Multi-Valent Protein Hybrid Pneumococcal Vaccines: A Strategy for the Next Generation of Vaccines
Abstract
:1. Introduction
Pneumococcal Capsule
2. The Rationale for Capsule-Based Vaccines and Their Success
2.1. Pneumococcal Polysaccharide Vaccine: PPSV23
2.2. Polysaccharide Conjugate Vaccines: PCV7,10, and 13
3. Limitations of Polysaccharide-Based Vaccines
3.1. Serotype Replacement
3.2. Antibiotic Resistance
3.3. Capsule Shedding and Phase Variation
3.4. Serotype 3
3.5. The Elderly Are Highly Susceptible to Pneumococcal Disease
4. Advantages of Multiprotein-Based Vaccines
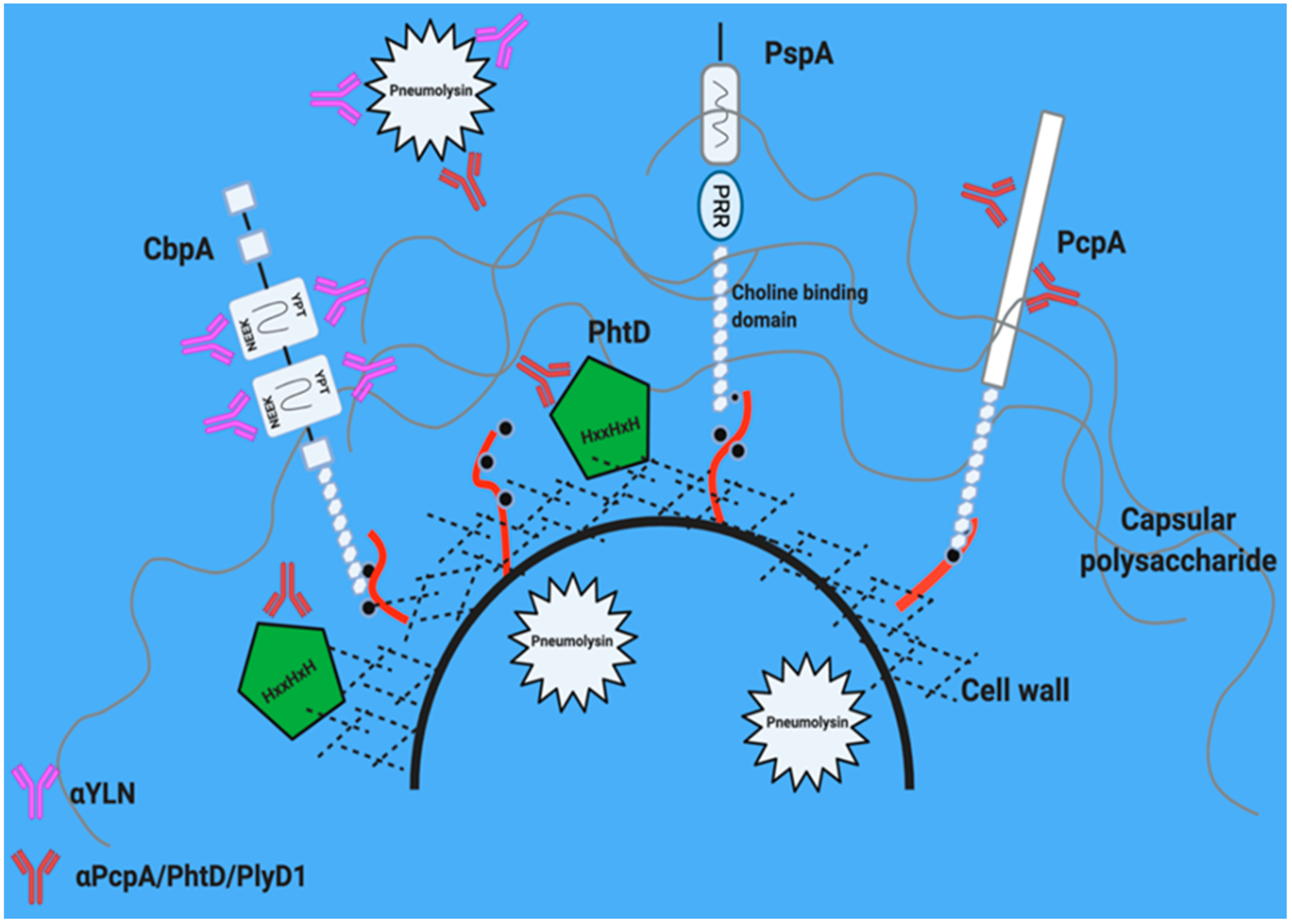
5. Leading Candidate Proteins
5.1. Pneumolysin
5.2. Pneumococcal Surface Protein A (PspA)
5.3. Choline Binding Protein A (CbpA/PspC)
5.4. Pneumococcal Choline Binding Protein A: PcpA
5.5. Histidine Triad Protein D: PhtD
6. Why Immunization with More Than One Protein Is Better. A Potential Role for Hybrid Antigens within the Conjugate Vaccine
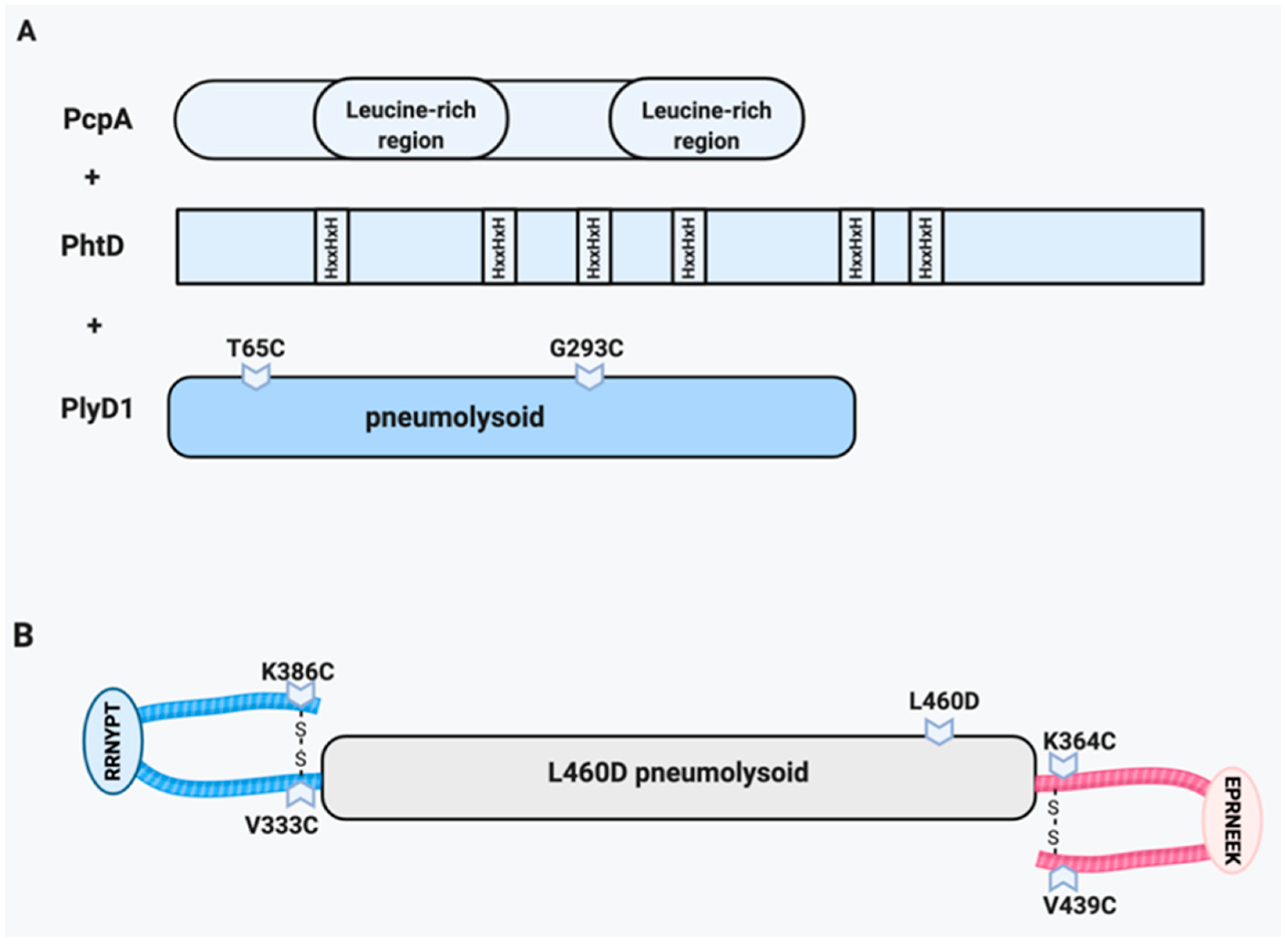
6.1. PcpA/PhtD/Pneumolysin
6.2. Pneumolysin/CbpA Hybrid (YLN)
6.3. Integrated Approaches
7. Live and Whole Cell Vaccines as Alternatives
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hoffman, J.A.; Mason, E.O.; Schutze, G.E.; Tan, T.Q.; Barson, W.J.; Givner, L.B.; Wald, E.R.; Bradley, J.S.; Yogev, R.; Kaplan, S.L. Streptococcus pneumoniae infections in the neonate. Pediatrics 2003, 112, 1095–1102. [Google Scholar] [CrossRef]
- Stupka, J.E.E.; Mortensen, E.M.; Anzueto, A.; Restrepo, M.I. Community-acquired pneumonia in elderly patients. Aging Health 2009, 5, 763–774. [Google Scholar] [CrossRef] [Green Version]
- Morrill, H.J.; Caffrey, A.R.; Noh, E.; Laplante, K.L. Epidemiology of pneumococcal disease in a national cohort of older adults. Infect. Dis. Ther. 2014, 3, 19–33. [Google Scholar] [CrossRef] [Green Version]
- van Aalst, M.; Lötsch, F.; Spijker, R.; van der Meer, J.T.M.; Langendam, M.W.; Goorhuis, A.; Grobusch, M.P.; de Bree, G.J. Incidence of invasive pneumococcal disease in immunocompromised patients: A systematic review and meta-analysis. Travel. Med. Infect. Dis. 2018, 24, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Backhaus, E.; Berg, S.; Andersson, R.; Ockborn, G.; Malmström, P.; Dahl, M.; Nasic, S.; Trollfors, B. Epidemiology of invasive pneumococcal infections: Manifestations, incidence and case fatality rate correlated to age, gender and risk factors. BMC Infect. Dis. 2016, 16, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regev-Yochay, G.; Raz, M.; Dagan, R.; Porat, N.; Shainberg, B.; Pinco, E.; Keller, N.; Rubinstein, E. Nasopharyngeal carriage of Streptococcus pneumoniae by adults and children in community and family settings. Clin. Infect. Dis. 2004, 38, 632–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahl, B.; O’Brien, K.L.; Greenbaum, A.; Majumder, A.; Liu, L.; Chu, Y.; Lukšić, I.; Nair, H.; McAllister, D.A.; Campbell, H.; et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: Global, regional, and national estimates for 2000–15. Lancet Glob. Heal. 2018, 6, e744–e757. [Google Scholar] [CrossRef] [Green Version]
- Troeger, C.; Forouzanfar, M.; Rao, P.C.; Khalil, I.; Brown, A.; Swartz, S.; Fullman, N.; Mosser, J.; Thompson, R.L.; Reiner, R.C.; et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017, 17, 1133–1161. [Google Scholar] [CrossRef] [Green Version]
- Ganaie, F.; Saad, J.S.; McGee, L.; Van Tonder, A.J.; Bentley, S.D.; Lo, S.W.; Gladstone, R.A.; Turner, P.; Keenan, J.D.; Breiman, R.F.; et al. A new pneumococcal capsule type, 10D, is the 100th serotype and has a large cps fragment from an oral streptococcus. mBio 2020, 11, e00937-20. [Google Scholar] [CrossRef]
- Weiser, J.N.; Ferreira, D.M.; Paton, J.C. Streptococcus pneumoniae: Transmission, colonization and invasion. Nat. Rev. Genet. 2018, 16, 355–367. [Google Scholar] [CrossRef]
- Li, Y.; Weinberger, D.M.; Thompson, C.M.; Trzciński, K.; Lipsitch, M. Surface charge of Streptococcus pneumoniae predicts serotype distribution. Infect. Immun. 2013, 81, 4519–4524. [Google Scholar] [CrossRef] [Green Version]
- Walsh, R.L.; Camilli, A. Streptococcus pneumoniae is desiccation tolerant and infectious upon rehydration. mBio 2011, 2, e00092-11. [Google Scholar] [CrossRef] [Green Version]
- Melin, M.; Trzciński, K.; Meri, S.; Käyhty, H.; Väkeväinen, M. The capsular serotype of Streptococcus pneumoniae is more important than the genetic background for resistance to complement. Infect. Immun. 2010, 78, 5262–5270. [Google Scholar] [CrossRef] [Green Version]
- de Vos, A.F.; Dessing, M.C.; Lammers, A.J.; de Porto, A.P.; Florquin, S.; de Boer, O.J.; de Beer, R.; Terpstra, S.; Bootsma, H.J.; Hermans, P.W.; et al. The polysaccharide capsule of Streptococcus pneumonia partially impedes MyD88-mediated immunity during pneumonia in mice. PLoS ONE 2015, 10, e0118181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hathaway, L.J.; Grandgirard, D.; Valente, L.G.; Täuber, M.G.; Leib, S.L. Streptococcus pneumoniae capsule determines disease severity in experimental pneumococcal meningitis. Open Biol. 2016, 6, 150269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geno, K.A.; Gilbert, G.L.; Song, J.Y.; Skovsted, I.C.; Klugman, K.P.; Jones, C.; Konradsen, H.B.; Nahm, M.H. Pneumococcal capsules and their types: Past, present, and future. Clin. Microbiol. Rev. 2015, 28, 871–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyams, C.; Camberlein, E.; Cohen, J.M.; Bax, K.; Brown, J.S. The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms. Infect. Immun. 2010, 78, 704–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, L.E.; Robinson, D.A.; McDaniel, L.S. Nonencapsulated Streptococcus pneumoniae: Emergence and pathogenesis. mBio 2016, 7, e01792-15. [Google Scholar] [CrossRef] [Green Version]
- Hamborsky, J.; Kroger, A. Epidemiology and prevention of vaccine-preventable diseases, E-Book: The Pink Book. Public Health Foundation, 2015. Available online: https://www.cdc.gov/vaccines/pubs/pinkbook/pneumo.html (accessed on 26 January 2021).
- Wright, A.; Parry, M.W.; Colebrook, L.; Dodgson, R.W. Observations on prophylactic inoculation against pneumococcus infections and the results of which have been achieved by it. Lancet 1914, 183, 87–95. [Google Scholar] [CrossRef]
- MacLeod, C.M.; Hodges, R.G.; Heidelberger, M.; Bernhard, W.G. Prevention of pneumococcal pneumonia by immunization with specific capsular polysaccharides. J. Exp. Med. 1945, 82, 445–465. [Google Scholar] [CrossRef] [Green Version]
- Daniels, C.C.; Rogers, P.D.; Shelton, C.M. A review of pneumococcal vaccines: Current polysaccharide vaccine recommendations and future protein antigens. J. Pediatr. Pharmacol. Ther. 2016, 21, 27–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alicino, C.; Paganino, C.; Orsi, A.; Astengo, M.; Trucchi, C.; Icardi, G.; Ansaldi, F. The impact of 10-valent and 13-valent pneumococcal conjugate vaccines on hospitalization for pneumonia in children: A systematic review and meta-analysis. Vaccine 2017, 35, 5776–5785. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.A.; Neuzil, K.M.; Yu, O.; Benson, P.; Barlow, W.E.; Adams, A.L.; Hanson, C.A.; Mahoney, L.D.; Shay, D.K.; Thompson, W.W. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N. Engl. J. Med. 2003, 348, 1747–1755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldman, C.; Anderson, R. Review: Current and new generation pneumococcal vaccines. J. Infect. 2014, 69, 309–325. [Google Scholar] [CrossRef] [Green Version]
- Zarei, A.E.; Almehdar, H.A.; Redwan, E.M. Hib vaccines: Past, present, and future perspectives. J. Immunol. Res. 2016, 2016, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Wiese, A.D.; Griffin, M.R.; Grijalva, C.G. Impact of pneumococcal conjugate vaccines on hospitalizations for pneumonia in the United States. Expert Rev. Vaccines 2019, 18, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Grijalva, C.G.; Nuorti, J.P.; Arbogast, P.G.; Martin, S.W.; Edwards, K.M.; Griffin, M.R. Decline in pneumonia admissions after routine childhood immunization with pneumococcal conjugate vaccine in the USA: A time-series analysis. Lancet 2007, 369, 1179–1186. [Google Scholar] [CrossRef]
- Zhou, F.; Kyaw, M.H.; Shefer, A.; Winston, C.A.; Nuorti, J.P. Health care utilization for pneumonia in young children after routine pneumococcal conjugate vaccine use in the United States. Arch. Pediatr. Adolesc. Med. 2007, 161, 1162–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barricarte, A.; Castilla, J.; Gil Setas, A.; Torroba, L.; Alonso, J.A.N.; Irisarri, F.; Arriazu, M. Effectiveness of the 7-Valent Pneumococcal Conjugate Vaccine: A population-based case-control study. Clin. Infect. Dis. 2007, 44, 1436–1441. [Google Scholar] [CrossRef] [Green Version]
- van der Linden, M.; Falkenhorst, G.; Perniciaro, S.; Imöhl, M. Effects of infant pneumococcal conjugate vaccination on serotype distribution in invasive pneumococcal disease among children and adults in Germany. PLoS ONE 2015, 10, e0131494. [Google Scholar] [CrossRef]
- Feikin, D.R.; Kagucia, E.W.; Loo, J.D.; Link-Gelles, R.; Puhan, M.A.; Cherian, T.; Levine, O.S.; Whitney, C.G.; O’Brien, K.L.; Moore, M.R.; et al. Serotype-specific changes in invasive pneumococcal disease after pneumococcal conjugate vaccine introduction: A pooled analysis of multiple surveillance sites. PLoS Med. 2013, 10, e1001517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonten, M.J.; Huijts, S.M.; Bolkenbaas, M.; Webber, C.; Patterson, S.; Gault, S.; Van Werkhoven, C.H.; Van Deursen, A.M.; Sanders, E.A.; Verheij, T.J.; et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N. Engl. J. Med. 2015, 372, 1114–1125. [Google Scholar] [CrossRef] [Green Version]
- Fattom, A.; Cho, Y.H.; Chu, C.; Fuller, S.; Fries, L.; Naso, R. Epitopic overload at the site of injection may result in suppression of the immune response to combined capsular polysaccharide conjugate vaccines. Vaccine 1999, 17, 126–133. [Google Scholar] [CrossRef]
- Carter, R.; Wolf, J.; Van Opijnen, T.; Muller, M.; Obert, C.; Burnham, C.; Mann, B.; Li, Y.; Hayden, R.T.; Pestina, T.; et al. Genomic analyses of pneumococci from children with sickle cell disease expose host-specific bacterial adaptations and deficits in current interventions. Cell Host Microbe 2014, 15, 587–599. [Google Scholar] [CrossRef] [Green Version]
- Hicks, L.A.; Harrison, L.H.; Flannery, B.; Hadler, J.L.; Schaffner, W.; Craig, A.S.; Jackson, D.; Thomas, A.; Beall, B.; Lynfield, R.; et al. Incidence of pneumococcal disease due to non–pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998–2004. J. Infect. Dis. 2007, 196, 1346–1354. [Google Scholar] [CrossRef]
- Løchen, A.; Croucher, N.J.; Anderson, R.M. Divergent serotype replacement trends and increasing diversity in pneumococcal disease in high income settings reduce the benefit of expanding vaccine valency. Sci. Rep. 2020, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Control CfD. Drug Resistance. 2020. Available online: https://www.cdc.gov/Pneumococcal/Drug-Resistance.html (accessed on 26 January 2021).
- De Croix, M.S.; Mitsi, E.; Morozov, A.; Glenn, S.; Andrew, P.W.; Ferreira, D.M.; Oggioni, M.R. Phase variation in pneumococcal populations during carriage in the human nasopharynx. Sci. Rep. 2020, 10, 1803. [Google Scholar] [CrossRef] [PubMed]
- Kietzman, C.C.; Gao, G.; Mann, B.; Myers, L.; Tuomanen, E.I. Dynamic capsule restructuring by the main pneumococcal autolysin LytA in response to the epithelium. Nat. Commun. 2016, 7, 10859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luck, J.N.; Tettelin, H.; Orihuela, C.J. Sugar-Coated Killer: Serotype 3 pneumococcal disease. Front. Cell. Infect. Microbiol. 2020, 10, 613287. [Google Scholar] [CrossRef]
- Choi, E.H.; Zhang, F.; Lu, Y.-J.; Malley, R. Capsular Polysaccharide (CPS) Release by serotype 3 pneumococcal strains reduces the protective effect of anti-type 3 CPS antibodies. Clin. Vaccine Immunol. 2015, 23, 162–167. [Google Scholar] [CrossRef] [Green Version]
- Andrews, N.J.A.; Waight, P.; Burbidge, P.; Pearce, E.; Roalfe, L.; Zancolli, M.; Slack, M.; Ladhani, S.N.; Miller, E.; Goldblatt, D. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: A postlicensure indirect cohort study. Lancet Infect. Dis. 2014, 14, 839–846. [Google Scholar] [CrossRef]
- Africano, H.F.; Serrano-Mayorga, C.C.; Ramirez-Valbuena, P.C.; Bustos, I.G.; Bastidas, A.A.; Vargas, H.; Gómez, S.; Rodriguez, A.; Orihuela, C.J.; Reyes, L.F. Major adverse cardiovascular events during invasive pneumococcal disease are serotype dependent. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Harboe, Z.B.; Thomsen, R.W.; Riis, A.; Valentiner-Branth, P.; Christensen, J.J.; Lambertsen, L.; Krogfelt, K.A.; Konradsen, H.B.; Benfield, T.L. Pneumococcal serotypes and mortality following invasive pneumococcal disease: A population-based cohort study. PLoS Med. 2009, 6, e1000081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva-Costa, C.; Brito, M.J.; Pinho, M.D.; Friães, A.; Aguiar, S.I.; Ramirez, M.; Melo-Cristino, J.; on behalf of the Portuguese Group for the Study of Streptococcal Infections. Pediatric complicated pneumonia caused by Streptococcus pneumoniae serotype 3 in 13-valent pneumococcal conjugate vaccinees, Portugal, 2010–2015. Emerg. Infect. Dis. 2018, 24, 1307–1314. [Google Scholar] [CrossRef] [Green Version]
- Pick, H.; Daniel, P.; Rodrigo, C.; Bewick, T.; Ashton, D.; Lawrence, H.; Baskaran, V.; Edwards-Pritchard, R.C.; Sheppard, C.; Eletu, S.D.; et al. Pneumococcal serotype trends, surveillance and risk factors in UK adult pneumonia, 2013–2018. Thorax 2019, 75, 38–49. [Google Scholar] [CrossRef]
- CDC. Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Streptococcus Pneumoniae. 2015. Available online: http://www.cdc.gov/abcs/reports-findings/survreports/spneu15.pdf (accessed on 26 January 2021).
- Loughran, A.J.; Orihuela, C.J.; Tuomanen, E.I. Streptococcus pneumoniae: Invasion and inflammation. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Christenson, B.; Hedlund, J.; Lundbergh, P.; Örtqvist, Å. Additive preventive effect of influenza and pneumococcal vaccines in elderly persons. Eur. Respir. J. 2004, 23, 363–368. [Google Scholar] [CrossRef]
- Welte, T.; Torres, A.; Nathwani, D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax 2010, 67, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Adler, H.; Ferreira, D.M.; Gordon, S.B.; Rylance, J. Pneumococcal capsular polysaccharide imunity in the elderly. Clin. Vaccine Immunol. 2017, 24, e00004-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, R.; Cohen, J.M.; Reglinski, M.; Jose, R.J.; Chan, W.Y.; Marshall, H.; de Vogel, C.; Gordon, S.; Goldblatt, D.; Petersen, F.C.; et al. Naturally acquired human immunity to pneumococcus is dependent on antibody to protein antigens. PLoS. Pathog 2017, 13, e1006137. [Google Scholar]
- D’Mello, A.; Riegler, A.N.; Martínez, E.; Beno, S.M.; Ricketts, T.D.; Foxman, E.F.; Orihuela, C.J.; Tettelin, H. An in vivo atlas of host–pathogen transcriptomes during Streptococcus pneumoniae colonization and disease. Proc. Natl. Acad. Sci. USA 2020, 117, 33507–33518. [Google Scholar] [CrossRef]
- Rowe, H.M.; Karlsson, E.; Echlin, H.; Chang, T.-C.; Wang, L.; Van Opijnen, T.; Pounds, S.B.; Schultz-Cherry, S.; Rosch, J.W. Bacterial factors required for transmission of Streptococcus pneumoniae in mammalian hosts. Cell. Host. Microbe 2019, 25, 884–891.e6. [Google Scholar] [CrossRef] [PubMed]
- Rosch, J.W. Promises and pitfalls of live attenuated pneumococcal vaccines. Hum. Vaccines Immunother. 2014, 10, 3000–3003. [Google Scholar] [CrossRef] [Green Version]
- Hurwitz, J.L.; Tuomanen, E. Unveiling unexpected immune activities induced by your pneumococcal vaccine. mBio 2016, 7, e00137-16. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Sevillano, E.; Ercoli, G.; Brown, J.S. Mechanisms of naturally acquired immunity to Streptococcus pneumoniae. Front. Immunol. 2019, 10, 358. [Google Scholar] [CrossRef] [Green Version]
- Ogunniyi, A.D.; Folland, R.L.; Briles, D.E.; Hollingshead, S.K.; Paton, J.C. Immunization of mice with combinations of pneumococcal virulence proteins elicits enhanced protection against challenge with Streptococcus pneumoniae. Infect. Immun. 2000, 68, 3028–3033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamel, J.; Charland, N.; Pineau, I.; Ouellet, C.; Rioux, S.; Martin, D.; Brodeur, B.R. Prevention of pneumococcal disease in mice immunized with conserved surface-accessible proteins. Infect. Immun. 2004, 72, 2659–2670. [Google Scholar] [CrossRef] [Green Version]
- Ogunniyi, A.D.; Grabowicz, M.; Briles, D.E.; Cook, J.; Paton, J.C. Development of a vaccine against invasive pneumococcal disease based on combinations of virulence proteins of Streptococcus pneumoniae. Infect. Immun. 2006, 75, 350–357. [Google Scholar] [CrossRef] [Green Version]
- Masomian, M.; Ahmad, Z.; Gew, L.T.; Poh, C.L. Development of next generation Streptococcus pneumoniae vaccines conferring broad protection. Vaccines 2020, 8, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, A.; Mann, B.; Gao, G.; Heath, R.; King, J.; Maissoneuve, J.; Alderson, M.; Tate, A.; Hollingshead, S.K.; Tweten, R.K.; et al. Multivalent pneumococcal protein vaccines comprising pneumolysoid with epitopes/fragments of CbpA and/or PspA elicit strong and broad protection. Clin. Vaccine Immunol. 2015, 22, 1079–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gor, D.O.; Ding, X.; Briles, D.E.; Jacobs, M.R.; Greenspan, N.S. Relationship between surface accessibility for PpmA, PsaA, and PspA and antibody-mediated immunity to systemic infection by Streptococcus pneumoniae. Infect. Immun. 2005, 73, 1304–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paton, J.C.; Andrew, P.W.; Boulnois, G.J.; Mitchell, T.J. Molecular analysis of the pathogenicity of Streptococcus pneumoniae: The role of pneumococcal proteins. Annu. Rev. Microbiol. 1993, 47, 89–115. [Google Scholar] [CrossRef]
- Marriott, T.J.M.A.D.H.D.H.M.; Mitchell, T.J.; Dockrell, D.H. Pneumolysin: A double-edged sword during the host-pathogen interaction. Curr. Mol. Med. 2008, 8, 497–509. [Google Scholar] [CrossRef]
- Johnson, M.K. Cellular location of pneumolysin. FEMS Microbiol. Lett. 1977, 2, 243–245. [Google Scholar] [CrossRef]
- Walker, J.A.; Allen, R.L.; Falmagne, P.; Johnson, M.K.; Boulnois, G.J. Molecular cloning, characterization, and complete nucleotide sequence of the gene for pneumolysin, the sulfhydryl-activated toxin of Streptococcus pneumoniae. Infect. Immun. 1987, 55, 1184–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, K.E.; Camilli, A. Pneumolysin localizes to the cell wall of Streptococcus pneumoniae. J. Bacteriol. 2009, 191, 2163–2168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alouf, J. Cholesterol-binding cytolytic protein toxins. Int. J. Med. Microbiol. 2000, 290, 351–356. [Google Scholar] [CrossRef]
- Gilbert, R.J.; Jiménez, J.L.; Chen, S.; Tickle, I.J.; Rossjohn, J.; Parker, M.; Andrew, P.W.; Saibil, H.R. Two structural transitions in membrane pore formation by pneumolysin, the pore-forming toxin of Streptococcus pneumoniae. Cell 1999, 97, 647–655. [Google Scholar] [CrossRef] [Green Version]
- Beno, S.M.; Riegler, A.N.; Gilley, R.P.; Brissac, T.; Wang, Y.; Kruckow, K.L.; Jadapalli, J.K.; Wright, G.M.; Shenoy, A.T.; Stoner, S.N.; et al. Inhibition of necroptosis to prevent long-term cardiac damage during pneumococcal pneumonia and invasive disease. J. Infect. Dis. 2020, 222, 1882–1893. [Google Scholar] [CrossRef]
- González-Juarbe, N.; Bradley, K.M.; Shenoy, A.T.; Gilley, R.P.; Reyes, L.F.; Hinojosa, C.A.; Restrepo, M.I.; Dube, P.H.; Bergman, M.A.; Orihuela, C.J. Pore-forming toxin-mediated ion dysregulation leads to death receptor-independent necroptosis of lung epithelial cells during bacterial pneumonia. Cell Death Differ. 2017, 24, 917–928. [Google Scholar] [CrossRef] [Green Version]
- Beurg, M.; Hafidi, A.; Skinner, L.; Cowan, G.; Hondarrague, Y.; Mitchell, T.J.; Dulon, D. The mechanism of pneumolysin-induced cochlear hair cell death in the rat. J. Physiol. 2005, 568, 211–227. [Google Scholar] [CrossRef] [Green Version]
- Witzenrath, M.; Pache, F.; Lorenz, D.; Koppe, U.; Gutbier, B.; Tabeling, C.; Reppe, K.; Meixenberger, K.; Dorhoi, A.; Ma, J.T.; et al. The NLRP3 inflammasome is differentially activated by pneumolysin variants and contributes to host defense in pneumococcal pneumonia. J. Immunol. 2011, 187, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Alhamdi, Y.; Neill, D.R.; Abrams, S.T.; Malak, H.A.; Yahya, R.; Barrett-Jolley, R.; Wang, G.; Kadioglu, A.; Toh, C.-H. Circulating pneumolysin is a potent inducer of cardiac injury during pneumococcal infection. PLOS Pathog. 2015, 11, e1004836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huo, Z.; Spencer, O.; Miles, J.; Johnson, J.; Holliman, R.; Sheldon, J.; Riches, P. Antibody response to pneumolysin and to pneumococcal capsular polysaccharide in healthy individuals and Streptococcus pneumoniae infected patients. Vaccine 2004, 22, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Musher, D.M.; Phan, H.M.; Baughn, R.E. Protection against bacteremic pneumococcal infection by antibody to pneumolysin. J. Infect. Dis. 2001, 183, 827–830. [Google Scholar] [CrossRef] [Green Version]
- Salha, D.; Szeto, J.; Myers, L.; Claus, C.; Sheung, A.; Tang, M.; Ljutic, B.; Hanwell, D.; Ogilvie, K.; Ming, M.; et al. Neutralizing antibodies elicited by a novel detoxified pneumolysin derivative, PlyD1, provide protection against both pneumococcal infection and lung injury. Infect. Immun. 2012, 80, 2212–2220. [Google Scholar] [CrossRef] [Green Version]
- Rosenow, C.; Ryan, P.; Weiser, J.N.; Johnson, S.; Fontan, P.; Ortqvist, A.; Masure, H.R. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol. Microbiol. 1997, 25, 819–829. [Google Scholar] [CrossRef]
- Hammerschmidt, S.; Bethe, G.; Remane, P.H.; Chhatwal, G.S. Identification of pneumococcal surface protein A as a lactoferrin-binding protein of Streptococcus pneumoniae. Infect. Immun. 1999, 67, 1683–1687. [Google Scholar] [CrossRef] [PubMed]
- Mukerji, R.; Mirza, S.; Roche, A.M.; Widener, R.W.; Croney, C.M.; Rhee, D.-K.; Weiser, J.N.; Szalai, A.J.; Briles, D.E. Pneumococcal Surface Protein A inhibits complement deposition on the pneumococcal surface by competing with the binding of C-Reactive Protein to cell-surface phosphocholine. J. Immunol. 2012, 189, 5327–5335. [Google Scholar] [CrossRef] [Green Version]
- Coats, M.T.; Benjamin, W.H.; Hollingshead, S.K.; Briles, D.E. Antibodies to the pneumococcal surface protein A, PspA, can be produced in splenectomized and can protect splenectomized mice from infection with Streptococcus pneumoniae. Vaccine 2005, 23, 4257–4262. [Google Scholar] [CrossRef] [PubMed]
- Briles, D.E.; Hollingshead, S.K.; King, J.; Swift, A.; Braun, P.A.; Park, M.K.; Ferguson, L.M.; Nahm, M.H.; Nabors, G.S. Immunization of Humans with Recombinant Pneumococcal Surface Protein A (rPspA) Elicits antibodies that passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J. Infect. Dis. 2000, 182, 1694–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollingshead, S.K.; Becker, R.; Briles, D.E. Diversity of PspA: Mosaic genes and evidence for past recombination in Streptococcus pneumoniae. Infect. Immun. 2000, 68, 5889–5900. [Google Scholar] [CrossRef] [Green Version]
- Rolo, D.; Ardanuy, C.; Fleites, A.; Martin, R.; Liñares, J. Diversity of pneumococcal surface protein A (PspA) among prevalent clones in Spain. BMC Microbiol. 2009, 9, 80. [Google Scholar] [CrossRef] [Green Version]
- Nabors, G.S.; Braun, P.A.; Herrmann, D.J.; Heise, M.L.; Pyle, D.J.; Gravenstein, S.; Schilling, M.; Ferguson, L.M.; Hollingshead, S.K.; Briles, D.E.; et al. Immunization of healthy adults with a single recombinant pneumococcal surface protein A (PspA) variant stimulates broadly cross-reactive antibodies to heterologous PspA molecules. Vaccine 2000, 18, 1743–1754. [Google Scholar] [CrossRef]
- Lu, L.; Lamm, M.E.; Li, H.; Corthesy, B.; Zhang, J.-R. The human polymeric immunoglobulin receptor binds to Streptococcus pneumoniae via Domains 3 and 4. J. Biol. Chem. 2003, 278, 48178–48187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orihuela, C.J.; Mahdavi, J.; Thornton, J.; Mann, B.; Wooldridge, K.G.; Abouseada, N.; Oldfield, N.J.; Self, T.; Ala’Aldeen, D.A.; Tuomanen, E.I. Laminin receptor initiates bacterial contact with the blood brain barrier in experimental meningitis models. J. Clin. Investig. 2009, 119, 1638–1646. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Ma, Y.; Zhang, J.-R. Streptococcus pneumoniae recruits Complement Factor H through the amino terminus of CbpA. J. Biol. Chem. 2006, 281, 15464–15474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, R.M.A.; Paton, J.C. Differential role of CbpA and PspA in modulation of in vitro CXC chemokine responses of respiratory epithelial cells to Iifection with Streptococcus pneumoniae. Infect. Immun. 2006, 74, 6739–6749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.R.; Mostov, K.E.; Lamm, M.E.; Nanno, M.; Shimida, S.; Ohwaki, M.; Tuomanen, E. The polymeric immunoglobulin receptor translocates pneumococci across human nasopharyngeal epithelial cells. Cell 2000, 102, 827–837. [Google Scholar] [CrossRef] [Green Version]
- Orihuela, C.J.; Gao, G.; Francis, K.P.; Yu, J.; Tuomanen, E.I. Tissue-specific contributions of pneumococcal virulence factors to pathogenesis. J. Infect. Dis. 2004, 190, 1661–1669. [Google Scholar] [CrossRef]
- Mann, B.; Thornton, J.; Heath, R.; Wade, K.R.; Tweten, R.K.; Gao, G.; El Kasmi, K.; Jordan, J.B.; Mitrea, D.M.; Kriwacki, R.; et al. Broadly protective protein-based pneumococcal vaccine composed of pneumolysin toxoid-CbpA peptide recombinant fusion protein. J. Infect. Dis. 2014, 209, 1116–1125. [Google Scholar] [CrossRef] [Green Version]
- Ogunniyi, A.D.; Woodrow, M.C.; Poolman, J.T.; Paton, J.C. Protection against Streptococcus pneumoniae elicited by immunization with pneumolysin and CbpA. Infect. Immun. 2001, 69, 5997–6003. [Google Scholar] [CrossRef] [Green Version]
- Balachandran, P.; Brooks-Walter, A.; Virolainen-Julkunen, A.; Hollingshead, S.K.; Briles, D.E. Role of pneumococcal Surface Protein C in nasopharyngeal carriage and pneumonia and its ability to elicit protection against carriage of Streptococcus pneumoniae. Infect. Immun. 2002, 70, 2526–2534. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Beato, A.R.; López, R.; García, J.L. Molecular characterization of PcpA: A novel choline-binding protein of Streptococcus pneumoniae. FEMS Microbiol. Lett. 1998, 164, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, J.W.; Briles, D.E.; Myers, L.E.; Hollingshead, S.K. Mn2+-dependent regulation of multiple genes in Streptococcus pneumoniae through PsaR and the resultant impact on virulence. Infect. Immun. 2006, 74, 1171–1180. [Google Scholar] [CrossRef] [Green Version]
- Scheuhammer, A.; Cherian, M. Binding of manganese in human and rat plasma. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1985, 840, 163–169. [Google Scholar] [CrossRef]
- Jakubovics, N.S.; Jenkinson, H.F. Out of the iron age: New insights into the critical role of manganese homeostasis in bacteria. Microbiology 2001, 147, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Sharma, S.K.; Filkins, L.M.; Pichichero, M.E. PcpA of Streptococcus pneumoniae mediates adherence to nasopharyngeal and lung epithelial cells and elicits functional antibodies in humans. Microbes Infect. 2012, 14, 1102–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hava, D.L.; Camilli, A. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol. Microbiol. 2002, 45, 1389–1406. [Google Scholar] [PubMed]
- Glover, D.T.; Hollingshead, S.K.; Briles, D.E. Streptococcus pneumoniae surface protein PcpA elicits protection against lung infection and fatal sepsis. Infect. Immun. 2008, 76, 2767–2776. [Google Scholar] [CrossRef] [Green Version]
- Walker, M.M.; Novak, L.; Widener, R.; Grubbs, J.A.; King, J.; Hale, J.Y.; Ochs, M.M.; Myers, L.E.; Briles, D.E.; Deshane, J. PcpA promotes higher levels of infection and modulates recruitment of myeloid-derived suppressor sells during pneumococcal pneumonia. J. Immunol. 2016, 196, 2239–2248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Masi, A.W.; Barniak, V.; Mountzouros, K.; Hostetter, M.K.; Green, B.A. Recombinant PhpA protein, a unique histidine motif-containing protein from Streptococcus pneumoniae, protects mice against intranasal pneumococcal challenge. Infect. Immun. 2001, 69, 3827–3836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamou, J.E.; Heinrichs, J.H.; Erwin, A.L.; Walsh, W.; Gayle, T.; Dormitzer, M.; Dagan, R.; Brewah, Y.A.; Barren, P.; Lathigra, R.; et al. Identification and characterization of a novel family of pneumococcal proteins that Are protective against sepsis. Infect. Immun. 2001, 69, 949–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melin, M.; Jarva, H.; Siira, L.; Meri, S.; Käyhty, H.; Väkeväinen, M. Streptococcus pneumoniae capsular Serotype 19F is more resistant to C3 deposition and less sensitive to opsonophagocytosis than Serotype 6B. Infect. Immun. 2008, 77, 676–684. [Google Scholar] [CrossRef] [Green Version]
- Verhoeven, D.; Xu, Q.; Pichichero, M.E. Vaccination with a Streptococcus pneumoniae trivalent recombinant PcpA, PhtD and PlyD1 protein vaccine candidate protects against lethal pneumonia in an infant murine model. Vaccine 2014, 32, 3205–3210. [Google Scholar] [CrossRef]
- Xu, Q.; Surendran, N.; Verhoeven, D.; Klapa, J.; Ochs, M.; Pichichero, M.E. Trivalent pneumococcal protein recombinant vaccine protects against lethal Streptococcus pneumoniae pneumonia and correlates with phagocytosis by neutrophils during early pathogenesis. Vaccine 2015, 33, 993–1000. [Google Scholar] [CrossRef]
- Leroux-Roels, G.; Maes, C.; De Boever, F.; Traskine, M.; Rüggeberg, J.U.; Borys, D. Safety, reactogenicity and immunogenicity of a novel pneumococcal protein-based vaccine in adults: A phase I/II randomized clinical study. Vaccine 2014, 32, 6838–6846. [Google Scholar] [CrossRef] [Green Version]
- Bologa, M.; Kamtchoua, T.; Hopfer, R.; Sheng, X.; Hicks, B.; Bixler, G.; Hou, V.; Pehlic, V.; Yuan, T.; Gurunathan, S. Safety and immunogenicity of pneumococcal protein vaccine candidates: Monovalent choline-binding protein A (PcpA) vaccine and bivalent PcpA–pneumococcal histidine triad protein D vaccine. Vaccine 2012, 30, 7461–7468. [Google Scholar] [CrossRef]
- Seiberling, M.; Bologa, M.; Brookes, R.; Ochs, M.; Go, K.; Neveu, D.; Kamtchoua, T.; Lashley, P.; Yuan, T.; Gurunathan, S. Safety and immunogenicity of a pneumococcal histidine triad protein D vaccine candidate in adults. Vaccine 2012, 30, 7455–7460. [Google Scholar] [CrossRef]
- Rowe, H.M.; Mann, B.; Iverson, A.; Poole, A.; Tuomanen, E.; Rosch, J.W. A cross-reactive protein vaccine combined with PCV-13 prevents Streptococcus pneumoniae and Haemophilus influenzae-mediated acute otitis media. Infect. Immun. 2019, 87, e00253-19. [Google Scholar] [CrossRef] [Green Version]
- Brooks, W.A.; Chang, L.-J.; Sheng, X.; Hopfer, R. Safety and immunogenicity of a trivalent recombinant PcpA, PhtD, and PlyD1 pneumococcal protein vaccine in adults, toddlers, and infants: A phase I randomized controlled study. Vaccine 2015, 33, 4610–4617. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, L.-A.S.; Wiertsema, S.P.; Corscadden, K.J.; Mateus, T.; Mullaney, G.L.; Zhang, G.; Richmond, P.C.; Thornton, R.B. Otitis-prone children produce functional antibodies to pneumolysin and pneumococcal polysaccharides. Clin. Vaccine Immunol. 2016, 24, e00497-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammitt, L.L.; Campbell, J.C.; Borys, D.; Weatherholtz, R.C.; Reid, R.; Goklish, N.; Moulton, L.H.; Traskine, M.; Song, Y.; Swinnen, K.; et al. Efficacy, safety and immunogenicity of a pneumococcal protein-based vaccine co-administered with 13-valent pneumococcal conjugate vaccine against acute otitis media in young children: A phase IIb randomized study. Vaccine 2019, 37, 7482–7492. [Google Scholar] [CrossRef]
- Odutola, A.; Ota, M.O.; Antonio, M.; Ogundare, E.O.; Saidu, Y.; Foster-Nyarko, E.; Owiafe, P.K.; Ceesay, F.; Worwui, A.; Idoko, O.T.; et al. Efficacy of a novel, protein-based pneumococcal vaccine against nasopharyngeal carriage of Streptococcus pneumoniae in infants: A phase 2, randomized, controlled, observer-blind study. Vaccine 2017, 35, 2531–2542. [Google Scholar] [CrossRef]
- Kamtchoua, T.; Bologa, M.; Hopfer, R.; Neveu, D.; Hu, B.; Sheng, X.; Corde, N.; Pouzet, C.; Zimmermann, G.; Gurunathan, S. Safety and immunogenicity of the pneumococcal pneumolysin derivative PlyD1 in a single-antigen protein vaccine candidate in adults. Vaccine 2013, 31, 327–333. [Google Scholar] [CrossRef]
- Farrand, A.J.; Lachapelle, S.; Hotze, E.M.; Johnson, A.E.; Tweten, R.K. Only two amino acids are essential for cytolytic toxin recognition of cholesterol at the membrane surface. Proc. Natl. Acad. Sci. USA 2010, 107, 4341–4346. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.L.; Hostetter, M.K.; Rogers, P.D.; Stiles, J.K.; Chapman, S.W.; Cleary, J.D. C3 as Substrate for Adhesion of Streptococcus pneumoniae. J. Infect. Dis. 2000, 182, 497–508. [Google Scholar] [CrossRef] [Green Version]
- Brissac, T.; Shenoy, A.T.; Patterson, L.A.; Orihuela, C.J. Cell invasion and pyruvate oxidase-derived H2O2 are critical for Streptococcus pneumoniae-mediated cardiomyocyte killing. Infect. Immun. 2017, 86, e00569-17. [Google Scholar] [CrossRef] [Green Version]
- Malley, R.; Lipsitch, M.; Stack, A.; Saladino, R.; Fleisher, G.; Pelton, S.; Thompson, C.; Briles, D.; Anderson, P. Intranasal immunization with killed unencapsulated whole cells prevents colonization and invasive disease by capsulated Pneumococci. Infect. Immun. 2001, 69, 4870–4873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babb, R.; Chen, A.; Hirst, T.R.; Kara, E.E.; McColl, S.R.; Ogunniyi, A.D.; Paton, J.C.; Alsharifi, M. Intranasal vaccination with γ-irradiated Streptococcus pneumoniae whole-cell vaccine provides serotype-independent protection mediated by B-cells and innate IL-17 responses. Clin. Sci. 2016, 130, 697–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campo, J.J.; Le, T.Q.; Pablo, J.V.; Hung, C.; Teng, A.A.; Tettelin, H.; Tate, A.; Hanage, W.P.; Alderson, M.R.; Liang, X.; et al. Panproteome-wide analysis of antibody responses to whole cell pneumococcal vaccination. eLife 2018, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Keech, C.A.; Morrison, R.; Anderson, P.; Tate, A.; Flores, J.; Goldblatt, D.; Briles, D.; Hural, J.; Malley, R.; Alderson, M.R. A phase 1 randomized, placebo-controlled, observer-blinded trial to evaluate the safety and immunogenicity of inactivated Streptococcus pneumoniae whole-cell vaccine in adults. Pediatr. Infect. Dis. J. 2020, 39, 345–351. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scott, N.R.; Mann, B.; Tuomanen, E.I.; Orihuela, C.J. Multi-Valent Protein Hybrid Pneumococcal Vaccines: A Strategy for the Next Generation of Vaccines. Vaccines 2021, 9, 209. https://doi.org/10.3390/vaccines9030209
Scott NR, Mann B, Tuomanen EI, Orihuela CJ. Multi-Valent Protein Hybrid Pneumococcal Vaccines: A Strategy for the Next Generation of Vaccines. Vaccines. 2021; 9(3):209. https://doi.org/10.3390/vaccines9030209
Chicago/Turabian StyleScott, Ninecia R., Beth Mann, Elaine I. Tuomanen, and Carlos J. Orihuela. 2021. "Multi-Valent Protein Hybrid Pneumococcal Vaccines: A Strategy for the Next Generation of Vaccines" Vaccines 9, no. 3: 209. https://doi.org/10.3390/vaccines9030209
APA StyleScott, N. R., Mann, B., Tuomanen, E. I., & Orihuela, C. J. (2021). Multi-Valent Protein Hybrid Pneumococcal Vaccines: A Strategy for the Next Generation of Vaccines. Vaccines, 9(3), 209. https://doi.org/10.3390/vaccines9030209





