Factors Associated with Post-Transplant Active Epstein-Barr Virus Infection and Lymphoproliferative Disease in Hematopoietic Stem Cell Transplant Recipients: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Graft-versus-Host Disease
3.2. Graft-versus-Host Disease Prophylaxis/Treatment
3.3. Other Risk Factors
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability
Acknowledgments
Conflicts of Interest
Abbreviations
| aGvHD | Acute graft-versus-host disease |
| ATG | anti-thymocyte globulin |
| cGvHD | chronic graft-versus-host disease |
| CCR5 | CC chemokine receptor-5 |
| CI | confidence interval |
| CMV | cytomegalovirus |
| EPHPP | Effective Public Health Practice Project |
| EBV | Epstein–Barr Virus |
| HR | the hazard ratio |
| HSCT | hemamiddleoietic stem cell transplant |
| IFNɣ | interferon-ɣ |
| OR | odds ratio |
| PCR | polymerase chain reaction |
| PTLD | post-transplant lymphoproliferative disease |
| SHR | subhazard ratio |
| TCD | T-cell depleted |
| TBI | total body irradiation |
| VL | viral load |
References
- Aalto, S.M.; Juvonen, E.; Tarkkanen, J.; Volin, L.; Haario, H.; Ruutu, T.; Hedman, K. Epstein-Barr viral load and disease prediction in a large cohort of allogeneic stem cell transplant recipients. Clin. Infect. Dis. 2007, 45, 1305–1309. [Google Scholar] [CrossRef] [Green Version]
- Faraci, M.; Caviglia, I.; Morreale, G.; Lanino, E.; Cuzzubbo, D.; Giardino, S.; Di Marco, E.; Cirillo, C.; Scuderi, F.; Dallorso, S.; et al. Viral-load and B-lymphocyte monitoring of EBV reactivation after allogeneic hemopoietic SCT in children. Bone Marrow Transplant. 2010, 45, 1052–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herreman, A.; Dierickx, D.; Morscio, J.; Camps, J.; Bittoun, E.; Verhoef, G.; De Wolf-Peeters, C.; Sagaert, X.; Tousseyn, T. Clinicopathological characteristics of posttransplant lymphoproliferative disorders of T-cell origin: Single-center series of nine cases and meta-analysis of 147 reported cases. Leuk. Lymphoma 2013, 54, 2190–2199. [Google Scholar] [CrossRef] [PubMed]
- Patriarca, F.; Medeot, M.; Isola, M.; Battista, M.L.; Sperotto, A.; Pipan, C.; Toffoletti, E.; Dozzo, M.; Michelutti, A.; Gregoraci, G.; et al. Prognostic factors and outcome of Epstein-Barr virus DNAemia in high-risk recipients of allogeneic stem cell transplantation treated with preemptive rituximab. Transpl. Infect. Dis. 2013, 15, 259–267. [Google Scholar] [CrossRef]
- Rasche, L.; Kapp, M.; Einsele, H.; Mielke, S. EBV-induced post transplant lymphoproliferative disorders: A persisting challenge in allogeneic hematopoetic SCT. Bone Marrow Transplant. 2014, 49, 163–167. [Google Scholar] [CrossRef] [Green Version]
- Reddy, N.; Rezvani, K.; Barrett, A.J.; Savani, B.N. Strategies to prevent EBV reactivation and posttransplant lymphoproliferative disorders (PTLD) after allogeneic stem cell transplantation in high-risk patients. Biol. Blood Marrow Transplant. 2011, 17, 591–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Styczynski, J.; Einsele, H.; Gil, L.; Ljungman, P. Outcome of treatment of Epstein-Barr virus-related post-transplant lymphoproliferative disorder in hematopoietic stem cell recipients: A comprehensive review of reported cases. Transpl. Infect. Dis. 2009, 11, 383–392. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). Epstein-Barr Virus and Kaposi’s Sarcoma Herpesvirus/Human Herpesvirus 8. In Proceedings of the IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Lyon, France, 17–24 June 1997; Volume 70, pp. 1–492. [Google Scholar]
- Allen, U.; Alfieri, C.; Preiksaitis, J.; Humar, A.; Moore, D.; Tapiero, B.; Tellier, R.; Green, M.; Davies, D.; Hebert, D.; et al. Epstein-Barr virus infection in transplant recipients: Summary of a workshop on surveillance, prevention and treatment. Can. J. Infect. Dis. 2002, 13, 89–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pariente, M.; Bartolome, J.; Lorente, S.; Crespo, M.D. Age distribution of serological profiles of Epstein-Barr virus infection: Review of results from a diagnostic laboratory. Enferm. Infecc. Microbiol. Clin. 2007, 25, 108–110. [Google Scholar] [CrossRef]
- Corssmit, E.P.; Hall, M.A.L.-V.; Portegies, P.; Bakker, P. Severe neurological complications in association with Epstein-Barr virus infection. J. Neurovirol. 1997, 3, 460–464. [Google Scholar] [CrossRef]
- Jenson, H.B. Acute complications of Epstein-Barr virus infectious mononucleosis. Curr. Opin. Pediatr. 2000, 12, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M. The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer 2006, 118, 3030–3044. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J.I. Epstein-Barr virus lymphoproliferative disease associated with acquired immunodeficiency. Medicine 1991, 70, 137–160. [Google Scholar] [CrossRef]
- Cohen, J.I. Epstein–Barr virus infection. N. Engl. J. Med. 2000, 343, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Xuan, L.; Jiang, X.; Sun, J.; Zhang, Y.; Huang, F.; Fan, Z.; Guo, X.; Dai, M.; Liu, C.; Yu, G.; et al. Spectrum of Epstein-Barr virus-associated diseases in recipients of allogeneic hematopoietic stem cell transplantation. Transplantation 2013, 96, 560–566. [Google Scholar] [CrossRef]
- Tanner, J.; Alfieri, C. The Epstein-Barr virus and post-transplant lymphoproliferative disease: Interplay of immunosuppression, EBV, and the immune system in disease pathogenesis. Transpl. Infect. Dis. 2001, 3, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Curtis, R.E.; Travis, L.B.; Rowlings, P.A.; Socie, G.; Kingma, D.W.; Banks, P.M.; Jaffe, E.S.; Sale, G.E.; Horowitz, M.M.; Witherspoon, R.P.; et al. Risk of lymphoproliferative disorders after bone marrow transplantation: A multi-institutional study. Blood 1999, 94, 2208–2216. [Google Scholar] [PubMed]
- Faye, A.; Vilmer, E. Post-Transplant Lymphoproliferative Disorder in Children. Paediatr. Drugs 2005, 7, 55–65. [Google Scholar] [CrossRef]
- Kinch, A.; Oberg, G.; Arvidson, J.; Falk, K.I.; Linde, A.; Pauksens, K. Post-transplant lymphoproliferative disease and other Epstein-Barr virus diseases in allogeneic haematopoietic stem cell transplantation after introduction of monitoring of viral load by polymerase chain reaction. Scand. J. Infect. Dis. 2007, 39, 235–244. [Google Scholar] [CrossRef]
- Hussein, K.; Tiede, C.; Maecker-Kolhoff, B.; Kreipe, H. Posttransplant lymphoproliferative disorder in pediatric patients. Pathobiology 2013, 80, 289–296. [Google Scholar] [CrossRef]
- Blaes, A.H.; Cao, Q.; Wagner, J.E.; Young, J.A.; Weisdorf, D.J.; Brunstein, C.G. Monitoring and preemptive rituximab therapy for Epstein-Barr virus reactivation after antithymocyte globulin containing nonmyeloablative conditioning for umbilical cord blood transplantation. Biol. Blood Marrow Transplant. 2010, 16, 287–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpenter, B.; Haque, T.; Dimopoulou, M.; Atkinson, C.; Roughton, M.; Grace, S.; Denovan, S.; Fielding, A.; Kottaridis, P.D.; Griffiths, P.; et al. Incidence and dynamics of Epstein-Barr virus reactivation after alemtuzumab-based conditioning for allogeneic hematopoietic stem-cell transplantation. Transplantation 2010, 90, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Zallio, F.; Primon, V.; Tamiazzo, S.; Pini, M.; Baraldi, A.; Corsetti, M.T.; Gotta, F.; Bertassello, C.; Salvi, F.; Rocchetti, A.; et al. Epstein-Barr virus reactivation in allogeneic stem cell transplantation is highly related to cytomegalovirus reactivation. Clin. Transplant. 2013, 27, E491–E497. [Google Scholar] [CrossRef] [PubMed]
- Effective Public Health Practice Project. Quality Assessment Tool for Quantitative Studies. Hamilton, on: Effective Public Health Practice Project. Available online: https://merst.ca/wp-content/uploads/2018/02/quality-assessment-tool_2010.pdf (accessed on 30 September 2019).
- Effective Public Health Practice Project. Dictionary for the Effective Public Health Practice Project Quality Assessment Tool For Quantitative Studies. Available online: https://merst.ca/wp-content/uploads/2018/02/qualilty-assessment-dictionary_2017.pdf (accessed on 30 September 2019).
- Liu, Q.F.; Ling, Y.W.; Fan, Z.P.; Jiang, Q.L.; Sun, J.; Wu, X.L.; Zhao, J.; Wei, Q.; Zhang, Y.; Yu, G.P.; et al. Epstein-Barr virus (EBV) load in cerebrospinal fluid and peripheral blood of patients with EBV-associated central nervous system diseases after allogeneic hematopoietic stem cell transplantation. Transpl. Infect. Dis. 2013, 15, 379–392. [Google Scholar] [CrossRef]
- Borenstein, M. Introduction to Meta-Analysis; John Wiley & Sons: Chichester, West Sussex, UK; Hoboken, NJ, USA, 2009; p. xxviii, 421p. [Google Scholar]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Harrer, M.; Cuijpers, P.; Furukawa, T.; Ebert, D. Doing Meta-Analysis in R: A Hands-on Guide; PROTECT Lab Erlangen: Erlangen, Germany, 2019. [Google Scholar]
- Ali, S.; Al Thubaiti, S.; Renzi, S.; Krueger, J.; Chiang, K.Y.; Naqvi, A.; Schechter, T.; Punnett, A.; Ali, M. Hemophagocytic lymphohistiocytosis is a sign of poor outcome in pediatric Epstein-Barr virus-associated post-transplant lymphoproliferative disease after allogeneic hematopoietic stem cell transplantation. Pediatr. Transplant. 2019, 23, e13319. [Google Scholar] [CrossRef] [Green Version]
- Althubaiti, S.; Ali, S.; Renzi, S.; Krueger, J.; Chiang, K.Y.; Schechter, T.; Punnett, A.; Ali, M. Lymphocyte subset at time of Epstein-Barr viremia post-allogeneic hematopoietic stem cell transplantation in children may predict development of post-transplant lymphoproliferative disease: CD8:CD20 ratio as a sensitive predictor. Pediatr. Transplant. 2019, 23. [Google Scholar] [CrossRef]
- Atay, D.; Akcay, A.; Erbey, F.; Ozturk, G. The impact of alternative donor types on viral infections in pediatric hematopoietic stem cell transplantation. Pediatr. Transplant. 2018, 22. [Google Scholar] [CrossRef]
- Auger, S.; Orsini, M.; Ceballos, P.; Fegueux, N.; Kanouni, T.; Caumes, B.; Klein, B.; Villalba, M.; Rossi, J.F. Controlled Epstein-Barr virus reactivation after allogeneic transplantation is associated with improved survival. Eur. J. Haematol. 2014, 92, 421–428. [Google Scholar] [CrossRef]
- Bogunia-Kubik, K.; Jaskula, E.; Lange, A. The presence of functional CCR5 and EBV reactivation after allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant. 2007, 40, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Bogunia-Kubik, K.; Mlynarczewska, A.; Jaskula, E.; Lange, A. The presence of IFNG 3/3 genotype in the recipient associates with increased risk for Epstein-Barr virus reactivation after allogeneic haematopoietic stem cell transplantation. Br. J. Haematol. 2005, 132, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Bordon, V.; Padalko, E.; Benoit, Y.; Dhooge, C.; Laureys, G. Incidence, kinetics, and risk factors of Epstein-Barr virus viremia in pediatric patients after allogeneic stem cell transplantation. Pediatr. Transplant. 2012, 16, 144–150. [Google Scholar] [CrossRef]
- Brunstein, C.G.; Weisdorf, D.J.; DeFor, T.; Barker, J.N.; Tolar, J.; van Burik, J.A.; Wagner, J.E. Marked increased risk of Epstein-Barr virus-related complications with the addition of antithymocyte globulin to a nonmyeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood 2006, 108, 2874–2880. [Google Scholar] [CrossRef]
- Burns, D.M.; Rana, S.; Martin, E.; Nagra, S.; Ward, J.; Osman, H.; Bell, A.I.; Moss, P.; Russell, N.H.; Craddock, C.F.; et al. Greatly reduced risk of EBV reactivation in rituximab-experienced recipients of alemtuzumab-conditioned allogeneic HSCT. Bone Marrow Transplant. 2016, 51, 825–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buyck, H.C.E.; Ball, S.; Junagade, P.; Marsh, J.; Chakrabarti, S. Prior immunosuppressive therapy with antithymocyte globulin increases the risk of EBV-related lymphoproliferative disorder following allo-SCT for acquired aplastic anaemia. Bone Marrow Transplant. 2009, 43, 813–816. [Google Scholar] [CrossRef]
- Cesaro, S.; Murrone, A.; Mengoli, C.; Pillon, M.; Biasolo, M.A.; Calore, E.; Tridello, G.; Varotto, S.; Alaggio, R.; Zanesco, L.; et al. The real-time polymerase chain reaction-guided modulation of immunosuppression enables the pre-emptive management of Epstein-Barr virus reactivation after allogeneic haematopoietic stem cell transplantation. Br. J. Haematol. 2004, 128, 224–233. [Google Scholar] [CrossRef]
- Cesaro, S.; Pegoraro, A.; Tridello, G.; Calore, E.; Pillon, M.; Varotto, S.; Abate, D.; Barzon, L.; Mengoli, C.; Carli, M.; et al. A prospective study on modulation of immunosuppression for Epstein-Barr virus reactivation in pediatric patients who underwent unrelated hematopoietic stem-cell transplantation. Transplantation 2010, 89, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Chiereghin, A.; Prete, A.; Belotti, T.; Gibertoni, D.; Piccirilli, G.; Gabrielli, L.; Pession, A.; Lazzarotto, T. Prospective Epstein-Barr virus-related post-transplant lymphoproliferative disorder prevention program in pediatric allogeneic hematopoietic stem cell transplant: Virological monitoring and first-line treatment. Transpl. Infect. Dis. 2016, 18, 44–54. [Google Scholar] [CrossRef]
- Chiereghin, A.; Piccirilli, G.; Belotti, T.; Prete, A.; Bertuzzi, C.; Gibertoni, D.; Gabrielli, L.; Turello, G.; Borgatti, E.C.; Barbato, F.; et al. Clinical utility of measuring Epstein-Barr virus-specific cell-mediated immunity after HSCT in addition to virological monitoring: Results from a prospective study. Med. Microbiol. Immunol. 2019, 208, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Christopeit, M.; Janssen, N.; Weber, T.; Bacher, U.; Lautenschlager, C.; Oehme, A.; Kekule, A.S.; Schmoll, H.J. Cyclosporine area under the curve after allogeneic hematopoietic stem cell transplantation is an indicator of Epstein-Barr virus DNAemia. Leuk. Lymphoma 2013, 54, 133–137. [Google Scholar] [CrossRef]
- Cohen, J.; Gandhi, M.; Naik, P.; Cubitt, D.; Rao, K.; Thaker, U.; Davies, E.G.; Gaspar, H.B.; Amrolia, P.J.; Veys, P. Increased incidence of EBV-related disease following paediatric stem cell transplantation with reduced-intensity conditioning. Br. J. Haematol. 2005, 129, 229–239. [Google Scholar] [CrossRef]
- Comoli, P.; Basso, S.; Zecca, M.; Pagliara, D.; Baldanti, F.; Bernardo, M.E.; Barberi, W.; Moretta, A.; Labirio, M.; Paulli, M.; et al. Preemptive therapy of EBV-related lymphoproliferative disease after pediatric haploidentical stem cell transplantation. Am. J. Transplant. 2007, 7, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Czyzewski, K.; Styczynski, J.; Giebel, S.; Fraczkiewicz, J.; Salamonowicz, M.; Zajac-Spychala, O.; Zaucha-Prazmo, A.; Drozd-Sokolowska, J.; Waszczuk-Gajda, A.; Dybko, J.; et al. Age-dependent determinants of infectious complications profile in children and adults after hematopoietic cell transplantation: Lesson from the nationwide study. Ann. Hematol. 2019, 98, 2197–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Aveni, M.; Aissi-Rothe, L.; Venard, V.; Salmon, A.; Falenga, A.; Decot, V.; Virion, J.M.; Wang, Y.; Clement, L.; Latger-Cannard, V.; et al. The clinical value of concomitant Epstein Barr virus (EBV)-DNA load and specific immune reconstitution monitoring after allogeneic hematopoietic stem cell transplantation. Transpl. Immunol. 2011, 24, 224–232. [Google Scholar] [CrossRef]
- Dumas, P.Y.; Ruggeri, A.; Robin, M.; Crotta, A.; Abraham, J.; Forcade, E.; Bay, J.O.; Michallet, M.; Bertrand, Y.; Socie, G.; et al. Incidence and risk factors of EBV reactivation after unrelated cord blood transplantation: A Eurocord and Societe Francaise de Greffe de Moelle-Therapie Cellulaire collaborative study. Bone Marrow Transplant. 2013, 48, 253–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duver, F.; Weisbrich, B.; Eyrich, M.; Wolfl, M.; Schlegel, P.G.; Wiegering, V. Viral reactivations following hematopoietic stem cell transplantation in pediatric patients—A single center 11-year analysis. PLoS ONE 2020, 15, e0228451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmahdi, S.; Muramatsu, H.; Narita, A.; Torii, Y.; Ismael, O.; Kawashima, N.; Okuno, Y.; Sekiya, Y.; Xu, Y.; Wang, X.; et al. Correlation of rabbit antithymocyte globulin serum levels and clinical outcomes in children who received hematopoietic stem cell transplantation from an alternative donor. Pediatr. Transplant. 2016, 20, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Jing, M.; Yang, M.; Xu, L.; Liang, H.; Huang, Y.; Yang, R.; Gui, G.; Wang, H.; Gong, S.; et al. Herpesvirus infections in hematopoietic stem cell transplant recipients seropositive for human cytomegalovirus before transplantation. Int. J. Infect. Dis. 2016, 46, 89–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figgins, B.; Hammerstrom, A.; Ariza-Heredia, E.; Oran, B.; Milton, D.R.; Yeh, J. Characterization of Viral Infections after Antithymocyte Globulin-Based Conditioning in Adults Undergoing Allogeneic Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2019, 25, 1837–1843. [Google Scholar] [CrossRef]
- Fujimoto, A.; Hiramoto, N.; Yamasaki, S.; Inamoto, Y.; Uchida, N.; Maeda, T.; Mori, T.; Kanda, Y.; Kondo, T.; Shiratori, S.; et al. Risk Factors and Predictive Scoring System For Post-Transplant Lymphoproliferative Disorder after Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2019, 25, 1441–1449. [Google Scholar] [CrossRef]
- Gao, X.N.; Lin, J.; Wang, L.J.; Li, F.; Li, H.H.; Wang, S.H.; Huang, W.R.; Gao, C.J.; Yu, L.; Liu, D.H. Risk factors and clinical outcomes of Epstein-Barr virus DNAemia and post-transplant lymphoproliferative disorders after haploidentical and matched-sibling PBSCT in patients with hematologic malignancies. Ann. Hematol. 2019, 98, 2163–2177. [Google Scholar] [CrossRef]
- Garcia-Cadenas, I.; Castillo, N.; Martino, R.; Barba, P.; Esquirol, A.; Novelli, S.; Orti, G.; Garrido, A.; Saavedra, S.; Moreno, C.; et al. Impact of Epstein Barr virus-related complications after high-risk allo-SCT in the era of pre-emptive rituximab. Bone Marrow Transplant. 2015, 50, 579–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.B.; Bae, E.Y.; Lee, J.W.; Jang, P.S.; Lee, D.G.; Chung, N.G.; Jeong, D.C.; Cho, B.; Lee, S.J.; Kang, J.H.; et al. Features of Epstein-Barr virus reactivation after allogeneic hematopoietic cell transplantation in Korean children living in an area of high seroprevalence against Epstein-Barr virus. Int. J. Hematol. 2014, 100, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Hiwarkar, P.; Gaspar, H.B.; Gilmour, K.; Jagani, M.; Chiesa, R.; Bennett-Rees, N.; Breuer, J.; Rao, K.; Cale, C.; Goulden, N.; et al. Impact of viral reactivations in the era of pre-emptive antiviral drug therapy following allogeneic haematopoietic SCT in paediatric recipients. Bone Marrow Transplant. 2013, 48, 803–808. [Google Scholar] [CrossRef]
- Hoegh-Petersen, M.; Goodyear, D.; Geddes, M.N.; Liu, S.; Ugarte-Torres, A.; Liu, Y.; Walker, J.T.; Fonseca, K.; Daly, A.; Duggan, P.; et al. High incidence of post transplant lymphoproliferative disorder after antithymocyte globulin-based conditioning and ineffective prediction by day 28 EBV-specific T lymphocyte counts. Bone Marrow Transplant. 2011, 46, 1104–1112. [Google Scholar] [CrossRef]
- Hoshino, Y.; Kimura, H.; Tanaka, N.; Tsuge, I.; Kudo, K.; Horibe, K.; Kato, K.; Matsuyama, T.; Kikuta, A.; Kojima, S.; et al. Prospective monitoring of the Epstein-Barr virus DNA by a real-time quantitative polymerase chain reaction after allogenic stem cell transplantation. Br. J. Haematol. 2001, 115, 105–111. [Google Scholar] [CrossRef]
- Islam, M.S.; Anoop, P.; Gordon-Smith, E.C.; Rice, P.; Datta-Nemdharry, P.; Marsh, J.C. Epstein-Barr virus infections after allogeneic stem cell transplantation: A comparison between non-malignant and malignant hematological disorders. Hematology 2010, 15, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Issa, H.; Sharma, N.; Zhao, Q.; Ruppert, A.S.; Elder, P.; Benson, D.M.; Penza, S.; Vasu, S.; William, B.; Jaglowski, S.; et al. Comparison of Two Doses of Antithymocyte Globulin in Reduced-Intensity Conditioning Allogeneic Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2019, 25, 1993–2001. [Google Scholar] [CrossRef]
- Kutnik, P.; Kwiatkowska, A.; Krawczyk, D.; Polak, O.; Jawoszek, P.; Puchala, D.; Zaucha-Prazmo, A.; Kowalczyk, J. The impact of donor-recipient sex matching on transplantrelated complications in children after allogeneic haematopoietic stem cell transplantation—A single-centre, retrospective study. Pediatr. Pol. 2019, 94, 158–161. [Google Scholar] [CrossRef]
- Jaskula, E.; Dlubek, D.; Sedzimirska, M.; Duda, D.; Tarnowska, A.; Lange, A. Reactivations of cytomegalovirus, human herpes virus 6, and Epstein-Barr virus differ with respect to risk factors and clinical outcome after hematopoietic stem cell transplantation. Transplant. Proc. 2010, 42, 3273–3276. [Google Scholar] [CrossRef]
- Juvonen, E.; Aalto, S.; Tarkkanen, J.; Volin, L.; Hedman, K.; Ruutu, T. Retrospective evaluation of serum Epstein Barr virus DNA levels in 406 allogeneic stem cell transplant patients. Haematologica 2007, 92, 819–825. [Google Scholar] [CrossRef]
- Kalra, A.; Roessner, C.; Jupp, J.; Williamson, T.; Tellier, R.; Chaudhry, A.; Khan, F.; Taparia, M.; Jimenez-Zepeda, V.H.; Stewart, D.A.; et al. Risk factors for post-transplant lymphoproliferative disorder after Thymoglobulin-conditioned hematopoietic cell transplantation. Clin. Transplant. 2018, 32, e13150. [Google Scholar] [CrossRef]
- Kullberg-Lindh, C.; Mellgren, K.; Friman, V.; Fasth, A.; Ascher, H.; Nilsson, S.; Lindh, M. Opportunistic virus DNA levels after pediatric stem cell transplantation: Serostatus matching, anti-thymocyte globulin, and total body irradiation are additive risk factors. Transpl. Infect. Dis. 2011, 13, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Laberko, A.; Bogoyavlenskaya, A.; Shelikhova, L.; Shekhovtsova, Z.; Balashov, D.; Voronin, K.; Kurnikova, E.; Boyakova, E.; Raykina, E.; Brilliantova, V.; et al. Risk Factors for and the Clinical Impact of Cytomegalovirus and Epstein-Barr Virus Infections in Pediatric Recipients of TCR-alpha/beta- and CD19-Depleted Grafts. Biol. Blood Marrow Transplant. 2017, 23, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Landgren, O.; Gilbert, E.S.; Rizzo, J.D.; Socie, G.; Banks, P.M.; Sobocinski, K.A.; Horowitz, M.M.; Jaffe, E.S.; Kingma, D.W.; Travis, L.B.; et al. Risk factors for lymphoproliferative disorders after allogeneic hematopoietic cell transplantation. Blood 2009, 113, 4992–5001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Wang, B.; Fu, L.; Pang, Y.; Zhu, G.; Zhou, X.; Ma, J.; Su, Y.; Qin, M.; Wu, R. Hematopoietic stem cell transplantation without in vivo T-cell depletion for pediatric aplastic anemia: A single-center experience. Pediatr. Transplant. 2018, 22, e13204. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Wang, Y.; Huang, F.; Fan, Z.; Zhang, S.; Yang, T.; Xu, Y.; Xu, N.; Xuan, L.; Ye, J.; et al. Two dose levels of rabbit antithymocyte globulin as graft-versus-host disease prophylaxis in haploidentical stem cell transplantation: A multicenter randomized study. BMC Med. 2019, 17, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Gao, H.; Xu, L.P.; Mo, X.D.; Liu, R.; Liang, S.; Wu, N.; Wang, M.; Wang, Z.; Chang, Y.J.; et al. Immunosuppressant indulges EBV reactivation and related lymphoproliferative disease by inhibiting Vdelta2+ T cells activities after hematopoietic transplantation for blood malignancies. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Xuan, L.; Liu, H.; Huang, F.; Zhou, H.; Fan, Z.; Zhao, K.; Wu, M.; Xu, L.; Zhai, X.; et al. Molecular monitoring and stepwise preemptive therapy for Epstein-Barr virus viremia after allogeneic stem cell transplantation. Am. J. Hematol. 2013, 88, 550–555. [Google Scholar] [CrossRef]
- Liu, J.; Bian, Z.; Wang, X.; Xu, L.P.; Fu, Q.; Wang, C.; Chang, Y.J.; Wang, Y.; Zhang, X.H.; Jiang, Z.; et al. Inverse correlation of Vdelta2(+) T-cell recovery with EBV reactivation after haematopoietic stem cell transplantation. Br. J. Haematol. 2018, 180, 276–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marinho-Dias, J.; Baldaque, I.; Pinho-Vaz, C.; Leite, L.; Branca, R.; Campilho, F.; Campos, A.; Medeiros, R.; Sousa, H. Association of Epstein-Barr virus infection with allogeneic hematopoietic stem cell transplantation in patients in Portugal. Mol. Med. Rep. 2019, 19, 1435–1442. [Google Scholar] [CrossRef] [Green Version]
- Meijer, E.; Dekker, A.W.; Lokhorst, H.M.; Petersen, E.J.; Nieuwenhuis, H.K.; Verdonck, L.F. Low incidence of infectious complications after nonmyeloablative compared with myeloablative allogeneic stem cell transplantation. Transpl. Infect. Dis. 2004, 6, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Mountjoy, L.; Jain, T.; Kunze, K.L.; Khera, N.; Sproat, L.Z.; Jennifer, W.; McCallen, M.; Leis, J.F.; Noel, P.; Slack, J.L.; et al. Clinical outcomes with low dose anti-thymocyte globulin in patients undergoing matched unrelated donor allogeneic hematopoietic cell transplantation. Leuk. Lymphoma 2020, 61, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Neumann, T.; Schneidewind, L.; Thiele, T.; Pink, D.; Schulze, M.; Schmidt, C.; Krüger, W. No indication of increased infection rates using low-dose alemtuzumab instead of anti-thymocyte globulin as graft-versus-host disease prophylaxis before allogeneic stem cell transplantation. Transpl. Infect. Dis. 2018, 20, e12822. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.; Gwozdowicz, S.; Graczyk-Pol, E.; Mika-Witkowska, R.; Rogatko-Koros, M.; Nestorowicz, K.; Szlendak, U.; Malinowska, A.; Kaczmarek, B.; Nasilowska-Adamska, B.; et al. Epstein-Barr virus infections are strongly dependent on activating and inhibitory KIR-HLA pairs after T-cell replate unrelated hematopoietic stem cell transplantation, the principles, and method of pairing analysis. HLA 2019, 94, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Omar, H.; Hagglund, H.; Gustafsson-Jernberg, A.; LeBlanc, K.; Mattsson, J.; Remberger, M.; Ringden, O.; Sparrelid, E.; Sundin, M.; Winiarski, J.; et al. Targeted monitoring of patients at high risk of post-transplant lymphoproliferative disease by quantitative Epstein-Barr virus polymerase chain reaction. Transpl. Infect. Dis. 2009, 11, 393–399. [Google Scholar] [CrossRef] [Green Version]
- Pagliuca, S.; Bommier, C.; Michonneau, D.; Meignin, V.; Salmona, M.; Robin, M.; Prata, P.H.; Xhaard, A.; de Fontbrune, F.S.; Feghoul, L.; et al. Epstein-Barr Virus-Associated Post-Transplantation Lymphoproliferative Disease in Patients Who Received Anti-CD20 after Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2019, 25, 2490–2500. [Google Scholar] [CrossRef]
- Park, J.; Lim, S.H.; Kim, S.H.; Yun, J.; Kim, C.K.; Lee, S.C.; Won, J.H.; Hong, D.S.; Park, S.K. Is immunological recovery clinically relevant at 100 days after allogeneic transplantation? Korean J. Intern. Med. 2020, 21. [Google Scholar] [CrossRef] [Green Version]
- Peric, Z.; Cahu, X.; Chevallier, P.; Brissot, E.; Malard, F.; Guillaume, T.; Delaunay, J.; Ayari, S.; Dubruille, V.; Le Gouill, S.; et al. Features of EBV reactivation after reduced intensity conditioning unrelated umbilical cord blood transplantation. Bone Marrow Transplant. 2012, 47, 251–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peric, Z.; Cahu, X.; Chevallier, P.; Brissot, E.; Malard, F.; Guillaume, T.; Delaunay, J.; Ayari, S.; Dubruille, V.; Le Gouill, S.; et al. Features of Epstein-Barr Virus (EBV) reactivation after reduced intensity conditioning allogeneic hematopoietic stem cell transplantation. Leukemia 2011, 25, 932–938. [Google Scholar] [CrossRef] [Green Version]
- Ru, Y.; Zhang, X.; Song, T.; Ding, Y.; Zhu, Z.; Fan, Y.; Xu, Y.; Sun, A.; Qiu, H.; Jin, Z.; et al. Epstein-Barr virus reactivation after allogeneic hematopoietic stem cell transplantation: Multifactorial impact on transplant outcomes. Bone Marrow Transplant. 2020, 55, 1754–1762. [Google Scholar] [CrossRef]
- Rustia, E.; Violago, L.; Jin, Z.; Foca, M.D.; Kahn, J.M.; Arnold, S.; Sosna, J.; Bhatia, M.; Kung, A.L.; George, D.; et al. Risk Factors and Utility of a Risk-Based Algorithm for Monitoring Cytomegalovirus, Epstein-Barr Virus, and Adenovirus Infections in Pediatric Recipients after Allogeneic Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2016, 22, 1646–1653. [Google Scholar] [CrossRef] [Green Version]
- Sanz, J.; Arango, M.; Senent, L.; Jarque, I.; Montesinos, P.; Sempere, A.; Lorenzo, I.; Martin, G.; Moscardo, F.; Mayordomo, E.; et al. EBV-associated post-transplant lymphoproliferative disorder after umbilical cord blood transplantation in adults with hematological diseases. Bone Marrow Transplant. 2014, 49, 397–402. [Google Scholar] [CrossRef]
- Bueltzingsloewen, A.S.-V.; Morand, P.; Buisson, M.; Souillet, G.; Chambost, H.; Bosson, J.L.; Bordigoni, P. A prospective study of Epstein-Barr virus load in 85 hematopoietic stem cell transplants. Bone Marrow Transplant. 2002, 29, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Styczynski, J.; Gil, L.; Tridello, G.; Ljungman, P.; Donnelly, J.P.; Van Der Velden, W.; Omar, H.; Martino, R.; Halkes, C.; Faraci, M.; et al. Response to rituximab-based therapy and risk factor analysis in epstein barr virus-related lymphoproliferative disorder after hematopoietic stem cell transplant in children and adults: A study from the infectious diseases working party of the european group for blood and marrow transplantation. Clin. Infect. Dis. 2013, 57, 794–802. [Google Scholar] [PubMed] [Green Version]
- Torre-Cisneros, J.; Roman, J.; Torres, A.; Herrera, C.; Caston, J.J.; Rivero, A.; Mingot, E.; Rojas, R.; Martin, C.; Martinez, F.; et al. Control of Epstein-Barr virus load and lymphoproliferative disease by maintenance of CD8+ T lymphocytes in the T lymphocyte-depleted graft after bone marrow transplantation. J. Infect. Dis. 2004, 190, 1596–1599. [Google Scholar] [CrossRef] [Green Version]
- Trottier, H.; Buteau, C.; Robitaille, N.; Duval, M.; Tucci, M.; Lacroix, J.; Alfieri, C. Transfusion-related Epstein-Barr virus infection among stem cell transplant recipients: A retrospective cohort study in children. Transfusion 2012, 52, 2653–2663. [Google Scholar] [CrossRef]
- Tsoumakas, K.; Giamaiou, K.; Goussetis, E.; Graphakos, S.; Kossyvakis, A.; Horefti, E.; Mentis, A.; Elefsiniotis, I.; Pavlopoulou, I.D. Epidemiology of viral infections among children undergoing hematopoietic stem cell transplant: Alpha prospective single-center study. Transpl. Infect. Dis. 2019, 21, e13095. [Google Scholar] [CrossRef]
- Uhlin, M.; Wikell, H.; Sundin, M.; Blennow, O.; Maeurer, M.; Ringden, O.; Winiarski, J.; Ljungman, P.; Remberger, M.; Mattsson, J. Risk factors for Epstein-Barr virus-related post-transplant lymphoproliferative disease after allogeneic hematopoietic stem cell transplantation. Haematologica 2014, 99, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Van Der Velden, W.J.F.M.; Mori, T.; Stevens, W.B.C.; De Haan, A.F.J.; Stelma, F.F.; Blijlevens, N.M.A.; Donnelly, J.P. Reduced PTLD-related mortality in patients experiencing EBV infection following allo-SCT after the introduction of a protocol incorporating pre-emptive rituximab. Bone Marrow Transplant. 2013, 48, 1465–1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Esser, J.W.J.; Van Der Holt, B.; Meijer, E.; Niesters, H.G.M.; Trenschel, R.; Thijsen, S.F.T.; Van Loon, A.M.; Frassoni, F.; Bacigalupo, A.; Schaefer, U.W.; et al. Epstein-Barr virus (EBV) reactivation is a frequent event after allogeneic stem cell transplantation (SCT) and quantitatively predicts EBV-lymphoproliferative disease following T-cell-depleted SCT. Blood 2001, 98, 972–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Zhang, T.T.; Qi, J.Q.; Chu, T.T.; Miao, M.; Qiu, H.Y.; Fu, C.C.; Tang, X.W.; Ruan, C.G.; Wu, D.P.; et al. Incidence, risk factors, and clinical significance of Epstein-Barr virus reactivation in myelodysplastic syndrome after allogeneic haematopoietic stem cell transplantation. Ann. Hematol. 2019, 98, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.P.; Zhang, C.L.; Mo, X.D.; Zhang, X.H.; Chen, H.; Han, W.; Chen, Y.H.; Wang, Y.; Yan, C.H.; Wang, J.Z.; et al. Epstein-Barr Virus-Related Post-Transplantation Lymphoproliferative Disorder after Unmanipulated Human Leukocyte Antigen Haploidentical Hematopoietic Stem Cell Transplantation: Incidence, Risk Factors, Treatment, and Clinical Outcomes. Biol. Blood Marrow Transplant. 2015, 21, 2185–2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuan, L.; Huang, F.; Fan, Z.; Zhou, H.; Zhang, X.; Yu, G.; Zhang, Y.; Liu, C.; Sun, J.; Liu, Q. Effects of intensified conditioning on Epstein-Barr virus and cytomegalovirus infections in allogeneic hematopoietic stem cell transplantation for hematological malignancies. J. Hematol. Oncol. 2012, 5, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.X.; Cao, X.H.; Yan, H.; Luo, X.Y.; Zhao, X.S.; Sun, Y.Q.; Wang, Y.; Xu, L.P.; Zhang, X.H.; Chang, Y.J.; et al. Delay expression of NKp30 on NK cells correlates with long-term mycophenolate mofetil treatment and higher EBV viremia post allogenic hematological stem cells transplantation. Clin. Immunol. 2019, 205, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Gao, Z.Y.; Lu, D.P. Incidence, risk factors, and clinical outcomes associated with Epstein-Barr virus-DNAemia and Epstein-Barr virus-associated disease in patients after haploidentical allogeneic stem cell transplantation: A single-center study. Clin. Transplant. 2020, 34. [Google Scholar] [CrossRef]
- Zhou, L.; Gao, Z.Y.; Lu, D.P. Comparison of ATG-thymoglobulin with ATG-Fresenius for Epstein-Barr virus infections and graft-versus-host-disease in patients with hematological malignances after haploidentical hematopoietic stem cell transplantation: A single-center experience. Ann. Hematol. 2020, 99, 1389–1400. [Google Scholar] [CrossRef]
- Sundin, M.; Le Blanc, K.; Ringden, O.; Barkholt, L.; Omazic, B.; Lergin, C.; Levitsky, V.; Remberger, M. The role of HLA mismatch, splenectomy and recipient Epstein-Barr virus seronegativity as risk factors in post-transplant lymphoproliferative disorder following allogeneic hematopoietic stem cell transplantation. Haematologica 2006, 91, 1059–1067. [Google Scholar]
- Hoegh-Petersen, M.; Amin, M.; Liu, Y.; Ugarte-Torres, A.; Williamson, T.; Podgorny, P.; Russell, J.; Grigg, A.; Ritchie, D.; Storek, J. Anti-thymocyte globulins capable of binding to T and B cells reduce graft-vs-host disease without increasing relapse. Bone Marrow Transplant. 2013, 48, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Mohty, M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia 2007, 21, 1387–1394. [Google Scholar] [CrossRef] [Green Version]
- Kekre, N.; Antin, J.H. ATG in allogeneic stem cell transplantation: Standard of care in 2017? Counterpoint. Blood Adv. 2017, 1, 573–576. [Google Scholar] [CrossRef] [Green Version]
- Ram, R.; Storb, R. Pharmacologic Prophylaxis Regimens for Acute Graft-Versus-Host Disease: Past, Present and Future; Taylor & Francis: Oxfordshire, UK, 2013. [Google Scholar]
- Shimabukuro-Vornhagen, A.; Hallek, M.J.; Storb, R.F.; von Bergwelt-Baildon, M.S. The role of B cells in the pathogenesis of graft-versus-host disease. Blood 2009, 114, 4919–4927. [Google Scholar] [CrossRef] [Green Version]
- Zeiser, R.; Blazar, B.R. Acute graft-versus-host disease—Biologic process, prevention, and therapy. N. Engl. J. Med. 2017, 377, 2167–2179. [Google Scholar] [CrossRef] [PubMed]
- Société Française de Greffe de Moelle et Thérapie Cellulaire. Greffe de Cellules Souches Hématopoïétiques: Livret D’information et D’aide à la Décision à L’usage des Patients; Société Française de Greffe de Moelle et Thérapie Cellulaire: Toulouse, France, 2013. [Google Scholar]
- Andréoli, A.L.; de Latour, R.P.; Thépot, S.; Socié, G. La maladie chronique du greffon contre l’hôte. Hématologie 2010, 16, 420–429. [Google Scholar]
- Enok Bonong, P.R.; Buteau, C.; Delage, G.; Tanner, J.E.; Lacroix, J.; Duval, M.; Laporte, L.; Tucci, M.; Robitaille, N.; Spinella, P.C.; et al. Transfusion-related Epstein-Barr virus (EBV) infection: A multicenter prospective cohort study among pediatric recipients of hematopoietic stem cell transplants (TREASuRE study). Transfusion 2021, 61, 144–158. [Google Scholar] [CrossRef]
- Čičkušić, E.; Mustedanagić-Mujanović, J.; Iljazović, E.; Karasalihović, Z.; Škaljić, I. Association of Hodgkin’s lymphoma with Epstein Barr virus infection. Bosn. J. Basic Med. Sci. 2007, 7, 58. [Google Scholar] [CrossRef] [Green Version]
- Glaser, S.L.; Lin, R.J.; Stewart, S.L.; Ambinder, R.F.; Jarrett, R.F.; Brousset, P.; Pallesen, G.; Gulley, M.L.; Khan, G.; O’Grady, J. Epstein-Barr virus-associated Hodgkin’s disease: Epidemiologic characteristics in international data. Int. J. Cancer 1997, 70, 375–382. [Google Scholar] [CrossRef]
- Hakim, H.; Gibson, C.; Pan, J.; Srivastava, K.; Gu, Z.; Bankowski, M.J.; Hayden, R.T. Comparison of various blood compartments and reporting units for the detection and quantification of Epstein-Barr virus in peripheral blood. J. Clin. Microbiol. 2007, 45, 2151–2155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenfield, H.M.; Gharib, M.I.; Turner, A.J.; Guiver, M.; Carr, T.; Will, A.M.; Wynn, R.F. The impact of monitoring Epstein-Barr virus PCR in paediatric bone marrow transplant patients: Can it successfully predict outcome and guide intervention? Pediatr. Blood Cancer 2006, 47, 200–205. [Google Scholar] [CrossRef] [PubMed]

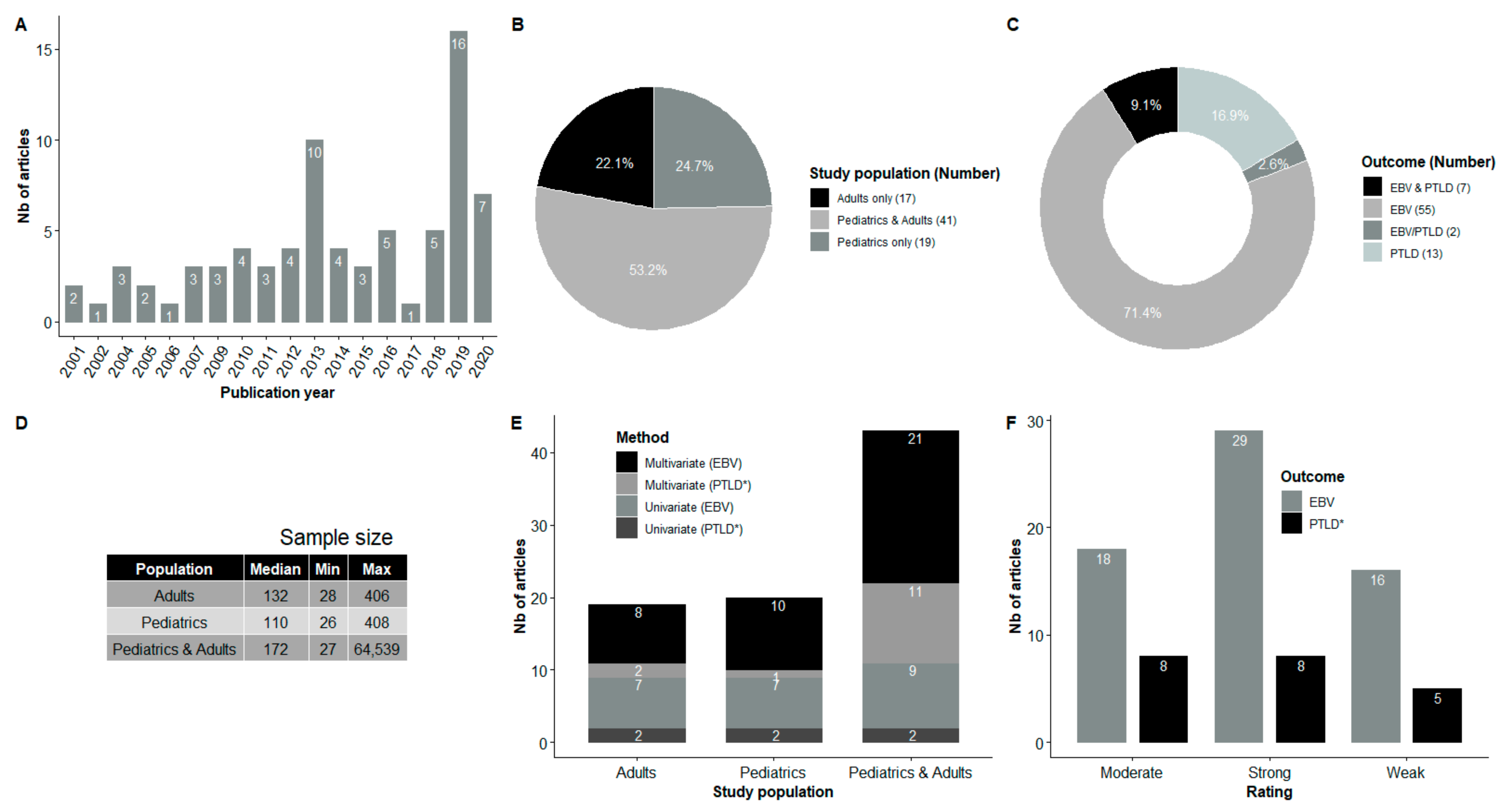


| First Author, Year | Country | Study Type | Study Population | Sample Size | Outcome | Median (Range) of Follow-Up | Statistical Analysis | Overall Rating (Table S2) |
|---|---|---|---|---|---|---|---|---|
| Ali, 2019 [31] | Canada | Retrospective | P | 408 | PTLD | NR | Univariate | Weak |
| Althubaiti, 2019 [32] | Canada | Retrospective | P | 26 | PTLD | NR | Univariate | Weak |
| Atay, 2018 [33] | Turkey | Retrospective | P | 171 | EBV ╧ | 14 months | Univariate | Weak |
| Auger, 2014 [34] | France | Retrospective | A | 190 | EBV | 36.6 months (95% IC 31.5–45.7) | Multivariate | Weak |
| Bogunia-Kubik, 2007 [35] | Poland | Retrospective | P and A | 92 | EBV | NR | Multivariate | Strong |
| Bogunia-Kubik, 2005 [36] | Poland | Retrospective | P and A | 83 | EBV | NR | Multivariate | Strong |
| Bordon, 2012 [37] | Belgium | Retrospective | P | 80 | EBV | NR | Multivariate | Moderate |
| Brunstein, 2006 [38] | USA | Multicenter retrospective | P and A | 335 | EBV/PTLD | 1.2 (77 days–9.2 years) | Multivariate | Moderate |
| Burns, 2016 [39] | United Kingdom | Retrospective | P and A | 186 | EBV | 28 months | Multivariate | Strong |
| Buyck, 2009 [40] | United Kingdom | Retrospective | P and A | 87 | PTLD | NR | Multivariate | Moderate |
| Carpenter, 2010 [23] | United Kingdom | Retrospective | P and A | 111 a | EBV | 2.4 years | Multivariate | Strong |
| Cesaro, 2004 [41] | Italy | Retrospective | P | 79 b | EBV | NR | Multivariate | Moderate |
| Cesaro, 2010 [42] | Italy | Retrospective | P | 89 | EBV | NR | Univariate | Weak |
| Chiereghin, 2016 [43] | Italy | Prospective | P | 28 | EBV | 7.1 months | Univariate | Weak |
| Chiereghin, 2019 [44] | Italy | Prospective | P and A | 51 | EBV | NR | Univariate | Weak |
| Christopeit, 2013 [45] | USA | Retrospective | A | 28 c | EBV | NR | Multivariate | Moderate |
| Cohen, 2005 [46] | United Kingdom | Prospective | P | 128 | EBV | NR | Multivariate | Moderate |
| Cohen, 2005 [46] | United Kingdom | Prospective | P | 128 | PTLD | NR | Multivariate | Moderate |
| Comoli, 2007 [47] | Italy | Prospective | P and A | 27 | EBV | 23 months | Univariate | Weak |
| Czyżewski, 2019 [48] | Poland | Retrospective multicenter study | P and A | 1569 | EBV | NR | Univariate | Weak |
| D’Aveni, 2011 [49] | France | Retrospective | P and A | 40 d | EBV | NR | Univariate | Weak |
| Dumas, 2013 [50] | France | Multicenter retrospective | P and A | 175 | EBV | NR | Multivariate | Moderate |
| Düver, 2020 [51] | Germany | Retrospective | P | 107 | EBV | 365 (range: 22–365) days | Multivariate | Strong |
| Elmahdi, 2016 [52] | Japan | Retrospective | P | 37 | EBV | NR | Multivariate | Moderate |
| Fan, 2016 [53] | China | Retrospective | P and A | 44 e | EBV ╧ | NR | Multivariate | Moderate |
| Figgins, 2019 [54] | USA | Retrospective | A | 123 | EBV | 12.8 (range: 1.0–23.1) months | Univariate | Weak |
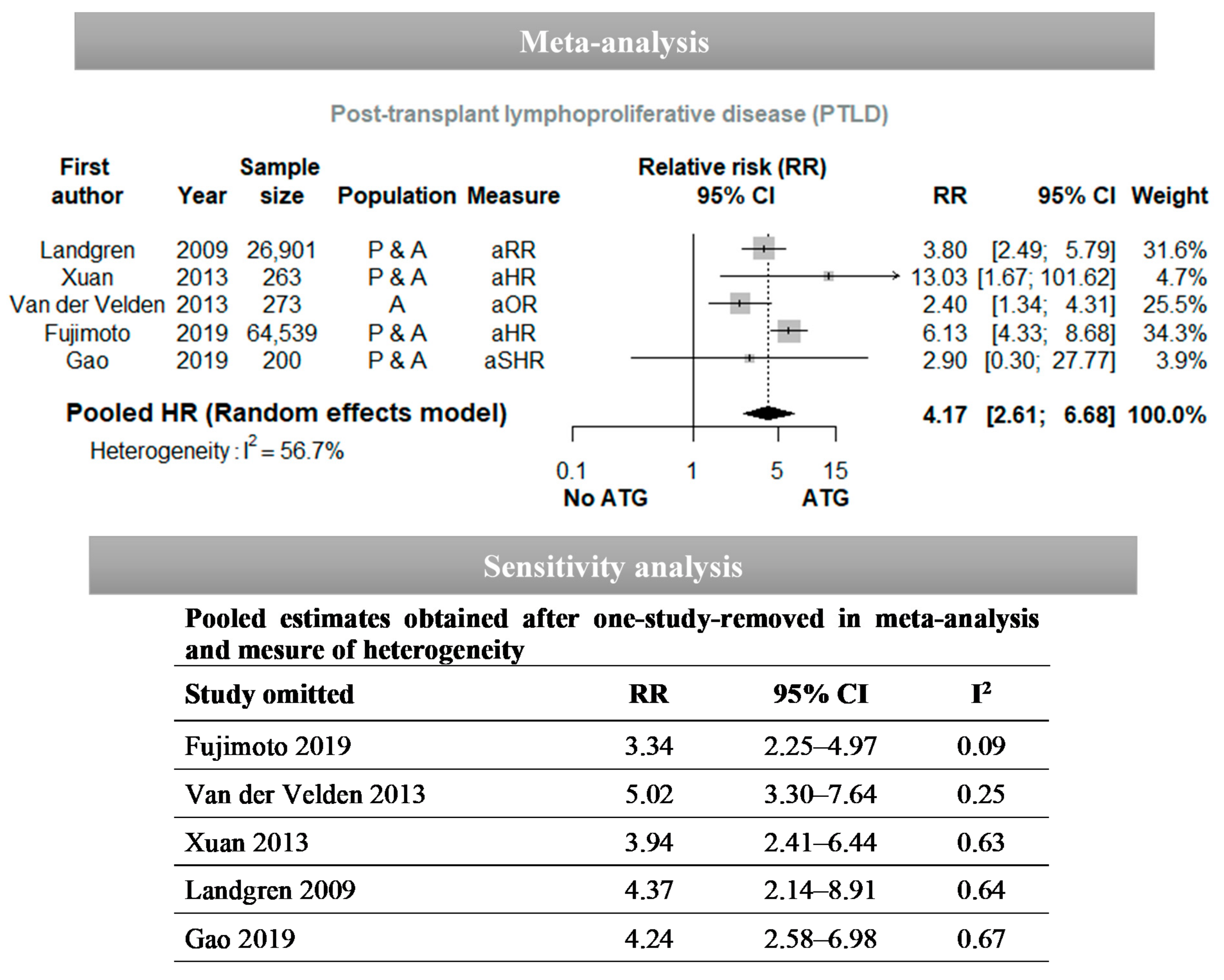
| Fujimoto, 2019 [55] | Japan | Multicenter retrospective | P and A | 64,539 | PTLD | NR | Multivariate | Strong |
| Gao, 2019 [56] | China | Retrospective | P and A | 200 | EBV | NR | Multivariate | Strong |
| Gao, 2019 [56] | China | Retrospective | P and A | 200 | PTLD | NR | Multivariate | Strong |
| Garcia-Cadenas, 2015 [57] | Spain | Prospective | A | 93 | EBV | NR | Multivariate | Strong |
| Garcia-Cadenas, 2015 [57] | Spain | Prospective | A | 93 | PTLD | NR | Multivariate | Strong |
| Han, 2014 [58] | Korea | Retrospective | P | 248 | EBV | NR | Univariate | Weak |
| Hiwarkar, 2013 [59] | United Kingdom | Retrospective | P | 278 | EBV | NR | Multivariate | Moderate |
| Hoegh-Petersen, 2011 [60] | Canada | Retrospective | A | 307 | PTLD | 375 (28–1727) days | Univariate | Weak |
| Hoshino, 2001 [61] | Japan | Prospective | P and A | 38 | EBV | NR | Univariate | Weak |
| Islam, 2010 [62] | United Kingdom | Retrospective | P and A | 83 | EBV | 4.2 (0.9–8.1) years | Univariate | Weak |
| Issa, 2019 [63] | USA | Retrospective | A | 357 | EBV | NR | Univariate | Weak |
| Kutnik, 2019 [64] | Poland | Retrospective | P | 198 | EBV | 12 months | Univariate | Weak |
| Jaskula, 2010 [65] | Poland | Prospective | P and A | 102 | EBV | NR | Multivariate | Moderate |
| Juvonen, 2007 [66] | Finland | Retrospective | A | 406 | EBV | NR | Multivariate | Strong |
| Kalra, 2018 [67] | Canada | Retrospective | P and A | 554 | PTLD | 509 days | Multivariate | Strong |
| Kullberg-Lindh, 2015 [68] | Sweden | Retrospective | P | 47 | EBV | NR | Multivariate | Strong |
| Laberko, 2017 [69] | Russia | Retrospective | P | 182 | EBV | 27 months | Multivariate | Strong |
| Landgren, 2009 [70] | CIBMTR | Multicenter retrospective | P and A | 26,901 | PTLD | >12 months | Multivariate | Strong |
| Li, 2018 [71] | China | Retrospective | P | 62 | EBV ╧ | 32.5 (0.5–132) months | Univariate | Weak |
| Lin, 2019 [72] | China | Multicenter randomized study | P and A | 408 | EBV | NR | Multivariate | Strong |
| Liu, 2020 [73] | China | Prospective | A | 170 | EBV | NR | Multivariate | Strong |
| Liu, 2020 [73] | China | Prospective | A | 170 | PTLD | NR | Univariate | Weak |
| Liu, 2013 [74] | China | Prospective | P and A | 251 f | EBV | 327 (27–1408) days | Multivariate | Strong |
| Liu, 2013 [27] | China | Prospective | P and A | 172 | EBV | 495 (45–1158) days | Multivariate | Strong |
| Liu, 2013 [27] | China | Prospective | P and A | 172 | PTLD | 495 (45–1158) days | Multivariate | Strong |
| Liu, 2018 [75] | China | Prospective | A | 132 | EBV | NR | Univariate ‡ | Strong |
| Marinho-Dias, 2019 [76] | Portugal | Prospective | P and A | 40 | EBV | >120 days | Multivariate | Strong |
| Meijer, 2004 [77] | Netherlands | Prospective | A | 78 g | EBV | (6–32) months | Univariate | Weak |
| Mountjoy, 2020 [78] | USA | Retrospective | A | 209 | EBV | Non-ATG group 677 (7–3147) days ATG group 504 (33–2156) days | Univariate | Weak |
| Neumann, 2018 [79] | Germany | Case–control | A | 44 | EBV ╧ | NR | Univariate § | Strong |
| Nowak, 2019 [80] | Poland | Retrospective | P and A | 239 | EBV | 2.1 (0.2–67.8) months | Univariate | Weak |
| Omar, 2009 [81] | Sweden | Prospective | P and A | 131 | EBV | NR | Multivariate | Moderate |
| Pagliuca, 2019 [82] | France | Retrospective | P and A | 208 | PTLD | 47.33 (3.18–126.20) months | Multivariate | Strong |
| Park, 2020 [83] | Korea | Retrospective | P and A | 114 | EBV | NR | Univariate | Weak |
| Patriarca, 2013 [4] | Italy | Prospective | A | 100 h | EBV | 7 (2–36) months | Multivariate | Strong |
| Peric, 2012 [84] | France | Retrospective | A | 33 | EBV | 468 (92–1277) days | Univariate | Weak |
| Peric, 2011 [85] | France | Retrospective | A | 175 | EBV | 655 (92–1542) days | Multivariate | Strong |
| Ru, 2020 [86] | China | Retrospective | P and A | 890 | EBV | NR | Multivariate | Strong |
| Rustia, 2016 [87] | USA | Retrospective | P | 140 | EBV | NR | Univariate | Weak |
| Sanz, 2014 [88] | Spain | Retrospective | P and A | 288 | EBV | >6 months | Multivariate | Strong |
| Sanz, 2014 [88] | Spain | Retrospective | P and A | 288 | PTLD | >6 months | Multivariate | Strong |
| Sirvent-von Bueltzingsloewen, 2002 [89] | France | Multicenter prospective | P and A | 85 i | EBV | 306 (26–867) days | Multivariate | Strong |
| Styczynski, 2013 [90] | EBMT | Multicenter retrospective | P and A | 4466 | PTLD | NR | Univariate | Weak |
| Torre-Cisneros, 2004 [91] | Spain | Prospective | P and A | 100 j | EBV | NR | Multivariate | Moderate |
| Trottier, 2012 [92] | Canada | Retrospective | P | 238 | EBV | NR | Multivariate | Moderate |
| Tsoumakas, 2019 [93] | Greece | Prospective | P | 110 | EBV | ≥1 year | Multivariate | Strong |
| Uhlin, 2014 [94] | Sweden | Retrospective | P and A | 1021 | PTLD | NR | Multivariate | Strong |
| Van der Velden, 2013 [95] | Netherlands | Retrospective | A | 273 | EBV/PTLD | ≥6 months | Multivariate | Moderate |
| Van Esser, 2001 [96] | Italy, Germany, Netherlands | Multicenter prospective | P and A | 152 | EBV | NR | Multivariate | Strong |
| Van Esser, 2001 [96] | Italy, Germany, Netherlands | Multicenter prospective | P and A | 152 | PTLD | NR | Multivariate | Strong |
| Wang, 2019 [97] | China | Retrospective | P and A | 186 | EBV | NR | Multivariate | Strong |
| Xu, 2015 [98] | China | Case–control | P and A | 180 | PTLD | NR | Multivariate | Strong |
| Xuan, 2012 [99] | China | Prospective | P and A | 185 | EBV | 319 (27–1194) days | Multivariate | Strong |
| Xuan, 2013 [16] | China | Prospective | P and A | 263 | PTLD | 374 (27–1554) days | Multivariate | Strong |
| Yu, 2019 [100] | China | Prospective | P and A | 90 | EBV | NR | Multivariate | Moderate |
| Zallio, 2013 [24] | Italy | Prospective | A | 100 | EBV | NR | Multivariate | Moderate |
| Zhou, 2020 [101] | China | Retrospective | P and A | 131 | EBV | 59.2 (range: 2.03–113.8) months | Multivariate | Strong |
| Zhou, 2020 [102] | China | Retrospective | P and A | 160 | PTLD | 64.7 (range: 2.03–113.8) months | Univariate | Weak |
| First Author, Year | Outcome | Study Population | Risk Factors | Estimate (95% CI); p-Value * | ||
|---|---|---|---|---|---|---|
| Recipient age | ||||||
| Bogunia-Kubik, 2007 [35] | EBV | P and A | > vs. ≤25 years | OR = 1.54 (1.136–2.703); p = 0.034 | ||
| Ru, 2020 [86] | EBV | P and A | <30 vs. ≥30 years | HR = 1.041 (0.763–1.420); p = 0.799 | ||
| Düver, 2020 [51] | EBV | P | Age (continuous) | OR = 1.08 (1.00–1.17); p = 0.057 | ||
| Kullberg-Lindh, 2011 [68] | EBV | P | Continuous | Slope = −0.06; p = 0.09 | ||
| Gao, 2019 [56] | PTLD | P and A | ≥40 vs. <40 years | HR = 0.4 (0.2–0.9); p = 0.032 | ||
| Landgren, 2009 [70] | PTLD | P and A | ≥50 years | RR = 5.1 (2.8–8.7) | ||
| Diagnosis | ||||||
| Burns, 2016 [39] | EBV | P and A | NHL vs. AML/MDS | HR = 0.18 (0.05–0.57); p = 0.004 | ||
| ALL vs. AML/MDS | HR = 0.89 (0.45–1.75); p = 0.734 | |||||
| HL vs. AML/MDS | HR = 1.63 (0.64–4.16); p = 0.308 | |||||
| CLL vs. AML/MDS | HR = 0.87 (0.41–1.85); p = 0.724 | |||||
| MPD vs. AML/MDS | HR = 0.95 (0.43–2.11); p = 0.907 | |||||
| Other vs. AML/MDS | HR = 3.01 (0.94–9.65); P = 0.063 | |||||
| Carpenter, 2010 [23] | EBV | P and A | HL vs. AML | HR = 3.53 (1.51–8.25); p = 0.004 | ||
| NHL vs. AML | HR = 0.678 (0.249–1.848); p = 0.448 | |||||
| MPD vs. AML | HR = 2.01 (0.828–4.858); p = 0.123 | |||||
| CLL vs. AML | HR = 3.767 (1.375–10.322); p = 0.01 | |||||
| Other disease vs. AML | HR = 1.449 (0.486–4.319); p = 0.506 | |||||
| Sanz, 2014 [88] | EBV | P and A | Hodgkin’s disease vs. other diagnosis | SHR = 11.6 (3.4–40.0); p < 0.0001 | ||
| Zhou, 2020 [101] | EBV | P and A | Underlying disease (AA vs. AL) | HR = 4.369 (0.484–39.451); p = 0.189 | ||
| Fujimoto, 2019 [55] | PTLD | P and A | ALL vs. AML/MDS | HR = 1.08 (0.75–1.57); p = 0.68 | ||
| CML/MPD vs. AML/MDS | HR = 1.55 (0.89–2.69); p = 0.12 | |||||
| Lymphoid malignancies vs. AML/MDS | HR = 1.33 (0.92–1.92); p = 0.13 | |||||
| AA vs. AML/MDS | HR = 5.19 (3.32–8.11); p < 0.001 | |||||
| Others vs. AML/MDS | HR = 1.94 (0.97–3.89); p = 0.06 | |||||
| Genotype | ||||||
| Bogunia-Kubik, 2005 [36] | EBV | P and A | Recipient having IFNG 3/3 genotype vs. other IFNG | OR = 7.28; p = 0.005 | ||
| Bogunia-Kubik, 2007 [35] | EBV | P and A | Presence of CCR5 deletion mutation (yes vs. no) | OR = 0.17 (0.034–0.803); p = 0.026 | ||
| Pagliuca, 2019 [82] | PTLD | P and A | Presence of HLA DRB1*11:01 (yes vs. no) | SHR = 4.85 (1.57–14.97); p = 0.006 | ||
| Recipient, donor EBV, CMV serostatus | ||||||
| Hiwarkar, 2013 [59] | EBV | P | D+ and R+ (CMV or EBV) or host adenoviral infection | Significant, but NR | ||
| Laberko, 2017 [69] | EBV | P and A | EBV D+/R− vs. D+/R+ | HR = 2.85 (1.12–7.28); p = 0.028 | ||
| EBV D−/R+ vs. D+/R+ | HR = 0.32 (0.05–2.0); p = 0.22 | |||||
| EBV D−/R− vs. D+/R+ | No events | |||||
| EBV Unknown vs. D+/R+ | HR = 1.23 (0.53–2.9); p = 0.63 | |||||
| Lin, 2019 [72] | EBV | P and A | D/R EBV serostatus (D−/R+ vs. Other) | HR = 1.58 (1.01–2.46); p = 0.046 | ||
| Uhlin, 2014 [94] | PTLD | P and A | EBV D+ R− vs. Other | SHR = 4.97 (2.30–10.7); p < 0.001 | ||
| Brunstein, 2006 [38] | EBV/PTLD | P and A | CMV (R− vs. R+) | HR = 3.0 (0.9–9.7) p = 0.07 | ||
| Donor sex | ||||||
| Fan, 2016 [53] | EBV | P and A | Male donor | OR = 13.24 (2.006–87.387); p = 0.007 | ||
| Jaskula, 2010 [65] | EBV | P and A | Female donor | OR = 2.816; p = 0.044 | ||
| Donor type | ||||||
| Düver, 2020 [51] | EBV | P | Unrelated donor vs. Related donor | OR = 5.05 (1.24–20.63); p = 0.024 | ||
| Marinho-Dias, 2019 [76] | EBV | P and A | Unrelated donor (yes vs. no) | HR = 8.8, p = 0.030 at D + 150 | ||
| Tsoumakas, 2019 [93] | EBV | P | Related donor vs. unrelated donor | HR = 0.38 (0.15–0.98); p = 0.045 | ||
| Omar, 2009 [81] | EBV | P and A | URD + MMRD vs. HLA-matched donor | p = 0.04 | ||
| Pagliuca, 2019 [82] | PTLD | P and A | Unrelated (yes vs. no) | SHR = 2.11 (1.00–4.45); p = 0.051 | ||
| Fujimoto, 2019 [55] | PTLD | P and A | MMRD vs. MRD | HR = 4.39 (2.39–8.07); p < 0.001 | ||
| MURD vs. MRD | HR = 4.08 (2.39–6.99); p < 0.001 | |||||
| MMURD vs. MRD | HR = 3.20 (1.58–6.47); p = 0.001 | |||||
| CB vs. MRD | HR = 8.03 (4.72–13.7); p < 0.001 | |||||
| Sirvent-von Bueltzingsloewen, 2002 [89] | EBV | P and A | HLA incompatibility (yes vs. no) | OR = 5 (1.5–16.4) | ||
| Torre-Cisneros, 2004 [91] | EBV | P and A | No HLA-matched sibling donor | HR = 2.1 (0.8–6.2); p = 0.069 | ||
| Gao, 2019 [56] | EBV | P and A | Haploidentical donors vs. matched sibling donors | HR = 2.0 (0.8–5.1); p = 0.130 | ||
| Ru, 2020 [86] | EBV | P and A | HLA-haploidentical vs. HLA-identical | HR = 1.830 (1.275–2.627); p = 0.001 | ||
| Gao, 2019 [56] | PTLD | P and A | Haploidentical donors vs. matched sibling donors | HR = 2.0 (0.5–8.3); p = 0.350 | ||
| Uhlin, 2014 [94] | PTLD | P and A | HLA mismatch vs. match | SHR = 5.89 (2.43–14.3) p < 0.001 | ||
| Graft source | ||||||
| Tsoumakas, 2019 [93] | EBV | P | PBSC vs. BM | HR = 2.51 (1.04–6.05); p = 0.041 | ||
| Wang, 2019 [97] | EBV | P and A | PB + BM vs. PB | HR = 7.89; p = 0.003 | ||
| BM vs. PB | HR = 18.69; p < 0.001 | |||||
| Graft content | ||||||
| Christopeit, 2013 [45] | EBV | A | CD3+ (≥ vs. < median) | OR = 0.11 (0.02–0.78); p = 0.027 | ||
| CD3+CD8+ (≥ vs. < median) | OR = 0.05 (0.006–0.431); p = 0.007 | |||||
| Van Esser, 2001 [96] | EBV | P and A | CD34+ (>1.35 × 106/kg) | HR = 2.6 (1.5–4.6); p = 0.001 | ||
| Conditioning regimens and GvHD prophylaxis/treatment | ||||||
| Kullberg-Lindh, 2011 [68] | EBV | P | TBI (yes vs. no) | Slope = 1.60; p = 0.001 | ||
| Liu, 2013 [74] | EBV | P and A | Intensified MAC vs. standard MAC | HR = 1.72 (1.03–2.88); p = 0.038 | ||
| Lin, 2019 [72] | EBV | P and A | Intensified conditioning vs. standard MAC | HR = 1.73 (1.18–2.54); p = 0.005 | ||
| Sanz, 2014 [88] | EBV | P and A | RIC vs. MAC | SHR = 6.0 (2.0–17.6); p = 0.001 | ||
| PTLD | RIC vs. MAC | SHR = 5.5 (1.8–17.1); p = 0.003 | ||||
| Fujimoto, 2019 [55] | PTLD | P and A | RIC vs. MAC | HR = 0.82 (0.60–1.12); p = 0.22 | ||
| Uhlin, 2014 [94] | PTLD | P and A | RIC vs. no RIC | SHR = 3.25 (1.53–6.89) p = 0.002 | ||
| Xuan, 2013 [17] | PTLD | P and A | Standard vs. intensified | HR = 4.46 (1.20–16.61); p = 0.026 | ||
| Liu, 2013 [27] | PTLD | P and A | Intensified MAC vs. standard MAC | p = 0.018 | ||
| Brunstein, 2006 [38] | EBV/PTLD | P and A | NMAC without ATG vs. MAC | HR = 0.7 (0.1–6.5); p = 0.51 | ||
| NMAC with ATG vs. MAC | HR = 15.4 (2.0–116.1); p < 0.01 | |||||
| Van der Velden, 2013 [95] | PTLD | A | MAC without ATG | OR = 2.6 (1.05–7.15); p = 0.01 | ||
| NMAC with ATG | OR = 2.1 (0.92–4.8); p = 0.08 | |||||
| Gao, 2019 [56] | PTLD | P and A | Use of fludarabine (yes vs. no) | HR = 3.8 (1.4–10.6); p = 0.010 | ||
| Cohen, 2005 [46] | EBV | P | ATG vs. Campath | OR = 2.09 (0.83–5.29) | ||
| Cesaro, 2004 [41] | EBV | P | Use of ATG (yes vs. no) | HR = 13.0 (2–96); p = 0.01 | ||
| Düver, 2020 [51] | EBV | P | Use of ATG (yes vs. no) | OR = 10.68 (1.15–98.86); p = 0.037 | ||
| Gao, 2019 [56] | EBV | P and A | Use of ATG (yes vs. no) | HR = 6.3 (1.6–24.0); p = 0.008 | ||
| Kullberg-Lindh, 2011 [68] | EBV | P | Use of ATG (yes vs. no) | Slope = 1.34; p = 0.004 | ||
| Juvonen, 2007 [66] | EBV | A | Use of ATG (yes vs. no) ╪ | HR = 5.78 (2.47–13.5); p < 0.001 | ||
| Peric, 2011 [85] | EBV | A | Use of ATG (yes vs. no) | SHR = 4.9 (1.1–21.0); p = 0.03 | ||
| Fan, 2016 [53] | EBV | P and A | Use of ATG (yes vs. no) | OR = 7.69 (1.17–50.49); p = 0.034 | ||
| Laberko, 2017 [69] | EBV | P and A | Horse ATG vs. no serotherapy | HR = 2.47 (0.95–6.38); p = 0.063 | ||
| Rabbit ATG vs. no serotherapy | HR = 1.22 (0.467–3.18); p = 0.69 | |||||
| Christopeit, 2013 [45] | EBV | A | Use of ATG (yes vs. no) | OR = 0.83 (0.17–4.01); p = 0.820 | ||
| Liu, 2013 [74] | EBV | P and A | Use of ATG (yes vs. no) | HR = 14.08 (6.02–32.92); p < 0.001 | ||
| Ru, 2020 [86] | EBV | P and A | Use of ATG (yes vs. no) | HR = 4.288(2.638–6.97); p < 0.001 | ||
| Liu, 2013 [27] | PTLD | P and A | Use of ATG (yes vs. no) | p = 0.038 | ||
| Van der Velden, 2013 [95] | PTLD | A | Use of ATG (yes vs. no) | OR = 2.4 (1.3–4.2) p = 0.001 | ||
| Landgren, 2009 [70] | PTLD | P and A | Use of ATG (yes vs. no) ╪ | RR = 3.8 (2.5–5.8) | ||
| Xuan, 2013 [16] | PTLD | P and A | Use of ATG (yes vs. no) | HR = 13.03 (1.67–101.58) p = 0.014 | ||
| Fujimoto, 2019 [55] | PTLD | P and A | Use of ATG in conditioning regimen (yes vs. no) | HR = 6.13 (4.33–8.68); p < 0.001 | ||
| Fujimoto, 2019 [55] | PTLD | P and A | Use of ATG for GvHD treatment (yes vs. no) ╪ | HR = 2.09 (1.17–3.72); p = 0.01 | ||
| Gao, 2019 [56] | PTLD | P and A | Use of ATG (yes vs. no) | HR = 2.9 (0.3–27.5); p = 0.350 | ||
| Lin, 2019 [72] | EBV | P and A | ATG dose (10.0 mg/kg vs. 7.5 mg/kg) | HR = 2.02 (1.37–2.97); p < 0.001 | ||
| Buyck, 2009 [40] | PTLD | P and A | Number of prior courses of ATG | HR = 7.23 (1.67–31.32); p = 0.008; | ||
| Fan, 2016 [53] | EBV | P and A | MMF + CsA + prednisone vs. MMF + CsA | OR = 23.68 (1.924–291.449); p = 0.013 | ||
| Christopeit, 2013 [45] | EBV | A | CsA AUC (≥ vs. <6000 ng/mL x days) | OR = 6.067 (1.107–33.238); p = 0.038 | ||
| T-cell depletion | ||||||
| Bordon, 2012 [37] | EBV | P | In vivo TCD (yes vs. no) | p = 0.04 | ||
| Torre-Cisneros, 2004 [91] | EBV | P and A | Use of CD4+ lymphocyte-depleted graft (yes vs. no) | HR = 11.5 (5.8–22.8); p < 0.0001 | ||
| Van Esser, 2001 [96] | EBV | P and A | TCD without ATG vs. non-TCD | HR = 1.5 (0.8–2.9); p = 0.3 | ||
| TCD with ATG vs. non-TCD | HR = 3.4 (1.6–7.1); p = 0.001 | |||||
| Landgren, 2009 [70] | PTLD | P and A | Broad lymphocyte depletion vs. no TCD | RR = 3.1 (1.2–6.7) | ||
| Selective TCD vs. no TCD | RR = 9.4 (6.0–14.7) | |||||
| Method of T-cell depletion | ||||||
| Landgren, 2009 [70] | PTLD | P and A | Alemtuzumab MoAb vs. no TCD | RR = 3.1 (0.7–8.4) | ||
| Elutriation/density gradient centrifugation vs. no TCD | RR = 3.2 (0.8–8.8) | |||||
| Anti-T or anti-T + NK MoAb vs. no TCD | RR = 8.4 (5.1–13) | |||||
| SRBC rosetting vs. no TCD | RR = 14.6 (5.9–31) | |||||
| Lectins with/without SRBC or anti-T MoAb vs. no TCD | RR = 15.8 (7.2–32) | |||||
| Unclassified/unknown method vs. no TCD | RR = 6.0 (0.96–20) | |||||
| Graft-versus-host disease | ||||||
| Cohen, 2005 [46] | EBV | P | aGvHD (yes vs. no) | OR = 2.20 (2.12–15.08) | ||
| Elmahdi, 2016 [52] | EBV | P | aGvHD (yes vs. no) | HR = 3.29 (1.26–8.58); p = 0.015 | ||
| Hiwarkar, 2013 [59] | EBV | P | aGvHD ≥ grade II | Significant, but NR | ||
| Kullberg-Lindh, 2011 [68] | EBV | P | cGvHD (yes vs. no) | Slope = −1.12; p = 0.023 | ||
| Juvonen, 2007 [66] | EBV | A | aGvHD ≥ grade III╪ | HR = 1.70 (1.11–2.62); p = 0.015 | ||
| Sirvent-von Bueltzingsloewen, 2002 [89] | EBV | P and A | aGvHD ≥ grade II | OR = 3.4 (1.2–9.7) | ||
| Omar, 2009 [81] | EBV | P and A | aGvHD (yes vs. no) | p = 0.009 | ||
| Gao, 2019 [56] | EBV | P and A | aGvHD (yes vs. no) | HR = 1.0 (0.7–1.6); p = 0.960 | ||
| Gao, 2019 [56] | PTLD | P and A | aGvHD (yes vs. no) | HR = 1.4 (0.5–3.8); p = 0.480 | ||
| Laberko, 2017 [69] | EBV | P and A | GvHD (yes vs. no) | HR = 1.97 (1.04–3.72); p = 0.037 | ||
| Landgren, 2009 [70] | PTLD | P and A | aGvHD ≥ grade II ╪ | RR = 1.7 (1.2–2.5) | ||
| Ru, 2020 [86] | EBV | P and A | aGvHD (grade II-IV vs. none or grade I) | HR = 1.26 (0.89–1.78); p = 0.193 | ||
| Fujimoto, 2019 [55] | PTLD | P and A | aGvHD grade II-IV (yes vs. no) ╪ | HR = 1.93 (1.48–2.52); p < 0.001 | ||
| Uhlin, 2014 [94] | PTLD | P and A | aGvHD ≥ grade II | SHR = 2.65 (1.32–5.35) p = 0.006 | ||
| Landgren, 2009 [70] | PTLD | P and A | cGvHD moderate/severe or clinical extensive ╪ | RR = 2.0 (1.1–3.2) | ||
| Ru, 2020 [86] | EBV | P and A | cGvHD (yes vs. no) | HR = 1.413 (1.013–1.971); p = 0.042 | ||
| Kalra, 2018 [67] | PTLD | P and A | aGvHD grade II-IV or chronic NST (yes vs. no) | SHR = 0.47, p = 0.04 | ||
| Immunological reconstitution | ||||||
| Patriarca, 2013 [4] | EBV | A | Peripheral blood CD4+ lymphocyte/µL at +1 month after HSCT (≥50 vs. <50) | OR = 0.1 (0.02–0.48); p = 0.004 | ||
| Yu, 2019 [100] | EBV | P and A | NKp30 in 1-month post-transplant (1 M) (% of total NK cells) | HR = 0.957 (0.918–0.998); p = 0.04 | ||
| Liu, 2020 [73] | EBV | A | Vδ2+ cell recovery at day 30 post-transplantation | HR = 0.347 (0.161–0.747); p = 0.007 | ||
| Liu, 2020 [73] | EBV | A | CD8+ cell recovery at day 30 post-transplantation | HR = 0.499 (0.207–1.201); p = 0.121 | ||
| Xu, 2015 [98] | PTLD | P and A | CD8+ cell count at day 30 after HSCT (≥median vs. < median) | HR = 0.34 (0.13–0.92) p = 0.033 | ||
| PTLD | P and A | IgM count at day 30 after HSCT (≥median vs. <median) | HR = 0.27 (0.10–0.75) p = 0.012 | |||
| CMV reactivation | ||||||
| Gao, 2019 [56] | EBV | P and A | CMV DNAemia (yes vs. no) | HR = 5.9 (2.5–13.9); p < 0.001 | ||
| Torre-Cisneros, 2004 [91] | EBV | P and A | CMV load > 2500 copies/mL | HR = 2.1 (0.9–7); p = 0.061 | ||
| Zallio, 2013 [24] | EBV | A | yes vs. no | Significant, but NR | ||
| Zhou, 2020 [101] | EBV | P and A | CMV DNAemia (yes vs. no) | HR = 97.754 (9.477–1008.304) | ||
| Gao, 2019 [56] | PTLD | P and A | CMV DNAemia (yes vs. no) | HR = 11.6 (1.2–114.4); p = 0.036 | ||
| Xu, 2015 [98] | PTLD | P and A | CMV DNAemia (yes vs. no) | HR = 5.68 (1.17–27.57) p = 0.031 | ||
| Transfusion | ||||||
| Trottier, 2012 [92] | EBV | P | RBC transfusion volume (mL) | <850 vs. 0 | HR = 1.99 (0.47–8.44) | p-value trend = 0.047 |
| 850–1890 vs. 0 | HR = 2.40 (0.56–10.24) | |||||
| >1890 vs. 0 | HR = 2.86 (0.68–12.11) | |||||
| P | FFP transfusion volume (mL) | ≤200 vs. 0 | HR = 0.70 (0.22–2.25) | p-value trend = 0.079 | ||
| >200 vs. 0 | HR = 3.16 (1.00–11.17) | |||||
| P | PLT transfusion volume (mL) | 1260–2530 vs. <1260 | HR = 1.65 (0.86–3.18) | p-value trend = 0.012 | ||
| >2530 vs. <1260 | HR = 2.19 (1.21–3.97) | |||||
| Other factors | ||||||
| Garcia-Cadenas, 2015 [57] | EBV | A | Prior SCT (yes vs. no) | HR: 2.6 (1.1–6.4); p = 0.04 | ||
| PTLD | A | Prior SCT (yes vs. no) | HR: 6.4 (1.3–31.9); p = 0.02 | |||
| Fujimoto, 2019 [55] | PTLD | P and A | Number of allogeneic HSCT (two or more vs. one) | HR = 1.50 (1.05–2.15); p = 0.03 | ||
| Landgren, 2009 [70] | PTLD | P and A | Second transplant (yes vs. no) ╪ | RR = 3.5 (1.7–6.3) | ||
| Uhlin, 2014 [94] | PTLD | P and A | Splenectomy (yes vs. no) | SHR = 4.81 (1.51–15.4) p = 0.008 | ||
| PTLD | P and A | MSC treatment (yes vs. no) | SHR = 3.05 (1.25–7.48) p = 0.015 | |||
| Landgren, 2009 [70] | PTLD | P and A | 2+ HLA MMRD or URD, no ATG, no selective TCD vs. matched sibling or 1 HLA-Ag mismatched relative | RR = 0.9 (0.3–2.2) | ||
| 2+ HLA MMRD or URD, ATG and/or selective TCD vs. matched sibling or 1 HLA-Ag mismatched relative | RR = 3.8 (2.4–6.1) | |||||
| Van Esser, 2001 [96] | PTLD | P and A | A stepwise increase of EBV-DNA by 1 log | HR = 2.9 (1.7–4.8); p < 0.001 | ||
| Pagliuca, 2019 [82] | PTLD | P and A | Fever at onset of EBV infection (yes vs. no) | SHR = 6.12 (1.74–21.58); p = 0.005 | ||
| Fujimoto, 2019 [55] | PTLD | P and A | Year of HSCT (2010–2015 vs. 1990–2009) | HR = 1.87 (1.38–2.52); p < 0.001 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enok Bonong, P.R.; Zahreddine, M.; Buteau, C.; Duval, M.; Laporte, L.; Lacroix, J.; Alfieri, C.; Trottier, H. Factors Associated with Post-Transplant Active Epstein-Barr Virus Infection and Lymphoproliferative Disease in Hematopoietic Stem Cell Transplant Recipients: A Systematic Review and Meta-Analysis. Vaccines 2021, 9, 288. https://doi.org/10.3390/vaccines9030288
Enok Bonong PR, Zahreddine M, Buteau C, Duval M, Laporte L, Lacroix J, Alfieri C, Trottier H. Factors Associated with Post-Transplant Active Epstein-Barr Virus Infection and Lymphoproliferative Disease in Hematopoietic Stem Cell Transplant Recipients: A Systematic Review and Meta-Analysis. Vaccines. 2021; 9(3):288. https://doi.org/10.3390/vaccines9030288
Chicago/Turabian StyleEnok Bonong, Pascal Roland, Monica Zahreddine, Chantal Buteau, Michel Duval, Louise Laporte, Jacques Lacroix, Caroline Alfieri, and Helen Trottier. 2021. "Factors Associated with Post-Transplant Active Epstein-Barr Virus Infection and Lymphoproliferative Disease in Hematopoietic Stem Cell Transplant Recipients: A Systematic Review and Meta-Analysis" Vaccines 9, no. 3: 288. https://doi.org/10.3390/vaccines9030288
APA StyleEnok Bonong, P. R., Zahreddine, M., Buteau, C., Duval, M., Laporte, L., Lacroix, J., Alfieri, C., & Trottier, H. (2021). Factors Associated with Post-Transplant Active Epstein-Barr Virus Infection and Lymphoproliferative Disease in Hematopoietic Stem Cell Transplant Recipients: A Systematic Review and Meta-Analysis. Vaccines, 9(3), 288. https://doi.org/10.3390/vaccines9030288






