Roles of the Fc Receptor γ-Chain in Inducing Protective Immune Responses after Heterologous Vaccination against Respiratory Syncytial Virus Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of RSV A2 Virus, RSV F-VLP, and FI-RSV
2.2. Immunization and RSV A2 Virus Challenge
2.3. Determination of Antibody Response, RSV Neutralization, and Lung Viral Titration
2.4. Cytokine-Expressing T-Cells and Analysis of BAL Cells Phenotype by FACS
2.5. Histopathology
2.6. Statistical Analysis
3. Results
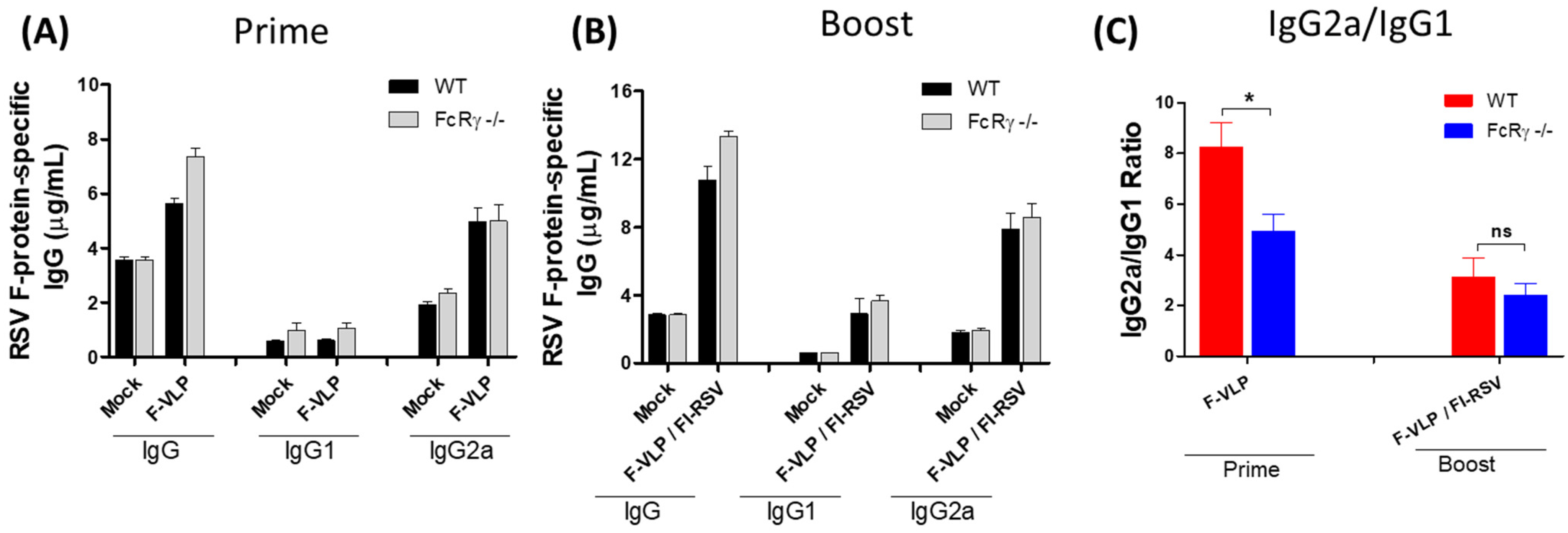
3.1. Lower Neutralizing Titers in FcRγ −/− Mice after RSV F-VLP/FI-RSV Vaccination
3.2. FcRγ Contributes to the Effective Clearance of Lung Viral Loads by F-VLP/FI-RSV Vaccination
3.3. FcRγ Is Important for Inducing RSV F-Specific CD8 T-Cell Responses
3.4. FcRγ Plays a Role in Recruiting CD8 T-Cells and NK Cells into Airway BALF in RSV F-VLP/FI-RSV-Vaccinated Mice after RSV Infection
3.5. FcRγ Plays a Role in Diminishing Histopathology in F-VLP/FI-RSV-Vaccinated Mice after Challenge
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, H.W.; Canchola, J.G.; Brandt, C.D.; Pyles, G.; Chanock, R.M.; Jensen, K.; Parrott, R.H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 1969, 89, 422–434. [Google Scholar] [CrossRef]
- Ruckwardt, T.J.; Morabito, K.M.; Graham, B.S. Immunological Lessons from Respiratory Syncytial Virus Vaccine Development. Immunity 2019, 51, 429–442. [Google Scholar] [CrossRef]
- McGinnes, L.W.; Morrison, T.G. Newcastle disease virus-like particles: Preparation, purification, quantification, and incorporation of foreign glycoproteins. Curr. Protoc. Microbiol. 2013, 30, 18 12 11–18 12 21. [Google Scholar] [CrossRef]
- Cimica, V.; Boigard, H.; Bhatia, B.; Fallon, J.T.; Alimova, A.; Gottlieb, P.; Galarza, J.M. Novel Respiratory Syncytial Virus-Like Particle Vaccine Composed of the Postfusion and Prefusion Conformations of the F Glycoprotein. Clin. Vaccine Immunol. 2016, 23, 451–459. [Google Scholar] [CrossRef] [PubMed]
- McGinnes, L.W.; Gravel, K.A.; Finberg, R.W.; Kurt-Jones, E.A.; Massare, M.J.; Smith, G.; Schmidt, M.R.; Morrison, T.G. Assembly and immunological properties of Newcastle disease virus-like particles containing the respiratory syncytial virus F and G proteins. J. Virol. 2011, 85, 366–377. [Google Scholar] [CrossRef]
- Murawski, M.R.; McGinnes, L.W.; Finberg, R.W.; Kurt-Jones, E.A.; Massare, M.J.; Smith, G.; Heaton, P.M.; Fraire, A.E.; Morrison, T.G. Newcastle disease virus-like particles containing respiratory syncytial virus G protein induced protection in BALB/c mice, with no evidence of immunopathology. J. Virol. 2010, 84, 1110–1123. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.S.; Henderson, G.S.; Tang, Y.W.; Lu, X.; Neuzil, K.M.; Colley, D.G. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J. Immunol. 1993, 151, 2032–2040. [Google Scholar] [PubMed]
- Kim, K.H.; Lee, Y.T.; Hwang, H.S.; Kwon, Y.M.; Kim, M.C.; Ko, E.J.; Lee, J.S.; Lee, Y.; Kang, S.M. Virus-Like Particle Vaccine Containing the F Protein of Respiratory Syncytial Virus Confers Protection without Pulmonary Disease by Modulating Specific Subsets of Dendritic Cells and Effector T Cells. J. Virol. 2015, 89, 11692–11705. [Google Scholar] [CrossRef]
- Ko, E.J.; Kwon, Y.M.; Lee, J.S.; Hwang, H.S.; Yoo, S.E.; Lee, Y.N.; Lee, Y.T.; Kim, M.C.; Cho, M.K.; Lee, Y.R.; et al. Virus-like nanoparticle and DNA vaccination confers protection against respiratory syncytial virus by modulating innate and adaptive immune cells. Nanomedicine 2015, 11, 99–108. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kwon, Y.M.; Lee, J.S.; Yoo, S.E.; Lee, Y.N.; Ko, E.J.; Kim, M.C.; Cho, M.K.; Lee, Y.T.; Jung, Y.J.; et al. Co-immunization with virus-like particle and DNA vaccines induces protection against respiratory syncytial virus infection and bronchiolitis. Antiviral Res. 2014, 110, 115–123. [Google Scholar] [CrossRef]
- Cannon, M.J.; Bangham, C.R. Recognition of respiratory syncytial virus fusion protein by mouse cytotoxic T cell clones and a human cytotoxic T cell line. J. Gen. Virol. 1989, 70(Pt. 1), 79–87. [Google Scholar] [CrossRef]
- Lambert, L.; Sagfors, A.M.; Openshaw, P.J.; Culley, F.J. Immunity to RSV in Early-Life. Front. Immunol. 2014, 5, 466. [Google Scholar] [CrossRef]
- Blanco, J.C.G.; Pletneva, L.M.; McGinnes-Cullen, L.; Otoa, R.O.; Patel, M.C.; Fernando, L.R.; Boukhvalova, M.S.; Morrison, T.G. Efficacy of a respiratory syncytial virus vaccine candidate in a maternal immunization model. Nat. Commun. 2018, 9, 1904. [Google Scholar] [CrossRef]
- Hoft, D.F.; Lottenbach, K.; Goll, J.B.; Hill, H.; Winokur, P.L.; Patel, S.M.; Brady, R.C.; Chen, W.H.; Edwards, K.; Creech, C.B.; et al. Priming Vaccination With Influenza Virus H5 Hemagglutinin Antigen Significantly Increases the Duration of T cell Responses Induced by a Heterologous H5 Booster Vaccination. J. Infect. Dis. 2016, 214, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Teng, M.N.; Collins, P.L.; Prince, G.A.; Exner, M.; Regele, H.; Lirman, D.D.; Rabold, R.; Hoffman, S.J.; Karp, C.L.; et al. A role for immune complexes in enhanced respiratory syncytial virus disease. J. Exp. Med. 2002, 196, 859–865. [Google Scholar] [CrossRef]
- Halstead, S.B.; Mahalingam, S.; Marovich, M.A.; Ubol, S.; Mosser, D.M. Intrinsic antibody-dependent enhancement of microbial infection in macrophages: Disease regulation by immune complexes. Lancet Infect. Dis. 2010, 10, 712–722. [Google Scholar] [CrossRef]
- Acevedo, O.A.; Diaz, F.E.; Beals, T.E.; Benavente, F.M.; Soto, J.A.; Escobar-Vera, J.; Gonzalez, P.A.; Kalergis, A.M. Contribution of Fcgamma Receptor-Mediated Immunity to the Pathogenesis Caused by the Human Respiratory Syncytial Virus. Front. Cell Infect. Microbiol. 2019, 9, 75. [Google Scholar] [CrossRef]
- Gomez, R.S.; Ramirez, B.A.; Cespedes, P.F.; Cautivo, K.M.; Riquelme, S.A.; Prado, C.E.; Gonzalez, P.A.; Kalergis, A.M. Contribution of Fcgamma receptors to human respiratory syncytial virus pathogenesis and the impairment of T-cell activation by dendritic cells. Immunology 2016, 147, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Mejias, A.; Ramilo, O. Review of palivizumab in the prophylaxis of respiratory syncytial virus (RSV) in high-risk infants. Biologics 2008, 2, 433–439. [Google Scholar] [CrossRef][Green Version]
- Bukreyev, A.; Yang, L.; Fricke, J.; Cheng, L.; Ward, J.M.; Murphy, B.R.; Collins, P.L. The secreted form of respiratory syncytial virus G glycoprotein helps the virus evade antibody-mediated restriction of replication by acting as an antigen decoy and through effects on Fc receptor-bearing leukocytes. J. Virol. 2008, 82, 12191–12204. [Google Scholar] [CrossRef]
- Radu, G.U.; Caidi, H.; Miao, C.; Tripp, R.A.; Anderson, L.J.; Haynes, L.M. Prophylactic treatment with a G glycoprotein monoclonal antibody reduces pulmonary inflammation in respiratory syncytial virus (RSV)-challenged naive and formalin-inactivated RSV-immunized BALB/c mice. J. Virol. 2010, 84, 9632–9636. [Google Scholar] [CrossRef]
- Tang, A.; Chen, Z.; Cox, K.S.; Su, H.P.; Callahan, C.; Fridman, A.; Zhang, L.; Patel, S.B.; Cejas, P.J.; Swoyer, R.; et al. A potent broadly neutralizing human RSV antibody targets conserved site IV of the fusion glycoprotein. Nat. Commun. 2019, 10, 4153. [Google Scholar] [CrossRef]
- van Erp, E.A.; Luytjes, W.; Ferwerda, G.; van Kasteren, P.B. Fc-Mediated Antibody Effector Functions During Respiratory Syncytial Virus Infection and Disease. Front. Immunol. 2019, 10, 548. [Google Scholar] [CrossRef] [PubMed]
- Jans, J.; Vissers, M.; Heldens, J.G.; de Jonge, M.I.; Levy, O.; Ferwerda, G. Fc gamma receptors in respiratory syncytial virus infections: Implications for innate immunity. Rev. Med. Virol. 2014, 24, 55–70. [Google Scholar] [CrossRef]
- Hwang, H.S.; Lee, Y.T.; Kim, K.H.; Ko, E.J.; Lee, Y.; Kwon, Y.M.; Kang, S.M. Virus-like particle vaccine primes immune responses preventing inactivated-virus vaccine-enhanced disease against respiratory syncytial virus. Virology 2017, 511, 142–151. [Google Scholar] [CrossRef]
- Quan, F.S.; Kim, Y.; Lee, S.; Yi, H.; Kang, S.M.; Bozja, J.; Moore, M.L.; Compans, R.W. Viruslike particle vaccine induces protection against respiratory syncytial virus infection in mice. J. Infect. Dis. 2011, 204, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Borthwick, N.J.; Morrison, P.; Gao, G.F.; Steward, M.W. Virus-specific CTL responses induced by an H-2K(d)-restricted, motif-negative 15-mer peptide from the fusion protein of respiratory syncytial virus. J. Gen. Virol. 2002, 83, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.R.; Varga, S.M. Pulmonary immunity and immunopathology: Lessons from respiratory syncytial virus. Expert Rev. Vaccines 2008, 7, 1239–1255. [Google Scholar] [CrossRef] [PubMed]
- Meyerholz, D.K.; Griffin, M.A.; Castilow, E.M.; Varga, S.M. Comparison of histochemical methods for murine eosinophil detection in an RSV vaccine-enhanced inflammation model. Toxicol Pathol. 2009, 37, 249–255. [Google Scholar] [CrossRef]
- Stokes, K.L.; Chi, M.H.; Sakamoto, K.; Newcomb, D.C.; Currier, M.G.; Huckabee, M.M.; Lee, S.; Goleniewska, K.; Pretto, C.; Williams, J.V.; et al. Differential pathogenesis of respiratory syncytial virus clinical isolates in BALB/c mice. J. Virol. 2011, 85, 5782–5793. [Google Scholar] [CrossRef]
- Storni, T.; Lechner, F.; Erdmann, I.; Bachi, T.; Jegerlehner, A.; Dumrese, T.; Kundig, T.M.; Ruedl, C.; Bachmann, M.F. Critical role for activation of antigen-presenting cells in priming of cytotoxic T cell responses after vaccination with virus-like particles. J. Immunol. 2002, 168, 2880–2886. [Google Scholar] [CrossRef] [PubMed]
- Win, S.J.; Ward, V.K.; Dunbar, P.R.; Young, S.L.; Baird, M.A. Cross-presentation of epitopes on virus-like particles via the MHC I receptor recycling pathway. Immunol. Cell Biol. 2011, 89, 681–688. [Google Scholar] [CrossRef]
- Tay, M.Z.; Wiehe, K.; Pollara, J. Antibody-Dependent Cellular Phagocytosis in Antiviral Immune Responses. Front. Immunol. 2019, 10, 332. [Google Scholar] [CrossRef]
- Lodoen, M.B.; Lanier, L.L. Viral modulation of NK cell immunity. Nat. Rev. Microbiol. 2005, 3, 59–69. [Google Scholar] [CrossRef] [PubMed]
- van Erp, E.A.; Lakerveld, A.J.; de Graaf, E.; Larsen, M.D.; Schepp, R.M.; Hipgrave Ederveen, A.L.; Ahout, I.M.; de Haan, C.A.; Wuhrer, M.; Luytjes, W.; et al. Natural killer cell activation by respiratory syncytial virus-specific antibodies is decreased in infants with severe respiratory infections and correlates with Fc-glycosylation. Clin. Transl. Immunol. 2020, 9, e1112. [Google Scholar]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef]
- Ko, E.J.; Lee, Y.; Lee, Y.T.; Hwang, H.S.; Park, Y.; Kim, K.H.; Kang, S.M. Natural Killer and CD8 T Cells Contribute to Protection by Formalin Inactivated Respiratory Syncytial Virus Vaccination under a CD4-Deficient Condition. Immune Netw. 2020, 20, e51. [Google Scholar] [CrossRef] [PubMed]
- Zepeda-Cervantes, J.; Ramirez-Jarquin, J.O.; Vaca, L. Interaction Between Virus-Like Particles (VLPs) and Pattern Recognition Receptors (PRRs) From Dendritic Cells (DCs): Toward Better Engineering of VLPs. Front. Immunol. 2020, 11, 1100. [Google Scholar] [CrossRef]
- Hemann, E.A.; Kang, S.M.; Legge, K.L. Protective CD8 T cell-mediated immunity against influenza A virus infection following influenza virus-like particle vaccination. J. Immunol. 2013, 191, 2486–2494. [Google Scholar] [CrossRef] [PubMed]
- DiLillo, D.J.; Palese, P.; Wilson, P.C.; Ravetch, J.V. Broadly neutralizing anti-influenza antibodies require Fc receptor engagement for in vivo protection. J. Clin. Investig. 2016, 126, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.C.; Lee, J.S.; Kwon, Y.M.; Eunju, O.; Lee, Y.J.; Choi, J.G.; Wang, B.Z.; Compans, R.W.; Kang, S.M. Multiple heterologous M2 extracellular domains presented on virus-like particles confer broader and stronger M2 immunity than live influenza A virus infection. Antiviral Res. 2013, 99, 328–335. [Google Scholar] [CrossRef]
- Kim, M.C.; Lee, Y.N.; Ko, E.J.; Lee, J.S.; Kwon, Y.M.; Hwang, H.S.; Song, J.M.; Song, B.M.; Lee, Y.J.; Choi, J.G.; et al. Supplementation of influenza split vaccines with conserved M2 ectodomains overcomes strain specificity and provides long-term cross protection. Mol. Ther. 2014, 22, 1364–1374. [Google Scholar] [CrossRef]
- Lee, Y.N.; Lee, Y.T.; Kim, M.C.; Hwang, H.S.; Lee, J.S.; Kim, K.H.; Kang, S.M. Fc receptor is not required for inducing antibodies but plays a critical role in conferring protection after influenza M2 vaccination. Immunology 2014, 143, 300–309. [Google Scholar] [CrossRef]
- Skibinski, D.A.G.; Jones, L.A.; Zhu, Y.O.; Xue, L.W.; Au, B.; Lee, B.; Naim, A.N.M.; Lee, A.; Kaliaperumal, N.; Low, J.G.H.; et al. Induction of Human T-cell and Cytokine Responses Following Vaccination with a Novel Influenza Vaccine. Sci. Rep. 2018, 8, 18007. [Google Scholar] [CrossRef]
- Swain, S.L.; McKinstry, K.K.; Strutt, T.M. Expanding roles for CD4(+) T cells in immunity to viruses. Nat. Rev. Immunol. 2012, 12, 136–148. [Google Scholar] [CrossRef]
- Deng, L.; Cho, K.J.; Fiers, W.; Saelens, X. M2e-Based Universal Influenza A Vaccines. Vaccines 2015, 3, 105–136. [Google Scholar] [CrossRef]
- Song, A.; Myojo, K.; Laudenslager, J.; Harada, D.; Miura, T.; Suzuki, K.; Kuni-Kamochi, R.; Soloff, R.; Ohgami, K.; Kanda, Y. Evaluation of a fully human monoclonal antibody against multiple influenza A viral strains in mice and a pandemic H1N1 strain in nonhuman primates. Antiviral Res. 2014, 111, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Bournazos, S.; Gupta, A.; Ravetch, J.V. The role of IgG Fc receptors in antibody-dependent enhancement. Nat. Rev. Immunol. 2020, 20, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Channappanavar, R.; Fehr, A.R.; Vijay, R.; Mack, M.; Zhao, J.; Meyerholz, D.K.; Perlman, S. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe. 2016, 19, 181–193. [Google Scholar] [CrossRef]
- Bournazos, S.; Ravetch, J.V. Fcgamma Receptor Function and the Design of Vaccination Strategies. Immunity 2017, 47, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.; Kotsias, F.; Visentin, G.; Bruhns, P.; Savina, A.; Amigorena, S. Autonomous phagosomal degradation and antigen presentation in dendritic cells. Proc. Natl. Acad. Sci. USA 2012, 109, 14556–14561. [Google Scholar] [CrossRef] [PubMed]
- Regnault, A.; Lankar, D.; Lacabanne, V.; Rodriguez, A.; Thery, C.; Rescigno, M.; Saito, T.; Verbeek, S.; Bonnerot, C.; Ricciardi-Castagnoli, P.; et al. Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J. Exp. Med. 1999, 189, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.B.; Farley, C.R.; Pinelli, D.F.; Adams, L.E.; Cragg, M.S.; Boss, J.M.; Scharer, C.D.; Fribourg, M.; Cravedi, P.; Heeger, P.S.; et al. Signaling through the Inhibitory Fc Receptor FcgammaRIIB Induces CD8(+) T Cell Apoptosis to Limit T Cell Immunity. Immunity 2020, 52, 136–150 e136. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, H.S.; Lee, Y.-T.; Kim, K.-H.; Seo, H.S.; Yang, K.S.; Cho, H.; Kang, S.-M. Roles of the Fc Receptor γ-Chain in Inducing Protective Immune Responses after Heterologous Vaccination against Respiratory Syncytial Virus Infection. Vaccines 2021, 9, 232. https://doi.org/10.3390/vaccines9030232
Hwang HS, Lee Y-T, Kim K-H, Seo HS, Yang KS, Cho H, Kang S-M. Roles of the Fc Receptor γ-Chain in Inducing Protective Immune Responses after Heterologous Vaccination against Respiratory Syncytial Virus Infection. Vaccines. 2021; 9(3):232. https://doi.org/10.3390/vaccines9030232
Chicago/Turabian StyleHwang, Hye Suk, Young-Tae Lee, Ki-Hye Kim, Ho Seong Seo, Kap Seung Yang, Hoonsung Cho, and Sang-Moo Kang. 2021. "Roles of the Fc Receptor γ-Chain in Inducing Protective Immune Responses after Heterologous Vaccination against Respiratory Syncytial Virus Infection" Vaccines 9, no. 3: 232. https://doi.org/10.3390/vaccines9030232
APA StyleHwang, H. S., Lee, Y.-T., Kim, K.-H., Seo, H. S., Yang, K. S., Cho, H., & Kang, S.-M. (2021). Roles of the Fc Receptor γ-Chain in Inducing Protective Immune Responses after Heterologous Vaccination against Respiratory Syncytial Virus Infection. Vaccines, 9(3), 232. https://doi.org/10.3390/vaccines9030232





