Allergic Reactions to Current Available COVID-19 Vaccinations: Pathophysiology, Causality, and Therapeutic Considerations
Abstract
:1. Introduction
2. Pathophysiology of Vaccine-Induced Allergic Reactions
3. Causality of Vaccine Allergy
4. Types of Current COVID-19 Vaccines Worldwide
5. Mode of Action of Current COVID-19 Vaccines
6. General Therapeutic Considerations
- Mild allergic reactions, such as hives, nasal congestion, rash, scratchy throat, watery or itchy eyes to any injection, or orally and locally given substance.
- Severe allergic reactions, such as angioedema, cardiovascular collapse, including Kounis syndrome, cerebral manifestations, chest tightness, flushing, hives, laryngeal edema, loss of consciousness, low blood pressure (anaphylactic shock), shortness of breath, swelling of mouth, lips, tongue, throat, or wheezing to any oral, local or injectable medication (intravenous, intramuscular, or subcutaneous).
- Severe allergic reaction to a previously injected vaccine.
- Severe allergic reaction to another substance acting as an allergen (e.g., food, venom, or latex
- Severe allergic reaction to PEG or polysorbate.
7. Treatment of Severe Systemic Allergic Reactions
8. Patients with Mastocytosis and Mast Cell Disorders
9. The Second Vaccine Dose in Case of Allergic Reaction to First Dose
10. Vasovagal Symptomatology Masquerading as Allergic Reaction
11. COVID-19 Vaccination in Pregnancy
12. The FDA Emergency Use Authorization (EUA) for Adverse COVID-19 Events after the First Dose of Pfizer-BioNTech and ModernaVaccines
13. Potential Mechanisms of the COVID-19 Induced Allergic Reactions
14. Are COVID-19 Vaccines Causing Deaths?
15. Antihistamines, Anti-IgEs, Bronchial Asthma, and COVID-19 Vaccines
16. “There Are More Questions Than Answers”
- What is the level of immunity after one shot?
- Which vaccine component is the culprit?
- What happens if the second shot is delayed due to lack of availability?
- How long is the immunity duration?
- How long is the duration of the vaccination?
- When will trials stop in order to have effective vaccines?
- How long is the course of the immunity after vaccination?
- If you already had COVID-19, should you still get vaccinated?
- Are reactions mediated by IgEs?
- Once you get vaccinated, can you transmit the virus or still get sick?
- Is post-vaccination surveillance and documentation a challenge?
- Will we need another vaccination if the virus mutates?
- Are the deaths after COVID-19 due to anaphylactic cardiac collapse and Kounis syndrome?
17. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE-2 | Angiotensin-Converting Enzyme 2 |
| ACIP | Advisory Committee on Immunization Practices |
| CARPA | Complement Activation-related Pseudo-Allergy |
| CDC | Centers for Disease Control |
| COVID-19 | Coronavirus Disease 2019 |
| EUA | Emergency Use Authorization |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| IgE | Immunoglobulin E |
| IgG | Immunoglobulin G |
| IgM | Immunoglobulin M |
| LNP | Lipid Nano Particles |
| MRGPRx2 | Mas-related G Protein-Coupled Receptor X2 |
| MMWR | Morbidity and Mortality Weekly Report |
| PAF | Platelet Activating Factor |
| PEG | Polyethylene Glycol |
| RNA | Ribonucleic Acid |
| mRNA | messenger Ribonucleic Acid |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| VAERS | Vaccine Adverse Events Reporting System (VAERS). |
References
- Fritsche, P.J.; Helbling, A.; Ballmer-Weber, B.K. Vaccine hypersensitivity—Update and overview. Swiss Med. Wkly. 2010, 140, 238–246. [Google Scholar]
- Chung, E.H. Vaccine allergies. Clin. Exp. Vaccine Res. 2014, 3, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Robinson, C.L.; Romero, J.R.; Kempe, A.; Pellegrini, C. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Children and Adolescents Aged 18 Years or Younger—United States, 2017. Morb. Mortal. Wkly. Rep. 2017, 66, 134–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNeil, M.M.; Weintraub, E.S.; Duffy, J.; Sukumaran, L.; Jacobsen, S.J.; Klein, N.P.; Hambidge, S.J.; Lee, G.M.; Jackson, L.A.; Irving, S.A.; et al. Risk of anaphylaxis after vaccination in children and adults. J. Allergy Clin. Immunol. 2016, 137, 868–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm093833.htm (accessed on 1 February 2021).
- Olivera, A.; Beaven, M.A.; Metcalfe, D.D. Mast cells signal their importance in health and disease. J. Allergy Clin. Immunol. 2018, 142, 381–393. [Google Scholar] [CrossRef] [Green Version]
- Khan, S. Mast cell tryptase level should be checked in all patients with suspected Kounis syndrome. Eur. Hear. J. 2020, 41, 3018. [Google Scholar] [CrossRef]
- Stone, C.A.; Rukasin, C.R.; Beachkofsky, T.M.; Phillips, E.J. Immune-mediated adverse reactions to vaccines. Br. J. Clin. Pharmacol. 2019, 85, 2694–2706. [Google Scholar] [CrossRef]
- Weiler, C.R. Mastocytosis, Quinolones, MRGPRX2, and Anaphylaxis. J. Allergy Clin. Immunol. Pract. 2019, 7, 2091–2092. [Google Scholar] [CrossRef]
- Porebski, G.; Kwiecien, K.; Pawica, M.; Kwitniewski, M. Mas-Related G Protein-Coupled Receptor-X2 (MRGPRX2) in Drug Hypersensitivity Reactions. Front. Immunol. 2018, 9, 3027. [Google Scholar] [CrossRef] [PubMed]
- Caballero, M.L.; Quirce, S. Delayed Hypersensitivity Reactions Caused by Drug Excipients: A Literature Review. J. Investig. Allergol. Clin. Immunol. 2020, 30, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Coors, E.A.; Seybold, H.; Merk, H.F.; Mahler, V. Polysorbate 80 in medical products and nonimmunologic anaphylactoid reactions. Ann. Allergy Asthma Immunol. 2005, 95, 593–599. [Google Scholar] [CrossRef]
- Banerji, A.; Wickner, P.G.; Saff, R.; Stone, C.A.; Robinson, L.B.; Long, A.A.; Wolfson, A.R.; Williams, P.; Khan, D.A.; Phillips, E.; et al. mRNA Vaccines to Prevent COVID-19 Disease and Reported Allergic Reactions: Current Evidence and Suggested Approach. J. Allergy Clin. Immunol. Pract. 2020, 31. [Google Scholar] [CrossRef]
- Neun, B.W.; Barenholz, Y.; Szebeni, J.; Dobrovolskaia, M.A. Understanding the Role of Anti-PEG Antibodies in the Complement Activation by Doxil in Vitro. Molecules 2018, 23, 1700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szebeni, J.; Fontana, J.L.; Wassef, N.M.; Mongan, P.D.; Morse, D.S.; Dobbins, D.E.; Stahl, G.L.; Bünger, R.; Alving, C.R. Hemodynamic Changes Induced by Liposomes and Liposome-Encapsulated Hemoglobin in Pigs: A model for pseudoallergic cardiopulmonary reactions to liposomes. Role of complement and inhibition by soluble CR1 and anti-C5a antibody. Circulation 1999, 99, 2302–2309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNeil, M.M.; DeStefano, F. Vaccine-associated hypersensitivity. J. Allergy Clin. Immunol. 2018, 141, 463–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caubet, J.-C.; Ponvert, C. Vaccine Allergy. Immunol. Allergy Clin. N. Am. 2014, 34, 597–613. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [Green Version]
- Stone, C.A.; Liu, Y.; Relling, M.V.; Krantz, M.S.; Pratt, A.L.; Abreo, A.; Hemler, J.A.; Phillips, E.J. Immediate Hypersensitivity to Polyethylene Glycols and Polysorbates: More Common Than We Have Recognized. J. Allergy Clin. Immunol. Pract. 2019, 7, 1533–1540.e8. [Google Scholar] [CrossRef]
- Calogiuri, G.; Foti, C.; Bilò, M.; Garvey, L. Macrogols: A Misleading Cause of Drug Hypersensitivity Diagnosis. Clin. Immunol. Endocr. Metab. Drugs 2018, 4, 9–13. [Google Scholar] [CrossRef]
- Hawe, A.; Filipe, V.; Jiskoot, W. Fluorescent Molecular Rotors as Dyes to Characterize Polysorbate-Containing IgG Formulations. Pharm. Res. 2010, 27, 314–326. [Google Scholar] [CrossRef] [Green Version]
- Sokolowska, M.; Eiwegger, T.; Ollert, M.; Torres, M.J.; Barber, D.; Del Giacco, S.; Jutel, M.; Nadeau, K.C.; Palomares, O.; Rabin, R.L.; et al. EAACI statement on the diagnosis, management and prevention of severe allergic reactions to COVID-19 vaccines. Allergy 2021. [Google Scholar] [CrossRef] [PubMed]
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatullin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G.; et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. Lancet 2020, 396, 887–897. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef]
- Forni, G.; Mantovani, A. COVID-19 Commission of Accademia Nazionale deiLincei, Rome. COVID-19 vaccines: Where we stand and challenges ahead. Cell Death Differ. 2021, 28, 626–639. [Google Scholar] [CrossRef]
- Sharma, O.; Sultan, A.A.; Ding, H.; Triggle, C.R. A Review of the Progress and Challenges of Developing a Vaccine for COVID-19. Front. Immunol. 2020, 11, 585354. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Sun, X.; Aikins, M.E.; Moon, J.J. Non-viral COVID-19 vaccine delivery systems. Adv. Drug Deliv. Rev. 2021, 169, 137–151. [Google Scholar] [CrossRef]
- Krammer, F. SARS-CoV-2 vaccines in development. Nat. Cell Biol. 2020, 586, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.J.; Ansotegui, I.J.; Campbell, D.E.; Cardona, V.; Ebisawa, M.; El-Gamal, Y.; Fineman, S.; Geller, M.; Gonzalez-Estrada, A.; Greenberger, P.A.; et al. COVID-19 vaccine-associated anaphylaxis: A statement of the World Allergy Organization Anaphylaxis Committee. World Allergy Organ. J. 2021, 14, 100517. [Google Scholar] [CrossRef]
- Demir, S.; Erdenen, F.; Gelincik, A.; Unal, D.; Olgac, M.; Coskun, R.; Colakoglu, B.; Buyukozturk, S. Evaluation of the Potential Risk Factors for Drug-Induced Anaphylaxis in Adult Patients. Int. Arch. Allergy Immunol. 2018, 178, 167–176. [Google Scholar] [CrossRef]
- Petrikova, M.; Jančinová, V.; Nosal, R.; Májeková, M.; Holomanova, D. H1- antihistamines and activated blood platelets. Inflamm. Res. 2006, 55, S51–S52. [Google Scholar] [CrossRef]
- Freedberg, D.E.; Conigliaro, J.; Wang, T.C.; Tracey, K.J.; Callahan, M.V.; Abrams, J.A.; Sobieszczyk, M.E.; Markowitz, D.D.; Gupta, A.; O’Donnell, M.R.; et al. Famotidine Use Is Associated With Improved Clinical Outcomes in Hospitalized COVID-19 Patients: A Propensity Score Matched Retrospective Cohort Study. Gastroenterology 2020, 159, 1129–1131.e3. [Google Scholar] [CrossRef]
- Ilie, P.C.; Stefanescu, S.; Smith, L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020, 32, 1195–1198. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine—United States. 2020. Available online: https://www.cdc.gov/mmwr/volumes/70/wr/mm7002e1.htm (accessed on 1 July 2020).
- Theoharides, T.C. Potential association of mast cells with coronavirus disease 2019. Ann. Allergy, Asthma Immunol. 2021, 126, 217–218. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Akin, C.; Bonadonna, P.; Brockow, K.; Niedoszytko, M.; Nedoszytko, B.; Butterfield, J.H.; Alvarez-Twose, I.; Sotlar, K.; Schwaab, J.; et al. Risk and management of patients with mastocytosis and MCAS in the SARS-CoV-2 (COVID-19) pandemic: Expert opinions. J. Allergy Clin. Immunol. 2020, 146, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Mohedano-Vicente, E.; González-Mancebo, E.; Matito, A.; Kounis, N.G.; Escribano, L. Kounis syndrome following the performance of skin test to amoxicillin. Int. J. Cardiol. 2014, 174, 856–857. [Google Scholar] [CrossRef]
- Netterlid , E.; Schwarze, J.; Sheikh, A.; Storsaeter, J.; Skevaki, C.; Terreehorst, I.; Zanoni, G. Vaccination and allergy: EAACI position paper, practical aspects. Pediatr. Allergy Immunol. 2017, 28, 628–640. [Google Scholar] [CrossRef]
- McMurtry, C.M. Managing immunization stress-related response: A contributor to sustaining trust in vaccines. Can. Commun. Dis. Rep. 2020, 4, 210–218. [Google Scholar] [CrossRef]
- Dayneka, N.; Jensen, C.; Hildebrand, K. Canadian Immunization Guide: “Anaphylaxis and other acute reactions following vaccination” chapter update. Can. Commun. Dis. Rep. 2020, 46, 384–386. [Google Scholar] [CrossRef]
- Jaffe, E.; Goldfarb, I.T.; Lyerly, A.D. The Costs of Contradictory Messages about Live Vaccines in Pregnancy. Am. J. Public Health 2021, 111, 498–503. [Google Scholar] [CrossRef]
- Laris-González, A.; Bernal-Serrano, D.; Jarde, A.; Kampmann, B. Safety of Administering Live Vaccines during Pregnancy: A Systematic Review and Meta-Analysis of Pregnancy Outcomes. Vaccines 2020, 8, 124. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention (CDC). Interim considerations: Preparing for the Potential Management of Anaphylaxis after COVID-19 Vaccination. Updated 31 December 2020. Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/anaphylaxis-management.html (accessed on 25 January 2021).
- Dooling, K.; Marin, M.; Wallace, M.; McClung, N.; Chamberland, M.; Lee, G.M.; Talbot, H.K.; Romero, J.R.; Bell, B.P.; Oliver, S.E. The Advisory Committee on Immunization Practices’ Updated Interim Recommendation for Allocation of COVID-19 Vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021, 69, 1657–1660. [Google Scholar] [CrossRef] [PubMed]
- Kleine-Tebbe, J.; Klimek, L.; Hamelmann, E.; Pfaar, O.; Taube, C.; Wagenmann, M.; Werfel, T.; Worm, M. Severe allergic reactions to the COVID-19 vaccine—statement and practical consequences. Allergol. Sel. 2021, 5, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, T.; Nair, N. Allergic Reactions Including Anaphylaxis after Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine. JAMA 2021, 325, 780. [Google Scholar] [CrossRef]
- Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Moderna COVID-19 Vaccine—United States, 21 December 2020–10 January 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 125–129. [CrossRef]
- Kounis, N.G.; Mazarakis, A.; Tsigkas, G.; Giannopoulos, S.; Goudevenos, J. Kounis syndrome: A new twist on an old disease. Futur. Cardiol. 2011, 7, 805–824. [Google Scholar] [CrossRef]
- Palmiere, C.; Tettamanti, C.; Scarpelli, M.P. Vaccination and anaphylaxis: A forensic perspective. Croat. Med. J. 2017, 58, 14–25. [Google Scholar] [CrossRef] [Green Version]
- Kounis, N.G.; Mazarakis, A.; Kounis, G.N.; Tsigkas, G.; Almpanis, G.; Gkouias, K. The more allergens an atopic patient is exposed to, the easier and quicker anaphylactic shock and Kounis syndrome appear: Clinical and therapeutic paradoxes. J. Nat. Sci. Biol. Med. 2014, 5, 240–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nopp, A.; Johansson, S.G.O.; Lundberg, M.; Oman, H. Simultaneous exposure of several allergens has an additive effect on multisensitized basophils. Allergy 2006, 61, 1366–1368. [Google Scholar] [CrossRef]
- Moghimi, S.M. Allergic Reactions and Anaphylaxis to LNP-Based COVID-19 Vaccines. Mol. Ther. 2021, 29, 898–900. [Google Scholar] [CrossRef]
- Vrieze, J. Suspicions grow that nanoparticles in Pfizer’s COVID-19 vaccine trigger rare allergic reactions. Science 2020, 21, 1126. [Google Scholar] [CrossRef]
- Torjesen, I. Covid-19: Norway investigates 23 deaths in frail elderly patients after vaccination. BMJ 2021, 372, n149. [Google Scholar] [CrossRef] [PubMed]
- Teraldsen, L.E. Norway Raises Concern over Vaccine Jabs for the Elderly. Bloomberg. 2021. Available online: https://www.bloombergquint.com/politics/norway-vaccine-fatalities-among-people-75-and-older-rise-to-29 (accessed on 16 January 2021).
- Zusammenhang Unwahrscheinlich: InstitutPrüftZehnTodesfälleNachImpfung [German]. NTV. Available online: www.n-tv.de/panorama/Institut-prueft-zehn-Todesfaelle-nach-Impfung-article22292066.html (accessed on 14 January 2021).
- Kounis, N.G.; Koniari, I.; Soufras, G.; Koutsogiannis, N.; Hahalis, G. Specific IgE levels in pericardial and cerebrospinal fluids in forensic casework: The presence of additional molecules for sudden cardiac death diagnosis. Forensic Sci. Int. 2018, 282, 79. [Google Scholar] [CrossRef]
- Leisman, D.E.; Deutschman, C.S.; Legrand, M. Facing COVID-19 in the ICU: Vascular dysfunction, thrombosis, and dysregulated inflammation. Intensiv. Care Med. 2020, 46, 1105–1108. [Google Scholar] [CrossRef]
- Aydin, S.; Aydin, S. Could Antihistamines Help in the Treatment and Spread of COVID-19 via re-Modulating Cytokines and by Reducing Sneezing? Sci. Nutr. Health 2020, 172–173. Available online: https://actascientific.com/ASNH/ASNH-04-0684.php (accessed on 14 January 2021).
- Ambrosch, A.; Borgmann, S.; Rihoux, J.-P.; Konig, W. Effect of the H1 receptor antagonist cetirizine on the stimulated expression of adhesion molecules and the activation of NFκB in human endothelial cells. Int. Arch. Allergy Immunol. 2001, 124, 362–364. [Google Scholar] [CrossRef]
- Cheng, H.; Schafer, A.; Soloveva, V.; Gharaibeh, D.; Kenny, T.; Retterer, C.; Zamani, R.; Bavari, S.; Peet, N.P.; Rong, L. Identification of a coumarin-based antihistamine-like small molecule as an anti-filoviral entry inhibitor. Antivir. Res. 2017, 145, 24–32. [Google Scholar] [CrossRef]
- He, S.; Lin, B.; Chu, V.; Hu, Z.; Hu, X.; Xiao, J.; Wang, A.Q.; Schweitzer, C.J.; Li, Q.; Imamura, M.; et al. Repurposing of the antihistamine chlorcyclizine and related compounds for treatment of hepatitis C virus infection. Sci. Transl. Med. 2015, 7, 282ra49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morán, B.J.I.; Alvarenga, B.J.A.; Homma, S.; Suzuki, K.; Fremont, S.-P.; Villar, G. Antihistamines and azithromycin as a treatment for COVID-19 on primary health care—A retrospective observational study in elderly patients. Pulm. Pharmacol. Ther. 2021, 67, 101989. [Google Scholar] [CrossRef]
- Palma, G.; Pasqua, T.; Silvestri, G.; Rocca, C.; Gualtieri, P.; Barbieri, A.; De Bartolo, A.; De Lorenzo, A.; Angelone, T.; Avolio, E.; et al. PI3Kδ Inhibition as a Potential Therapeutic Target in COVID-19. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Kounis, N.G.; Hahalis, G. Serum IgE levels in coronary artery disease. Atherosclerosis 2016, 251, 498–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Yuan, S.; Liu, Y.; Zeng, Y.; Xie, H.; Liu, Z.; Zhang, S.; Fang, Q.; Wang, J.; Shen, Z. Serum IgE levels are associated with coronary artery disease severity. Atherosclerosis 2016, 251, 355–360. [Google Scholar] [CrossRef]
- Min, K.-B.; Min, J.-Y. Risk of Cardiovascular Mortality in Relation to Increased Total Serum IgE Levels in Older Adults: A Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2019, 16, 4350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castells, M.C.; Phillips, E.J. Maintaining Safety with SARS-CoV-2 Vaccines. N. Engl. J. Med. 2021, 384, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Hellman, L. Is Vaccination Against IgE Possible? Results Probl. Cell Differ. 1996, 409, 337–342. [Google Scholar] [CrossRef]
- Halsey, N.A.; Griffioen, M.; Dreskin, S.C.; Dekker, C.L.; Wood, R.; Sharma, D.; Jones, J.F.; LaRussa, P.S.; Garner, J.; Berger, M.; et al. Immediate hypersensitivity reactions following monovalent 2009 pandemic influenza A (H1N1) vaccines: Reports to VAERS. Vaccine 2013, 31, 6107–6112. [Google Scholar] [CrossRef]

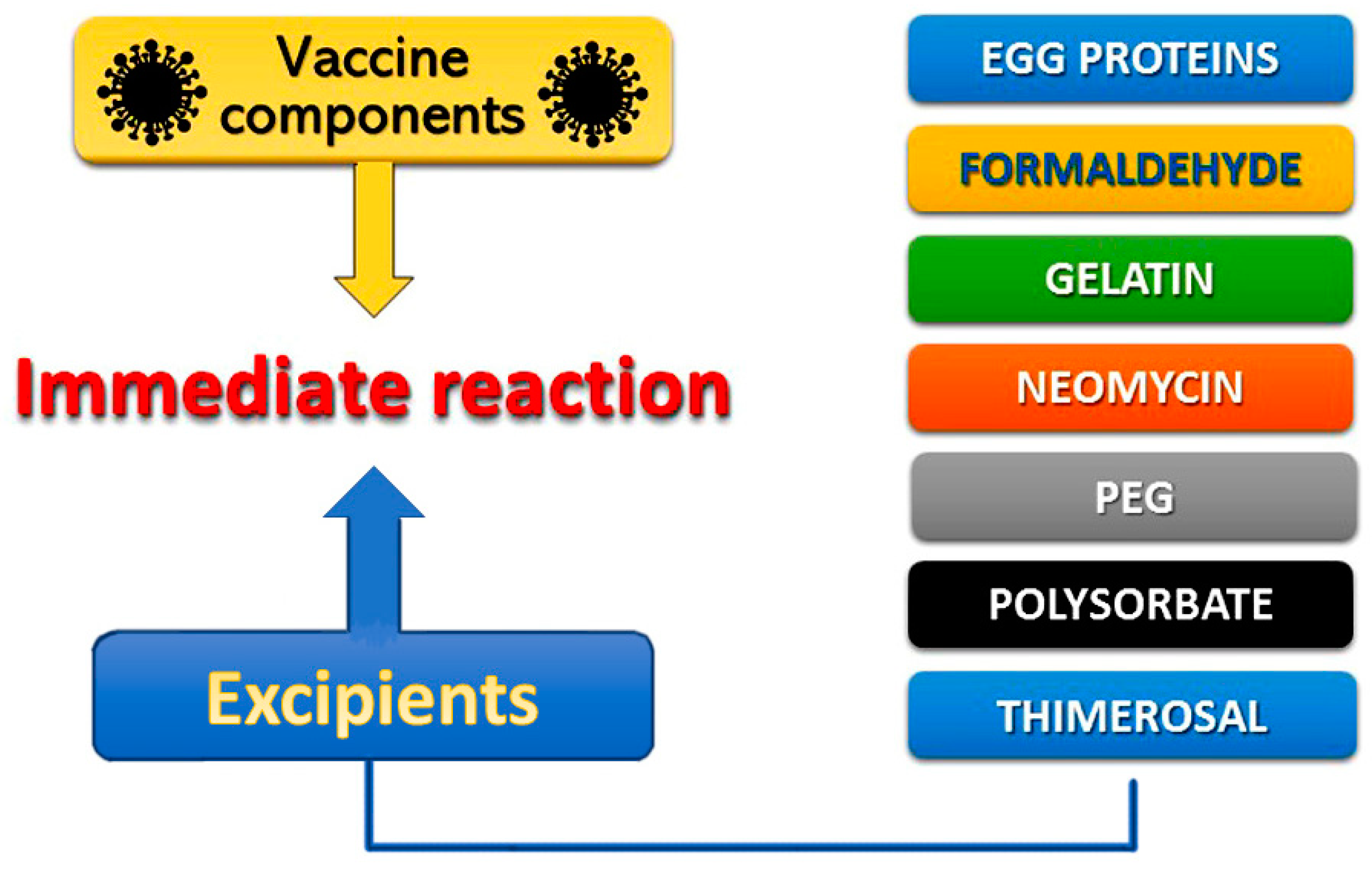

| Ascorbyl tetraisopalmitate (topical medications) |
| Benzalkonium chloride (corticosteroids, ophthalmic medications) |
| Benzyl alcohol (parenteral medications, topical medications) |
| Carboxymethylcellulose (corticosteroids, wound dressings) |
| Cetostearyl alcohol (topical medications) |
| Chlorocresol (topical medications) |
| Colloidal silica (NSAID) |
| Colophonium (wound dressings) |
| Dioctyl sodium sulfosuccinate (topical medications) |
| Ethylenediaminetetraacetic acid (EDTA) (topical medications) |
| Formaldehyde (VACCINES) |
| 1,2,6-Hexanetriol (topical medications) |
| Imidazolidinyl urea (ultrasound gels) |
| Isopropyl palmitate (topical medications) |
| Metacresol (insulin) |
| Methyldibromo glutaronitrile (ultrasound gels) |
| Parabens (topical medications) |
| Polyethylene glycol (VACCINES, foods, lubricants, medications, skin creams) |
| Polysorbate (VACCINES, anticancer agents, creams, lotions, medications, ointments, vitamin oils) |
| Propyl gallate (topical medications) |
| Propylene glycol (antihistamines, anxiolytics, lubricants, topical medications, ultrasound gels) |
| Sodium metabisulfite (local anesthetics, topical medications) |
| Sodium sulfite (topical medications) |
| Sorbitansesquioleate (topical medications) |
| Sunset Yellow (mineral supplements) |
| Thimerosal (ophthalmic medications) |
| A. The Pfizer-BioNTech COVID-19 Vaccine Excipients-Ingredients |
| 1. mRNA, nucleoside-modified messenger RNA (modRNA) encoding the viral spike (S) glycoprotein of SARS-CoV-2and constitutes the active ingredient |
| 2. Electrolytes potassium chloride, monobasic potassium phosphate, sodium chloride, dibasic sodiumphosphate dihydrate, |
| 3. Lipids ((4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate), [(polyethyleneglycol [PEG])-2000]-N,N-ditetradecylacetamide, 1,2-distearoyl-sn-glycero-3-phosphocholine, and 0.2 mgcholesterol) |
| 4. polyethyleneglycol |
| 5. Sugar (sucrose) |
| 6. Saline (Sodium Chloride) acting as adiluent, added to the vaccine forinjection |
| B. The Moderna COVID-19 Vaccine Excipients-Ingredients |
| 1. Messenger ribonucleic acid (mRNA) as an active ingredient |
| 2. Acetic acid |
| 3. Lipids (SM-102, polyethylene glycol [PEG] 2000 dimyristoyl glycerol [DMG], cholesterol, and 1,2-distearoyl-sn-glycero-3-phosphocholine [DSPC]) |
| 4. polyethylene glycol |
| 5. Sodium acetate |
| 6. Sugar (sucrose) |
| 7. Tromethamine (treat or prevent acidosis) |
| 8. Tromethamine hydrochloride |
| 1. Recombinant, replication-deficient chimpanzee adenovirus vector encoding the SARS CoV 2 Spike glycoprotein. Produced in genetically modified human embryonic kidney (HEK) 293 cells |
| 2. Histidine |
| 3. L-histidine hydrochloride monohydrat |
| 4. Magnesium chloride hexahydrate |
| 5. Polysorbate 80 |
| 6. Ethanol |
| 7. Sugar (sucrose) |
| 8. Sodium chloride |
| 9. Disodium edetate dihydrate |
| 10. Water for injections |
| 1. Patient in recline position with legs up and administer intramuscular epinephrine |
| 2. Intravenous line for volume replacement with intravenous 0.9% NaCl |
| 3. Airways clearing, vital signs checking, oxygen via facial mask at least 10 L/minute administration |
| 4. 2–3 L of intravenous 0.9% NaCl in 10–20 min if hypotension and rapid volume loss |
| 5. Repeat intramuscular epinephrine if no improvement within 5–10 min and call emergency assistance |
| 6. Short-acting beta-agonists [salbutamol) puffs via large volume spacer for severe dyspnea/wheezing |
| 7. Glucagon in patients on beta-blockers who are unresponsive to epinephrine |
| 8. Nebulized epinephrine and nebulized short-acting beta-agonists in cases of signs of severe upper airway obstruction (laryngeal/uvula/tongue edema) |
| 9. Oral or intravenous glucocorticoids and oral or intravenous antihistamines |
| 10. Measuring mast cell tryptase2–3 h after the beginning of the reaction to confirm anaphylaxis |
| Symptoms and Signs |
| 1. Precede or occur after a few seconds to a few minutes after the injection |
| 2. Fainting sensation, light-headedness dizziness, blurry vision, loss of consciousness in some cases |
| 3. Grey or pale skin appearance |
| 4. Vertigo, weakness |
| 5. Blurred, faded, narrowing, or tunnel vision |
| 6. Paresthesias |
| 7. Feeling of warmth |
| 8. Slow breathing, with a few seconds of apnea in some cases |
| 9. Regular butslow and weak pulse |
| 10. Cold, sweaty, clammy skin |
| 11. Transient hypotension |
| 12. Uncontrollable yawning, nausea, vomiting nausea, vomiting, epigastric discomfort, abdominal pains, diarrhea |
| Management |
| Continuous reassurance, well-ventilated room, cold and damp cloth on forehead and face, recumbent position with elevated legs above head or have the patientput their head between their knees |
| Prevention |
| Vaccination should be performed in a sitting position. Before vaccinating, ask if the patient tends to faint; if so, ask the patient to lie down |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kounis, N.G.; Koniari, I.; de Gregorio, C.; Velissaris, D.; Petalas, K.; Brinia, A.; Assimakopoulos, S.F.; Gogos, C.; Kouni, S.N.; Kounis, G.N.; et al. Allergic Reactions to Current Available COVID-19 Vaccinations: Pathophysiology, Causality, and Therapeutic Considerations. Vaccines 2021, 9, 221. https://doi.org/10.3390/vaccines9030221
Kounis NG, Koniari I, de Gregorio C, Velissaris D, Petalas K, Brinia A, Assimakopoulos SF, Gogos C, Kouni SN, Kounis GN, et al. Allergic Reactions to Current Available COVID-19 Vaccinations: Pathophysiology, Causality, and Therapeutic Considerations. Vaccines. 2021; 9(3):221. https://doi.org/10.3390/vaccines9030221
Chicago/Turabian StyleKounis, Nicholas G., Ioanna Koniari, Cesare de Gregorio, Dimitris Velissaris, Konstantinos Petalas, Aikaterini Brinia, Stelios F. Assimakopoulos, Christos Gogos, Sophia N. Kouni, George N. Kounis, and et al. 2021. "Allergic Reactions to Current Available COVID-19 Vaccinations: Pathophysiology, Causality, and Therapeutic Considerations" Vaccines 9, no. 3: 221. https://doi.org/10.3390/vaccines9030221
APA StyleKounis, N. G., Koniari, I., de Gregorio, C., Velissaris, D., Petalas, K., Brinia, A., Assimakopoulos, S. F., Gogos, C., Kouni, S. N., Kounis, G. N., Calogiuri, G., & Hung, M.-Y. (2021). Allergic Reactions to Current Available COVID-19 Vaccinations: Pathophysiology, Causality, and Therapeutic Considerations. Vaccines, 9(3), 221. https://doi.org/10.3390/vaccines9030221












