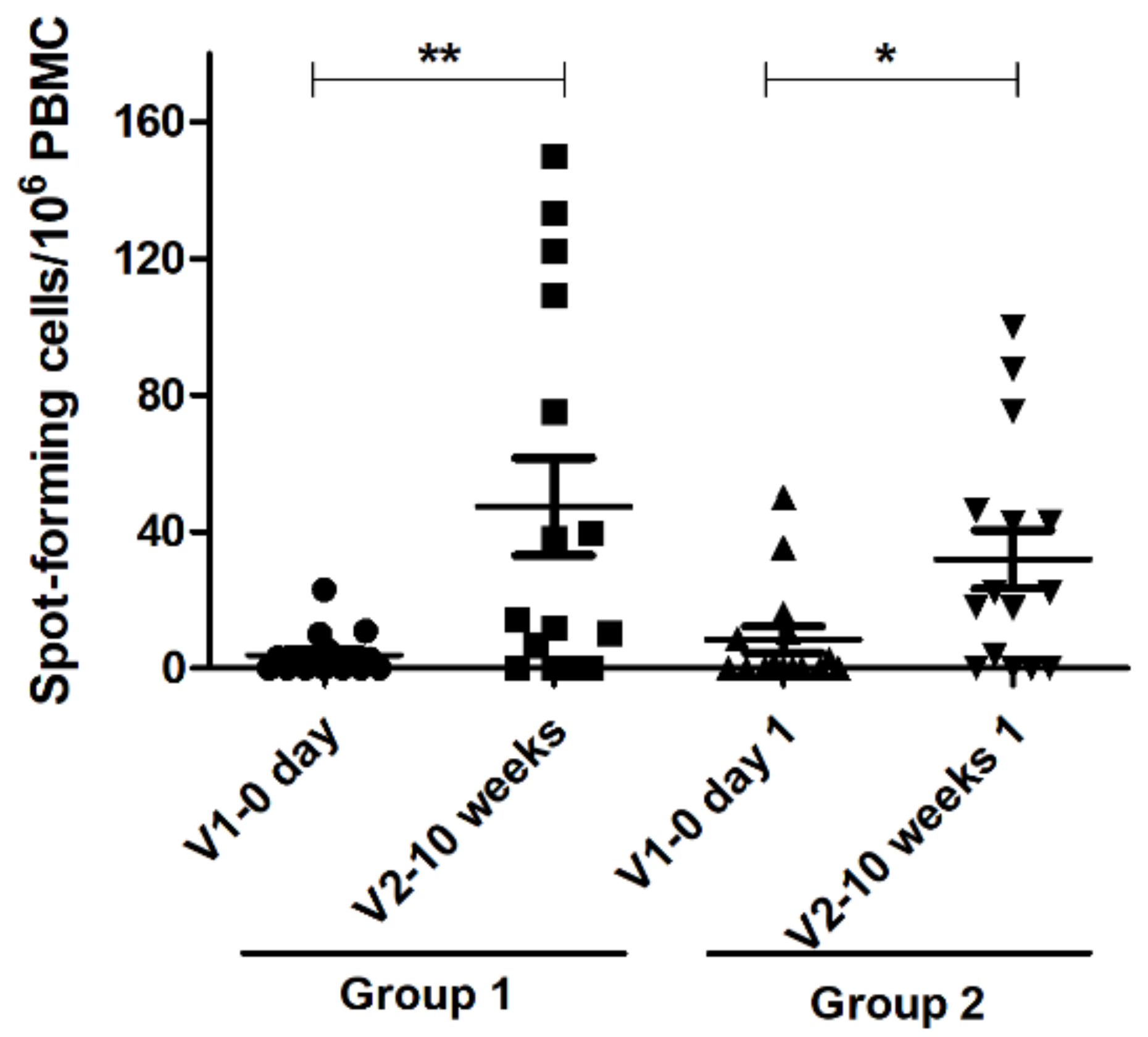
3.4. Interferon Gamma Assay Result
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paillot, R.; Prowse, L.; Montesso, F.; Stewart, B.; Jordon, L.; Newton, J.; Gilkerson, J. Duration of equine influenza virus shedding and infectivity in immunised horses after experimental infection with EIV A/eq2/Richmond/1/07. Vet. Microbiol. 2013, 166, 22–34. [Google Scholar] [CrossRef]
- Paillot, R.; Pitel, P.-H.; Pronost, S.; Legrand, L.; Fougerolle, S.; Jourdan, M.; Marcillaud-Pitel, C. Florida clade 1 equine influenza virus in France. Vet. Rec. 2019, 184, 101. [Google Scholar] [CrossRef] [PubMed]
- Mena, J.; Brito, B.; Moreira, R.; Tadich, T.; González, I.; Cruces, J.; Ortega, R.; Van Bakel, H.; Rathnasinghe, R.; Pizarro-Lucero, J.; et al. Reemergence of H3N8 Equine Influenza A virus in Chile, 2018. Transbound. Emerg. Dis. 2018, 65, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Fougerolle, S.; Fortier, C.; Legrand, L.; Jourdan, M.; Marcillaud-Pitel, C.; Pronost, S.; Paillot, R.; Marcillaud-Pitel, C. Success and Limitation of Equine Influenza Vaccination: The First Incursion in a Decade of a Florida Clade 1 Equine Influenza Virus that Shakes Protection Despite High Vaccine Coverage. Vaccines 2019, 7, 174. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xiao, Y.; Meng, F.; Sun, F.; Chen, M.; Cheng, Z.; Chen, Y.; Liu, S.; Chen, H. Emergence of H3N8 equine influenza virus in donkeys in China in 2017. Vet. Microbiol. 2018, 214, 1–6. [Google Scholar] [CrossRef]
- Miño, S.; Mojsiejczuk, L.; Guo, W.; Zhang, H.; Qi, T.; Du, C.; Zhang, X.; Wang, J.; Campos, R.; Wang, X. Equine Influenza Virus in Asia: Phylogeographic Pattern and Molecular Features Reveal Circulation of an Autochthonous Lineage. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Shittu, I.; Meseko, C.A.; Sulaiman, L.P.; Inuwa, B.; Mustapha, M.; Zakariya, P.S.; Muhammad, A.A.; Muhammad, U.; Atuman, Y.J.; Barde, I.J.; et al. Fatal multiple outbreaks of equine influenza H3N8 in Nigeria, 2019: The first introduction of Florida clade 1 to West Africa. Vet. Microbiol. 2020, 248, 108820. [Google Scholar] [CrossRef]
- Sreenivasan, C.C.; Jandhyala, S.S.; Luo, S.; Hause, B.M.; Thomas, M.; Knudsen, D.E.B.; Leslie-Steen, P.; Clement, T.; Reedy, S.E.; Chambers, T.M.; et al. Phylogenetic Analysis and Characterization of a Sporadic Isolate of Equine Influenza A H3N8 from an Unvaccinated Horse in 2015. Viruses 2018, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Rash, A.; Morton, R.; Woodward, A.; Maes, O.; McCauley, J.; Bryant, N.; Elton, D. Evolution and Divergence of H3N8 Equine Influenza Viruses Circulating in the United Kingdom from 2013 to 2015. Pathogens 2017, 6, 6. [Google Scholar] [CrossRef]
- Perglione, C.O.; Golemba, M.D.; Torres, C.; Barrandeguy, M. Molecular Epidemiology and Spatio-Temporal Dynamics of the H3N8 Equine Influenza Virus in South America. Pathogens 2016, 5, 61. [Google Scholar] [CrossRef]
- Daly, J.M.; Newton, J.R.; Mumford, J.A. Current perspectives on control of equine influenza. Vet. Res. 2004, 35, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Reemers, S.; Sonnemans, D.; Horspool, L.; Van Bommel, S.; Cao, Q.; Van De Zande, S. Determining Equine Influenza Virus Vaccine Efficacy—The Specific Contribution of Strain Versus Other Vaccine Attributes. Vaccines 2020, 8, 501. [Google Scholar] [CrossRef]
- Pavulraj, S.; Virmani, N.; Bera, B.C.; Joshi, A.; Anand, T.; Virmani, M.; Singh, R.; Singh, R.K.; Tripathi, B.N. Immunogenicity and protective efficacy of inactivated equine influenza (H3N8) virus vaccine in murine model. Vet. Microbiol. 2017, 210, 188–196. [Google Scholar] [CrossRef]
- Gamoh, K.; Nakamura, S. Update of inactivated equine influenza vaccine strain in Japan. J. Vet. Med. Sci. 2017, 79, 649–653. [Google Scholar] [CrossRef][Green Version]
- Paillot, R.; Prowse, L.; Montesso, F.; Huang, C.; Barnes, H.; Escala, J. Whole inactivated equine influenza vaccine: Efficacy against a representative clade 2 equine influenza virus, IFNgamma synthesis and duration of humoral immunity. Vet. Microbiol. 2013, 162, 396–407. [Google Scholar] [CrossRef]
- Paillot, R.; Rash, N.L.; Garrett, D.; Prowse-Davis, L.; Montesso, F.; Cullinane, A.; Lemaitre, L.; Thibault, J.-C.; Wittreck, S.; Dancer, A. How to Meet the Last OIE Expert Surveillance Panel Recommendations on Equine Influenza (EI) Vaccine Composition: A Review of the Process Required for the Recombinant Canarypox-Based EI Vaccine. Pathogens 2016, 5, 64. [Google Scholar] [CrossRef]
- Entenfellner, J.; Gahan, J.; Garvey, M.; Walsh, C.; Venner, M.; Cullinane, A. Response of Sport Horses to Different Formulations of Equine Influenza Vaccine. Vaccines 2020, 8, 372. [Google Scholar] [CrossRef]
- Chambers, T.M.; Holland, R.E.; Tudor, L.R.; Townsend, H.G.G.; Cook, A.; Bogdan, J.; Lunn, D.P.; Hussey, S.; Whitaker-Dowling, P.; Youngner, J.S.; et al. A new modified live equine influenza virus vaccine: Phenotypic stability, restricted spread and efficacy against heterologous virus challenge. Equine Vet. J. 2010, 33, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Youngner, J.S.; Whitaker-Dowling, P.; Chambers, T.M.; Rushlow, K.E.; Sebring, R. Derivation and characterization of a live attenuated equine influenza vaccine virus. Am. J. Vet. Res. 2001, 62, 1290–1294. [Google Scholar] [CrossRef] [PubMed]
- Tabynov, K.; Kydyrbayev, Z.; Ryskeldinova, S.; Assanzhanova, N.; Kozhamkulov, Y.; Inkarbekov, D.; Sansyzbay, A. Safety and immunogenicity of a novel cold-adapted modified-live equine influenza virus vaccine. Aust. Vet. J. 2014, 92, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Lobo, P.; Rodriguez, L.; Reedy, S.; Oladunni, F.S.; Nogales, A.; Murcia, P.R.; Chambers, T.M.; Martinez-Sobrido, L. A Bivalent Live-Attenuated Vaccine for the Prevention of Equine Influenza Virus. Viruses 2019, 11, 933. [Google Scholar] [CrossRef]
- Mathew, M.K.; Virmani, N.; Bera, B.C.; Anand, T.; Kumar, R.; Balena, V.; Sansanwal, R.; Pavulraj, S.; Sundaram, K.; Virmani, M.; et al. Protective efficacy of inactivated reverse genetics based equine influenza vaccine candidate adjuvanted with MontanideTM Pet Gel in murine model. J. Vet. Med. Sci. 2019, 81, 1753–1762. [Google Scholar] [CrossRef] [PubMed]
- Gildea, S.; Garvey, M.; Lyons, P.; Lyons, R.; Gahan, J.; Walsh, C.; Cullinane, A. Multifocal Equine Influenza Outbreak with Vaccination Breakdown in Thoroughbred Racehorses. Pathogens 2018, 7, 43. [Google Scholar] [CrossRef]
- Daly, J.M.; Elton, D. Potential of a sequence-based antigenic distance measure to indicate equine influenza vaccine strain efficacy. Vaccine 2013, 31, 6043–6045. [Google Scholar] [CrossRef] [PubMed]
- Daly, J.M.; Macrae, S.; Newton, J.R.; Wattrang, E.; Elton, D.M. Equine influenza: A review of an unpredictable virus. Vet. J. 2011, 189, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Mumford, J.; Wood, J.M.; Scott, A.M.; Folkers, C.; Schild, G.C. Studies with inactivated equine influenza vaccine: 2. Protection against experimental infection with influenza virus A/equine/Newmarket/79 (H3N8). J. Hyg. 1983, 90, 385–396. [Google Scholar] [CrossRef]
- Mumford, J.A.; Wood, J.M.; Folkers, C.; Schild, G.C. Protection against experimental infection with influenza virus A/equine/Miami/63 (H3N8) provided by inactivated whole virus vaccines containing homologous virus. Epidemiol. Infect. 1988, 100, 501–510. [Google Scholar] [CrossRef]
- Mumford, J.A.; Wood, J. Establishing an acceptability threshold for equine influenza vaccines. Dev. Biol. Stand. 1992, 79, 137–146. [Google Scholar]
- Holmes, M.A.; Townsend, H.G.; Kohler, A.K.; Hussey, S.; Breathnach, C.; Barnett, C.; Holland, R.; Lunn, D. Immune responses to commercial equine vaccines against equine herpesvirus-1, equine influenza virus, eastern equine encephalomyelitis, and tetanus. Vet. Immunol. Immunopathol. 2006, 111, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; Kydd, J.; Macrae, S.; Minke, J.; Hannant, D.; Daly, J. New assays to measure equine influenza virus-specific Type 1 immunity in horses. Vaccine 2007, 25, 7385–7398. [Google Scholar] [CrossRef]
- Equine Influenza. Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.05.07_EQ_INF.pdf (accessed on 17 February 2021).
- Morley, P.S.; Bogdan, J.R.; Townsend, H.G.; Haines, D.M. The effect of changing single radial hemolysis assay method when quantifying influenza A antibodies in serum. Vet. Microbiol. 1995, 44, 101–110. [Google Scholar] [CrossRef]
- Tangri, S.; Ishioka, G.Y.; Huang, X.; Sidney, J.; Southwood, S.; Fikes, J.; Sette, A. Structural Features of Peptide Analogs of Human Histocompatibility Leukocyte Antigen Class I Epitopes That Are More Potent and Immunogenic than Wild-Type Peptide. J. Exp. Med. 2001, 194, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R. A Systematic Review of Recent Advances in Equine Influenza Vaccination. Vaccines 2014, 2, 797–831. [Google Scholar] [CrossRef]
- Gildea, S.; Quinlivan, M.; Murphy, B.A.; Cullinane, A. Humoral response and antiviral cytokine expression following vaccination of thoroughbred weanlings—A blinded comparison of commercially available vaccines. Vaccine 2013, 31, 5216–5222. [Google Scholar] [CrossRef] [PubMed]
- Dilai, M.; Piro, M.; El Harrak, M.; Fougerolle, S.; Dehhaoui, M.; Dikrallah, A.; Legrand, L.; Paillot, R.; Fihri, O.F. Impact of Mixed Equine Influenza Vaccination on Correlate of Protection in Horses. Vaccines 2018, 6, 71. [Google Scholar] [CrossRef]
- Yamanaka, T.; Nemoto, M.; Bannai, H.; Tsujimura, K.; Matsumura, T.; Kokado, H.; Gildea, S.; Cullinane, A. Neutralization antibody response to booster/priming immunization with new equine influenza vaccine in Japan. J. Vet. Med. Sci. 2018, 80, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; Kydd, J.; Sindle, T.; Hannant, D.; Toulemonde, C.E.; Audonnet, J.; Minke, J.; Daly, J. Antibody and IFN-γ responses induced by a recombinant canarypox vaccine and challenge infection with equine influenza virus. Vet. Immunol. Immunopathol. 2006, 112, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Bryant, N.A.; Paillot, R.; Rash, A.S.; Medcalf, E.; Montesso, F.; Ross, J.; Watson, J.; Jeggo, M.; Lewis, N.S.; Newton, J.R.; et al. Comparison of two modern vaccines and previous influenza infection against challenge with an equine influenza virus from the Australian 2007 outbreak. Vet. Res. 2009, 41, 1–15. [Google Scholar] [CrossRef] [PubMed]
| S. No.: | Vaccine | Company | Adjuvant | Virus Strain | Type of Vaccine | Region in Use |
|---|---|---|---|---|---|---|
| 1. | Calvenza-03 EIV/EHV® | Boehringer Ingelheim | Carbimmune | A/Equi-2/Newmarket/93 A/Equi-2/Kentucky/95 A/Equi-2/Ohio/03 EHV-1 souche KyA | Inactivated vaccine | USA |
| 2. | Duvaxyn IE-T® | Elanco Animal Health | Carbomer, Aluminium hydroxide | A/Equi-1/Prague/56 A/Equi-2/Newmarket/93 A/Equi-2/Suffolk/89 | Inactivated vaccine | Europe |
| 3. | Equilis® Prequenza-TE | MSD Animal Health | Saponin, Cholesterol, Phosphatidylcholine | A/Equi-2/South Africa/03 A/Equi-2/Newmarket/93 | Inactivated vaccine | Europe |
| 4. | Equi N Tect FLU® | Nisseiken Co Ltd. | Nil | A/equi-2/Ibaraki/1/07 A/equi-2/Yokohama/10 | Inactivated vaccine | Japan |
| 5. | Fluvac Innovator® | Zoetis LLC | MetaStim® | A/equi-2/ Kentucky/97 EHV-1 and EHV-4 | Inactivated vaccine | USA |
| 6. | Equip-FT® | Pfizer | ISCOM, Quillaic Acid derivative, Aluminium phosphate | A/Equi-1/Newmarket/77 A/Equi-2/Borlange/91 A/Equi-2/Kentucky/98 | Subunit vaccine | Europe |
| 7. | Flu Avert® I.N. | MSD Animal Health | Nil | Cold adapted A/Equi-2/Kentucky/91 | Modified live viral vaccine | Europe USA |
| 8. | Proteqflu-TE® | Boehringer Ingelheim | Carbomer | A/Equi-2/Ohio/03 A/Equi-2/Richmond/07 | Vector based canary pox vaccine | Europe |
| Group | Test | Positive | Negative | Total | Fisher’s Exact Test p-Value |
|---|---|---|---|---|---|
| G1: V1-0 day | SRH | 5 | 10 | 15 | 0.2723; NS |
| VN | 9 | 6 | 15 | ||
| G1: V2 - 10 week | SRH | 10 | 5 | 15 | 1.0000; NS |
| VN | 10 | 5 | 15 | ||
| G1: V1 - 0 day + V2 - 10 weeks | SRH | 15 | 15 | 30 | 0.4348; NS |
| VN | 19 | 11 | 30 | ||
| G2: V1-0 day | SRH | 0 | 15 | 15 | 0.0996; NS |
| VN | 4 | 11 | 15 | ||
| G2: V2 - 10 week | SRH | 8 | 7 | 15 | 1.0000; NS |
| VN | 7 | 8 | 15 | ||
| G2: V1 - 0 day + V2 - 10 weeks | SRH | 8 | 22 | 30 | 0.5796; NS |
| VN | 11 | 19 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavulraj, S.; Bergmann, T.; Trombetta, C.M.; Marchi, S.; Montomoli, E.; Alami, S.S.E.; Ragni-Alunni, R.; Osterrieder, N.; Azab, W. Immunogenicity of Calvenza-03 EIV/EHV® Vaccine in Horses: Comparative In Vivo Study. Vaccines 2021, 9, 166. https://doi.org/10.3390/vaccines9020166
Pavulraj S, Bergmann T, Trombetta CM, Marchi S, Montomoli E, Alami SSE, Ragni-Alunni R, Osterrieder N, Azab W. Immunogenicity of Calvenza-03 EIV/EHV® Vaccine in Horses: Comparative In Vivo Study. Vaccines. 2021; 9(2):166. https://doi.org/10.3390/vaccines9020166
Chicago/Turabian StylePavulraj, Selvaraj, Tobias Bergmann, Claudia Maria Trombetta, Serena Marchi, Emanuele Montomoli, Sidi Sefiane El Alami, Roberto Ragni-Alunni, Nikolaus Osterrieder, and Walid Azab. 2021. "Immunogenicity of Calvenza-03 EIV/EHV® Vaccine in Horses: Comparative In Vivo Study" Vaccines 9, no. 2: 166. https://doi.org/10.3390/vaccines9020166
APA StylePavulraj, S., Bergmann, T., Trombetta, C. M., Marchi, S., Montomoli, E., Alami, S. S. E., Ragni-Alunni, R., Osterrieder, N., & Azab, W. (2021). Immunogenicity of Calvenza-03 EIV/EHV® Vaccine in Horses: Comparative In Vivo Study. Vaccines, 9(2), 166. https://doi.org/10.3390/vaccines9020166






