The Bacterial and Viral Agents of BRDC: Immune Evasion and Vaccine Developments
Abstract
1. Introduction
2. BRDC Pathogenesis
3. Cellular Response
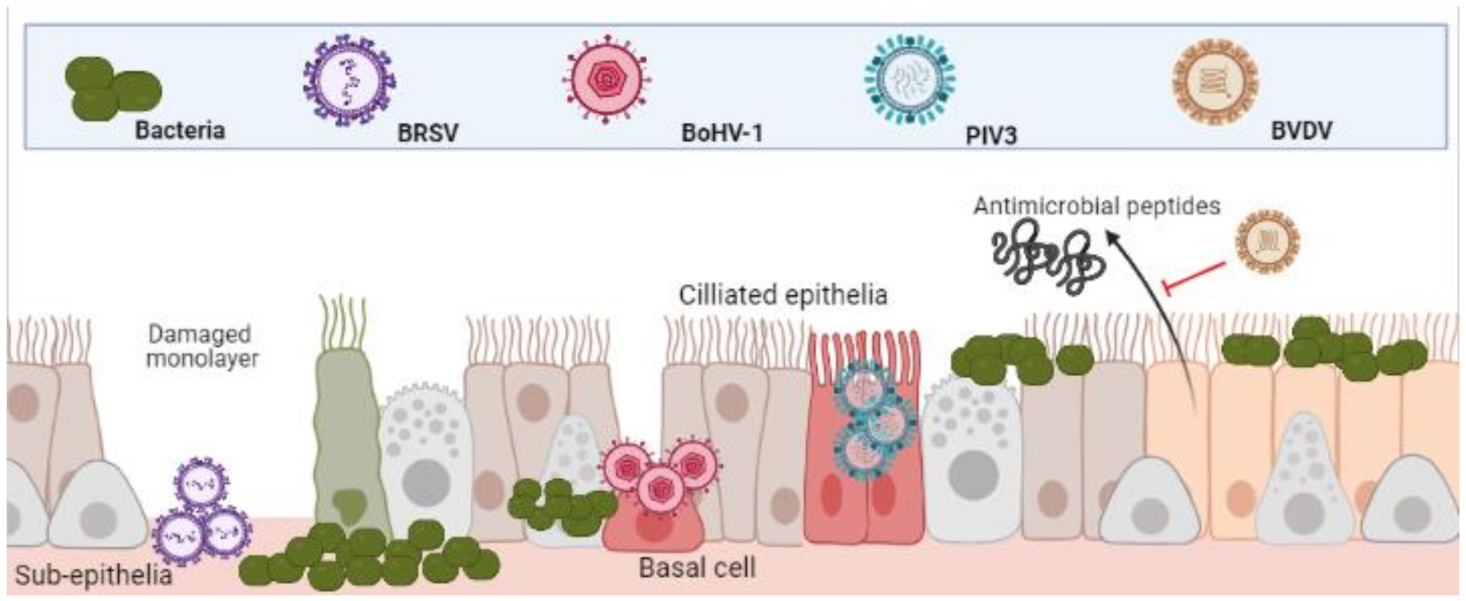
3.1. Epithelial Cells
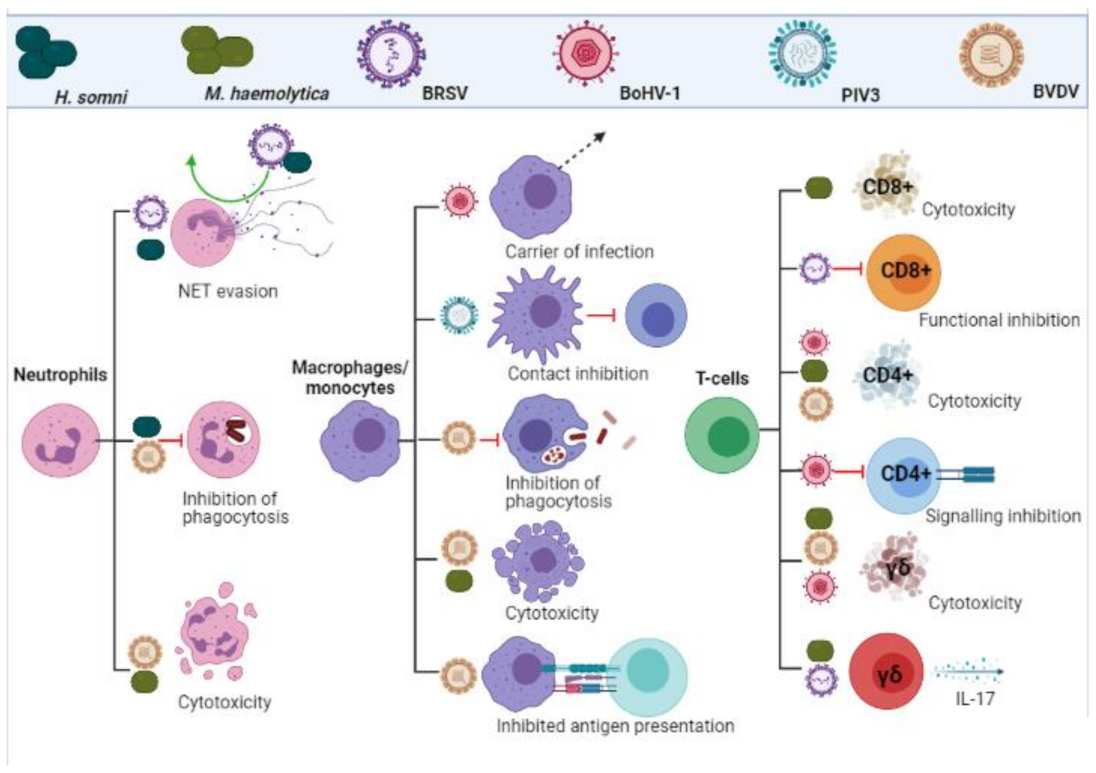
3.2. Neutrophils, Monocytes and Macrophages
3.3. T-Cells
4. Cell Signalling
4.1. MHC Signalling
4.2. Cytokines and Chemokines
4.3. Inflammasomes
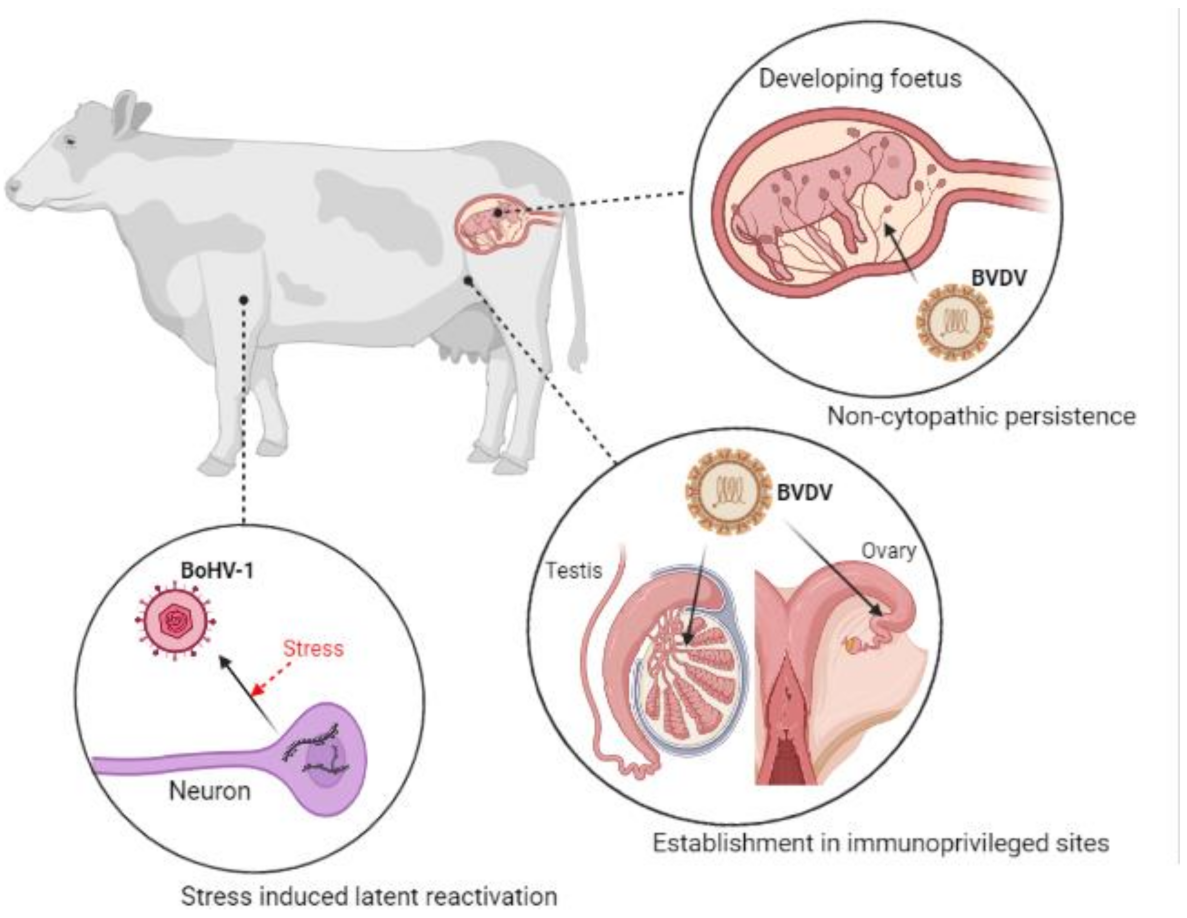
5. Viral Latency and Immune Tolerance
6. Genetic Predisposition
7. Current Vaccines
8. Vaccination Challenges
8.1. Lack of Evidence for Efficacy in the Field
8.2. Importance of BRDC-Causing Pathogens Included in Vaccines
8.3. Timing of Vaccination and Maternal Antibodies
8.4. Timing of Vaccination and Immunological Stress
8.5. Additional Issues
9. Conclusions and Necessary Developments
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Urban-Chmiel, R.; Grooms, D.L. Prevention and Control of Bovine Respiratory Disease. J. Livest Sci. 2012, 3, 27–36. [Google Scholar]
- Ellis, J.A. The immunology of the bovine respiratory disease complex. Vet. Clin. N. Am. Food Anim. Pract. 2001, 17, 535–550. [Google Scholar] [CrossRef]
- Veterinary Laboratory Service; Agri-Food & Biosciences Institute. All-Island Animal Disease Surveillance 2019; Department of Agriculture, Food and the Marine: Dublin, Ireland, 2020. [Google Scholar]
- Dubrovsky, S.A.; van Eenennaam, A.L.; Aly, S.S.; Karle, B.M.; Rossitto, P.V.; Overton, M.W.; Lehenbauer, T.W.; Fadel, J.G. Preweaning cost of bovine respiratory disease (BRD) and cost-benefit of implementation of preventative measures in calves on California dairies: The BRD 10K study. J. Dairy Sci. 2020, 103, 1583–1597. [Google Scholar] [CrossRef] [PubMed]
- Edwards, T.A. Control methods for bovine respiratory disease for feedlot cattle. Vol. 26, Veterinary Clinics of North America—Food Animal Practice. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Loneragan, G.H.; Dargatz, D.A.; Morley, P.S.; Smith, M.A. Trends in mortality ratios among cattle in US feedlots. J. Am. Vet. Med. Assoc. 2001, 219, 1122–1127. [Google Scholar] [CrossRef]
- Taylor, J.D.; Fulton, R.W.; Lehenbauer, T.W.; Step, D.L.; Confer, A.W. The epidemiology of bovine respiratory disease: What is the evidence for predisposing factors? Can. Vet. J. 2010, 51, 1095–1102. [Google Scholar]
- Ackermann, M.R.; Derscheid, R.; Roth, J.A. Innate immunology of bovine respiratory disease. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 215–228. [Google Scholar] [CrossRef]
- McGill, J.L.; Sacco, R.E. The Immunology of Bovine Respiratory Disease: Recent Advancements. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 333. [Google Scholar] [CrossRef]
- Grissett, G.P.; White, B.J.; Larson, R.L. Structured Literature Review of Responses of Cattle to Viral and Bacterial Pathogens Causing Bovine Respiratory Disease Complex. J. Vet. Int. Med. 2015, 29, 770–780. [Google Scholar] [CrossRef]
- Darbyshire, J.H.; Jennings, A.R.; Omar, A.R.; Dawson, P.S.; Lamont, P.H. Association of Adenoviruses with Bovine Respiratory Disease. Nature 1965, 208, 307–308. [Google Scholar] [CrossRef]
- Hick, P.M.; Read, A.J.; Lugton, I.; Busfield, F.; Dawood, K.E.; Gabor, L.; Hornitzky, M.; Kirkland, P.D. Coronavirus infection in intensively managed cattle with respiratory disease. Aust. Vet. J. 2012, 90, 381–386. [Google Scholar] [CrossRef]
- Ng, T.F.F.; Kondov, N.O.; Deng, X.; Van Eenennaam, A.; Neibergs, H.L.; Delwart, E. A Metagenomics and Case-Control Study to Identify Viruses Associated with Bovine Respiratory Disease. J. Virol. 2015, 89, 5340–5349. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.; Chengappa, M.M.; Kuszak, J.; McVey, D.S. Bacterial pathogens of the bovine respiratory disease complex. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 381–394. [Google Scholar] [CrossRef] [PubMed]
- McMullen, C.; Alexander, T.W.; Léguillette, R.; Workentine, M.; Timsit, E. Topography of the respiratory tract bacterial microbiota in cattle. Microbiome 2020, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Guzman, E.; Taylor, G. Immunology of bovine respiratory syncytial virus in calves. Mol. Immunol. 2015, 66, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Viuff, B.; Uttenthal, A.; Tegtmeier, C.; Alexandersen, S. Sites of Replication of Bovine Respiratory Syncytial Virus in Naturally Infected Calves as Determined by In Situ Hybridization. Vet. Pathol. 1996, 33, 383–390. [Google Scholar] [CrossRef]
- Goris, K.; Uhlenbruck, S.; Schwegmann-Wessels, C.; Köhl, W.; Niedorf, F.; Stern, M.; Hewicker-Trautwein, M.; Bals, R.; Taylor, G.; Braun, A.; et al. Differential Sensitivity of Differentiated Epithelial Cells to Respiratory Viruses Reveals Different Viral Strategies of Host Infection. J. Virol. 2009, 83, 1962–1968. [Google Scholar] [CrossRef]
- Zhang, L.; Bukreyev, A.; Thompson, C.I.; Watson, B.; Peeples, M.E.; Collins, P.L.; Pickles, R.J. Infection of Ciliated Cells by Human Parainfluenza Virus Type 3 in an In Vitro Model of Human Airway Epithelium. J. Virol. 2005, 79, 1113–1124. [Google Scholar] [CrossRef]
- Muylkens, B.; Thiry, J.; Kirten, P.; Schynts, F.; Thiry, E. Bovine herpesvirus 1 infection and infectious bovine rhinotracheitis. Vet. Res. 2007, 38, 181–209. [Google Scholar] [CrossRef] [PubMed]
- Nyaga, P.N.; McKercher, D.G. Pathogenesis of Bovine herpesvirus-1 (BHV-1) infections: Interactions of the virus with peripheral bovine blood cellular components. Comp. Immunol. Microbiol. Infect Dis. 1979, 2, 587–602. [Google Scholar] [CrossRef]
- Kirchhoff, J.; Uhlenbruck, S.; Goris, K.; Keil, G.M.; Herrler, G. Three viruses of the bovine respiratory disease complex apply different strategies to initiate infection. Vet. Res. 2014, 45, 1–12. [Google Scholar] [CrossRef]
- Kirchhoff, J.; Uhlenbruck, S.; Keil, G.M.; Schwegmann-Wessels, C.; Ganter, M.; Herrler, G. Infection of differentiated airway epithelial cells from caprine lungs by viruses of the bovine respiratory disease complex. Vet. Microbiol. 2014, 170, 58–64. [Google Scholar] [CrossRef]
- Sudaryatma, P.E.; Mekata, H.; Kubo, M.; Subangkit, M.; Goto, Y.; Okabayashi, T. Co-infection of epithelial cells established from the upper and lower bovine respiratory tract with bovine respiratory syncytial virus and bacteria. Vet. Microbiol. 2019, 235, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Rivas, J.J.; Kisiela, D.; Czuprynski, C.J. Bovine herpesvirus type 1 infection of bovine bronchial epithelial cells increases neutrophil adhesion and activation. Vet. Immunol. Immunopathol. 2009, 131, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Al-Haddawi, M.; Mitchell, G.B.; Clark, M.E.; Wood, R.D.; Caswell, J.L. Impairment of innate immune responses of airway epithelium by infection with bovine viral diarrhea virus. Vet. Immunol. Immunopathol. 2007, 116, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.B.; Al-Haddawi, M.H.; Clark, M.E.; Beveridge, J.D.; Caswell, J.L. Effect of corticosteroids and neuropeptides on the expression of defensins in bovine tracheal epithelial cells. Infect. Immun. 2007, 75, 1325–1334. [Google Scholar] [CrossRef]
- Babiuk, L.A.; Van Drunen Littel-Van Den Hurk, S.; Tikoo, S.K. Immunology of bovine herpesvirus 1 infection. In Veterinary Microbiology; Elsevier: New York, NY, USA, 1996; pp. 31–42. [Google Scholar]
- Olafson, P.; MacCallum, A.D.; Fox, F.H. An apparently new transmissible disease of cattle. Cornell Vet. 1946, 36, 205–213. [Google Scholar]
- Childs, T. X Disease of Cattle—Saskatchewan. Can. J. Comp. Med. Vet. Sci. 1946, 10, 316–319. [Google Scholar]
- Walz, P.H.; Grooms, D.L.; Passler, T.; Ridpath, J.F.; Tremblay, R.; Step, D.L.; Callan, R.J.; Givens, M.D. Control of bovine viral diarrhea virus in ruminants. J. Vet. Intern. Med. 2010, 24, 476–486. [Google Scholar] [CrossRef]
- Apley, M.D. Treatment of Calves with Bovine Respiratory Disease: Duration of Therapy and Posttreatment Intervals. Vet. Clin. N. Am. Food Anim. Pract. 2015, 31, 441–453. [Google Scholar] [CrossRef]
- Corbeil, L.B. Histophilus somni host–parasite relationships. Anim. Health Res. Rev. 1996, 10, 151–160. [Google Scholar]
- Orr, J.P. Haemophilus somnus infection: A retrospective analysis of cattle necropsied at the Western College of Veterinary Medicine from 1970 to 1990. Can. Vet. J. 1992, 33, 719–722. [Google Scholar]
- Zeilhofer, H.U.; Schorr, W. Role of interleukin-8 in neutrophil signaling. Curr. Opin. Hematol. 2000, 7, 178–182. [Google Scholar] [CrossRef]
- Brown, G.B.; Bolin, S.R.; Frank, D.E.; Roth, J.A. Defective function of leukocytes from cattle persistently infected with bovine viral diarrhea virus, and the influence or recombinant cytokines. Am. J. Vet. Res. 1991, 52, 381–387. [Google Scholar] [PubMed]
- Cortjens, B.; de Boer, O.J.; de Jong, R.; Antonis, A.F.G.; Sabogal Piñeros, Y.S.; Lutter, R.; van Woensel, J.B.M.; Bem, R.A. Neutrophil extracellular traps cause airway obstruction during respiratory syncytial virus disease. J. Pathol. 2016, 238, 401–411. [Google Scholar] [CrossRef]
- Singh, K.; Ritchey, J.W.; Confer, A.W. Mannheimia haemolytica: Bacterial-host interactions in bovine Pneumonia. Vet. Pathol. 2011, 48, 338–348. [Google Scholar] [CrossRef]
- Caswell, J.L.; Middleton, D.M.; Gordon, J.R. The importance of interleukin-8 as a neutrophil chemoattractant in the lungs of cattle with pneumonic pasteurellosis. Can. J. Vet. Res. 2001, 65, 229–232. [Google Scholar] [PubMed]
- Radi, Z.A.; Caverly, J.M.; Dixon, R.A.; Brogden, K.A.; Ackermann, M.R. Effects of the synthetic selectin inhibitor TBC1269 on tissue damage during acute Mannheimia haemolytica-induced pneumonia in neonatal calves. Am. J. Vet. Res. 2001, 62, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Puttur, F.; Gregory, L.G.; Lloyd, C.M. Airway macrophages as the guardians of tissue repair in the lung. Immunol. Cell Biol. 2019, 97, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Adler, B.; Adler, H.; Pfister, H.; Jungi, T.W.; Peterhans, E. Macrophages infected with cytopathic bovine viral diarrhea virus release a factor(s) capable of priming uninfected macrophages for activation-induced apoptosis. J. Virol. 1997, 71, 3255–3258. [Google Scholar] [CrossRef]
- Glew, E.J.; Carr, B.V.; Brackenbury, L.S.; Hope, J.C.; Charleston, B.; Howard, C.J. Differential effects of bovine viral diarrhoea virus on monocytes and dendritic cells. J. Gen. Virol. 2003, 84, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
- Schaut, R.G.; Ridpath, J.F.; Sacco, R.E. Bovine viral diarrhea virus type 2 impairs macrophage responsiveness to toll-like receptor ligation with the exception of toll-like receptor 7. PLoS ONE 2016, 11, e0159491. [Google Scholar] [CrossRef] [PubMed]
- Hesse, R.A.; Toth, T.E. Effects of bovine parainfluenza-3 virus on phagocytosis and phagosome-lysosome fusion of cultured bovine alveolar macrophages. Am. J. Vet. Res. 1983, 44, 1901–1907. [Google Scholar] [PubMed]
- Adair, B.M.; McNulty, M.S. Effect of “in vitro” exposure of bovine alveolar macrophages to different strains of bovine respiratory syncytial virus. Vet. Immunol. Immunopathol. 1992, 30, 193–206. [Google Scholar] [CrossRef]
- Moreno-López, J. Cell-Mediated Immunity to Parainfluenza-3 (PIV-3) in Cattle Evaluation of in vivo and in vitro tests. Zentralblatt Vet. R. B 1977, 24, 231–240. [Google Scholar] [CrossRef]
- Johnson, K.; Morein, B. In vitro stimulation of bovine circulating lymphocytes by parainfluenza type 3 virus. Res. Vet. Sci. 1977, 22, 83–85. [Google Scholar] [CrossRef]
- Basaraba, R.J.; Brown, P.R.; Laegreid, W.W.; Silflow, R.M.; Evermann, J.F.; Leid, R.W. Suppression of lymphocyte proliferation by parainfluenza virus type 3-infected bovine alveolar macrophages. Immunology 1993, 79, 179–188. [Google Scholar]
- Roth, J.A.; Kaeberle, M.L. Suppression of neutrophil and lymphocyte function induced by a vaccinal strain of bovine viral diarrhea virus with and without the administration of ACTH. Am. J. Vet. Res. 1983, 44, 2366–2372. [Google Scholar]
- Walz, P.H.; Bell, T.G.; Wells, J.L.; Grooms, D.L.; Kaiser, L.; Maes, R.K.; Baker, J.C. Relationship between degree of viremia and disease manifestations in calves with experimentally induced bovine viral diarrhea virus infection. Am. J. Vet. Res. 2001, 62, 1095–1103. [Google Scholar] [CrossRef]
- Kelling, C.L.; Steffen, D.J.; Topliff, C.L.; Eskridge, K.M.; Donis, R.O.; Higuchi, D.S. Comparative virulence of isolates of bovine viral diarrhea virus type II in experimentally inoculated six- to nine-month-old calves. Am. J. Vet. Res. 2002, 63, 1379–1384. [Google Scholar] [CrossRef]
- Carlos-Valdez, L.; Wilson, B.K.; Burciaga-Robles, L.O.; Step, D.L.; Holland, B.P.; Richards, C.J.; Montelongo, M.A.; Confer, A.W.; Fulton, R.W.; Krehbiel, C.R. Effect of timing of Mannheimia haemolytica challenge following short-term natural exposure to bovine viral diarrhea virus type 1b on animal performance and immune response in beef steers. J. Anim. Sci. 2016, 94, 4799–4808. [Google Scholar] [CrossRef]
- Burciaga-Robles, L.O.; Step, D.L.; Krehbiel, C.R.; Holland, B.P.; Richards, C.J.; Montelongo, M.A.; Confer, A.W.; Fulton, R.W. Effects of exposure to calves persistently infected with bovine viral diarrhea virus type 1b and subsequent infection with mannheima haemolytica on clinical signs and immune variables: Model for bovinerespiratory disease via viral and bacterial interaction. J. Anim. Sci. 2010, 88, 2166–2178. [Google Scholar] [CrossRef]
- Griebel, P.J.; Qualtiere, L.; Davis, W.C.; Gee, A.; Ohmann, H.B.; Lawman, M.J.; Babiuk, L.A. T lymphocyte population dynamics and function following a primary bovine herpesvirus type-1 infection. Viral Immunol. 1987, 1, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Czuprynski, C.J.; Leite, F.; Sylte, M.; Kuckleburg, C.; Schultz, R.; Inzana, T.; Behling-Kelly, E.; Corbeil, L. Complexities of the pathogenesis of Mannheimia haemolytica and Haemophilus somnus infections: Challenges and potential opportunities for prevention? Anim. Heal. Res. Rev. 2004, 5, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Inzana, T.J.; Balyan, R.; Howard, M.D. Decoration of Histophilus somni lipooligosaccharide with N-acetyl-5-neuraminic acid enhances bacterial binding of complement factor H and resistance to killing by serum and polymorphonuclear leukocytes. Vet. Microbiol. 2012, 161, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.F.; Sylte, M.J.; Czuprynski, C.J. Apoptosis: A possible tactic of Haemophilus somnus for evasion of killing by bovine neutrophils? Microb. Pathog. 1998, 24, 351–359. [Google Scholar] [CrossRef]
- Howard, M.D.; Boone, J.H.; Buechner-Maxwell, V.; Schurig, G.G.; Inzana, T.J. Inhibition of bovine macrophage and polymorphonuclear leukocyte superoxide anion production by Haemophilus somnus. Microb. Pathog. 2004, 37, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Hellenbrand, K.M.; Forsythe, K.M.; Rivera-Rivas, J.J.; Czuprynski, C.J.; Aulik, N.A. Histophilus somni causes extracellular trap formation by bovine neutrophils and macrophages. Microb. Pathog. 2013, 54, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Czuprynski, C.J.; Hamilton, H.L. Bovine neutrophils ingest but do not kill Haemophilus somnus in vitro. Infect. Immun. 1985, 50, 431–436. [Google Scholar] [CrossRef]
- Pennock, N.D.; White, J.T.; Cross, E.W.; Cheney, E.E.; Tamburini, B.A.; Kedl, R.M. T cell responses: Naïve to memory and everything in between. Am. J. Physiol. Adv. Physiol. Educ. 2013, 37, 273–283. [Google Scholar] [CrossRef]
- Gaddum, R.M.; Cook, R.S.; Furze, J.M.; Ellis, S.A.; Taylor, G. Recognition of bovine respiratory syncytial virus proteins by bovine CD8+ T lymphocytes. Immunology 2003, 108, 220–229. [Google Scholar] [CrossRef]
- Koppers-Lalic, D.; Rychlowski, M.; van Leeuwen, D.; Rijsewijk, F.A.M.; Ressing, M.E.; Neefjes, J.J.; Bienkowska-Szewczyk, K.; Wiertz, E.J.H.J. Bovine herpesvirus 1 interferes with TAP-dependent peptide transport and intracellular trafficking of MHC class I molecules in human cells. Arch. Virol. 2003, 148, 2023–2037. [Google Scholar] [CrossRef]
- Winkler, M.T.C.; Doster, A.; Jones, C. Bovine Herpesvirus 1 Can Infect CD4+ T Lymphocytes and Induce Programmed Cell Death during Acute Infection of Cattle. J. Virol. 1999, 73, 8657–8668. [Google Scholar] [CrossRef]
- Molina, V.; Risalde, M.A.; Sánchez-Cordón, P.J.; Pedrera, M.; Romero-Palomo, F.; Luzzago, C.; Gómez-Villamandos, J.C. Effect of infection with BHV-1 on peripheral blood leukocytes and lymphocyte subpopulations in calves with subclinical BVD. Res. Vet. Sci. 2013, 95, 115–122. [Google Scholar] [CrossRef]
- Guerra-Maupome, M.; Slate, J.R.; McGill, J.L. Gamma Delta T Cell Function in Ruminants. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 453–469. [Google Scholar] [CrossRef]
- McGill, J.L.; Sacco, R.E. γδ T cells and the immune response to respiratory syncytial virus infection. Vet. Immunol. Immunopathol. 2016, 181, 24–29. [Google Scholar] [CrossRef]
- Mcgill, J.L.; Nonnecke, B.J.; Lippolis, J.D.; Reinhardt, T.A.; Sacco, R.E. Differential chemokine and cytokine production by neonatal bovine γδ T-cell subsets in response to viral toll-like receptor agonists and in vivo respiratory syncytial virus infection. Immunology 2013, 139, 227–244. [Google Scholar] [CrossRef] [PubMed]
- Mcinnes, E.; Sopp, P.; Howard, C.J.; Taylor, G. Phenotypic analysis of local cellular responses in calves infected with bovine respiratory syncytial virus. Immunology 1999, 96, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Saravia, J.; You, D.; Shaw, A.J.; Cormier, S.A. Impaired gamma delta T cell-derived IL-17A and inflammasome activation during early respiratory syncytial virus infection in infants. Immunol. Cell Biol. 2015, 93, 126–135. [Google Scholar] [CrossRef]
- Bystrom, J.; Al-Adhoubi, N.; Al-Bogami, M.; Jawad, A.; Mageed, R. Th17 Lymphocytes in Respiratory Syncytial Virus Infection. Viruses 2013, 5, 777–791. [Google Scholar] [CrossRef] [PubMed]
- McGill, J.L.; Rusk, R.A.; Guerra-Maupome, M.; Briggs, R.E.; Sacco, R.E. Bovine Gamma Delta T Cells Contribute to Exacerbated IL-17 Production in Response to Co-Infection with Bovine RSV and Mannheimia haemolytica. PLoS ONE 2016, 11, e0151083. [Google Scholar] [CrossRef] [PubMed]
- Abele, R.; Tampé, R. The ABCs of immunology: Structure and function of TAP, the transporter associated with antigen processing. Physiology 2004. [Google Scholar] [CrossRef] [PubMed]
- Guermonprez, P.; Valladeau, J.; Zitvogel, L.; Théry, C.; Amigorena, S. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 2002, 20, 621–667. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, S.J.; Kumar, P. Cross-talk between antigen presenting cells and T cells impacts intestinal homeostasis, bacterial infections, and tumorigenesis. Front. Immunol. 2019. [Google Scholar] [CrossRef]
- Nataraj, C.; Eidmann, S.; Hariharan, M.J.; Sur, J.H.; Perry, G.A.; Srikumaran, S. Bovine herpesvirus 1 downregulates the expression of bovine MHC class I molecules. Viral Immunol. 1997, 10, 21–34. [Google Scholar] [CrossRef]
- Hinkley, S.; Hill, A.B.; Srikumaran, S. Bovine herpesvirus-1 infection affects the peptide transport activity in bovine cells. Virus Res. 1998, 53, 91–96. [Google Scholar] [CrossRef]
- Brodersen, B.W.; Kelling, C.L. Alteration of leukocyte populations in calves concurrently infected with bovine respiratory syncytial virus and bovine viral diarrhea virus. Viral Immunol. 1999, 12, 323–334. [Google Scholar] [CrossRef]
- Chang, J.; Braciale, T.J. Respiratory syncytial virus infection suppresses lung CD8+ T-cell effector activity and peripheral CD8+ T-cell memory in the respiratory tract. Nat. Med. 2002, 8, 54–60. [Google Scholar] [CrossRef]
- Chang, J.; Srikiatkhachorn, A.; Braciale, T.J. Visualization and Characterization of Respiratory Syncytial Virus F-Specific CD8 + T Cells During Experimental Virus Infection. J. Immunol. 2001, 167, 4254–4260. [Google Scholar] [CrossRef]
- Fach, S.J.; Olivier, A.; Gallup, J.M.; Waters, T.E.; Ackermann, M.R.; Lehmkuhl, H.D.; Sacco, R.E. Differential expression of cytokine transcripts in neonatal and adult ovine alveolar macrophages in response to respiratory syncytial virus or toll-like receptor ligation. Vet. Immunol. Immunopathol. 2010, 136, 55–64. [Google Scholar] [CrossRef]
- Singh, K.; Confer, A.W.; Hope, J.C.; Rizzi, T.; Wyckoff, J.H., III; Weng, H.; Ritchey, J.W. Cytotoxicity and cytokine production by bovine alveolar macrophages challenged with wild type and leukotoxin-deficient Mannheimia haemolytica. Vet. J. 2011, 188, 221–227. [Google Scholar] [CrossRef]
- Miller-Edge, M.; Splitter, G. Detection of impaired T cell-mediated immune responses to herpesvirus (BHV-1) in cattle. Vet. Immunol. Immunopathol. 1986, 13, 1–18. [Google Scholar] [CrossRef]
- Bryant, N.A.; Davis-Poynter, N.; Vanderplasschen, A.; Alcami, A. Glycoprotein G isoforms from some alphaherpesviruses function as broad-spectrum chemokine binding proteins. EMBO J. 2003, 22, 833–846. [Google Scholar] [CrossRef]
- Lan, H.C.; Chambers, M.A.; Ferguson, J.A.; Srivastava, K.K.; Reddy, P.G. Effect of bovine herpesvirus-1 on expression of interleukin-2 receptors and effect of interleukin-12 on lymphocyte proliferation. Vet. Microbiol. 1996, 49, 59–66. [Google Scholar] [CrossRef]
- Jones, C.; Chowdhury, S. A review of the biology of bovine herpesvirus type 1 (BHV-1), its role as a cofactor in the bovine respiratory disease complex and development of improved vaccines. Anim. Health Res. Rev. 2007, 8, 187–205. [Google Scholar] [CrossRef]
- Steimle, V.; Siegrist, C.A.; Mottet, A.; Lisowska-Grospierre, B.; Mach, B. Regulation of MHC class II expression by interferon-γ mediated by the transactivator gene CIITA. Science 1994, 265, 106–109. [Google Scholar] [CrossRef]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-γ: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef]
- Jones, C. Bovine herpesvirus 1 counteracts immune responses and immune-surveillance to enhance pathogenesis and virus transmission. Front. Immunol. 2019, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, T.; Takeuchi, K.; Gotoh, B. Bovine parainfluenza virus type 3 accessory proteins that suppress beta interferon production. Microbes Infect. 2007, 9, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Theofilopoulos, A.N.; Baccala, R.; Beutler, B.; Kono, D.H. Type I interferons (α/β) in immunity and autoimmunity. Ann. Rev. Immunol. 2005, 23, 307–336. [Google Scholar] [CrossRef] [PubMed]
- Jobe, F.; Simpson, J.; Hawes, P.; Guzman, E.; Bailey, D. Respiratory Syncytial Virus Sequesters NF-κB Subunit p65 to Cytoplasmic Inclusion Bodies to Inhibit Innate Immune Signaling. J. Virol. 2020, 94, e01380-20. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Burns, K.; Tschopp, J. The Inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P.Y. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef]
- Wang, J.; Alexander, J.; Wiebe, M.; Jones, C. Bovine herpesvirus 1 productive infection stimulates inflammasome formation and caspase 1 activity. Virus Res. 2014, 185, 72–76. [Google Scholar] [CrossRef]
- Segovia, J.; Sabbah, A.; Mgbemena, V.; Tsai, S.; Chang, T.; Berton, M.T.; Morris, I.R.; Allen, I.C.; Ting, J.P.; Bose, S. TLR2/MyD88/NF-κB pathway, reactive oxygen species, potassium efflux activates NLRP3/ASC inflammasome during respiratory syncytial virus infection. PLoS ONE 2012, 7, e29695. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.E.; Nonnecke, B.J.; Palmer, M.V.; Waters, W.R.; Lippolis, J.D.; Reinhardt, T.A. Differential expression of cytokines in response to respiratory syncytial virus infection of calves with high or low circulating 25-hydroxyvitamin D 3. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Taylor, G.; Wyld, S.; Valarcher, J.; Guzman, E.; Thom, M.; Widdison, S.; Buchholz, U.J. Recombinant bovine respiratory syncytial virus with deletion of the SH gene induces increased apoptosis and pro-inflammatory cytokines in vitro, and is attenuated and induces protective immunity in calves. J. Gen. Virol. 2014, 95, 1244–1254. [Google Scholar] [CrossRef]
- Nakanishi, K.; Yoshimoto, T.; Tsutsui, H.; Okamura, H. Interleukin-18 Regulates Both Th1 and Th2 Responses. Annu. Rev. Immunol. 2001, 19, 423–474. [Google Scholar] [CrossRef]
- Chen, M.; Wang, H.; Chen, W.; Meng, G. Regulation of adaptive immunity by the NLRP3 inflammasome. Int. Immunopharmacol. 2011, 11, 549–554. [Google Scholar] [CrossRef]
- Gershwin, L.J. Immunology of bovine respiratory syncytial virus infection of cattle. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 253–257. [Google Scholar] [CrossRef]
- Kalina, W.V.; Woolums, A.R.; Berghaus, R.D.; Gershwin, L.J. Formalin-inactivated bovine RSV vaccine enhances a Th2 mediated immune response in infected cattle. Vaccine 2004, 22, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, N.; Kurrer, M.; Bachmann, M.F.; Kopf, M. Interleukin-1 Is Responsible for Acute Lung Immunopathology but Increases Survival of Respiratory Influenza Virus Infection. J. Virol. 2005, 79, 6441–6448. [Google Scholar] [CrossRef] [PubMed]
- Ichinohe, T.; Lee, H.K.; Ogura, Y.; Flavell, R.; Iwasaki, A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J. Exp. Med. 2009, 206, 79–87. [Google Scholar] [CrossRef]
- Jones, C. Alphaherpesvirus latency: Its role in disease and survival of the virus in nature. Adv. Virus Res. 1998, 51, 81–133. [Google Scholar] [PubMed]
- Gilliam, S.E.; Thackray, A.M.; Brown, G.A.; Field, H.J. The pathogenesis of wild type and drug resistant mutant strains of bovine herpesvirus-1 (BHV-1) in the natural host. Arch. Virol. 1993, 128, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Levings, R.L.; Roth, J.A. Immunity to bovine herpesvirus 1: II. Adaptive immunity and vaccinology. In Animal Health Research Reviews; Cambridge University Press: Cambridge, UK, 2013; Volume 14, pp. 103–123. [Google Scholar]
- Levings, R.L.; Roth, J.A. Immunity to bovine herpesvirus 1: I. Viral lifecycle and innate immunity. In Animal Health Research Reviews; Cambridge University Press: Cambridge, UK, 2013; Volume 14, pp. 88–102. [Google Scholar]
- Barnes, P.J. Anti-inflammatory actions of glucocorticoids: Molecular mechanisms. Clin. Sci. 1998, 94, 557–572. [Google Scholar] [CrossRef]
- Funder, J.W. Glucocorticoid and mineralocorticoid receptors: Biology and clinical relevance. Annu. Rev. Med. 1997, 48, 231–240. [Google Scholar] [CrossRef]
- Schoneveld, O.J.L.M.; Gaemers, I.C.; Lamers, W.H. Mechanisms of glucocorticoid signalling. Biochim. Biophys. Acta Gene Struct. Expr. 2004, 1680, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Voges, H.; Horner, G.W.; Rowe, S.; Wellenberg, G.J. Persistent bovine pestivirus infection localized in the testes of an immuno-competent, non-viraemic bull. Vet. Microbiol. 1998, 61, 165–175. [Google Scholar] [CrossRef]
- Charleston, B.; Fray, M.D.; Baigent, S.; Carr, B.V.; Morrison, W.I. Establishment of persistent infection with non-cytopathic bovine viral diarrhoea virus in cattle is associated with a failure to induce type I interferon. J. Gen. Virol. 2001, 82, 1893–1897. [Google Scholar] [CrossRef]
- Babcock, A.H.; White, B.J.; Renter, D.G.; Dubnicka, S.R.; Morgan Scott, H. Predicting cumulative risk of bovine respiratory disease complex (BRDC) using feedlot arrival data and daily morbidity and mortality counts. Can. J. Vet. Res. 2013, 77, 33–44. [Google Scholar]
- Alvarez, A.E.; De Lima Marson, F.A.; Bertuzzo, C.S.; Arns, C.W.; Ribeiro, J.D. Epidemiological and genetic characteristics associated with the severity of acute viral bronchiolitis by respiratory syncytial virus. J. Pediatr. 2013, 89, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Larkin, E.K.; Hartert, T.V. Genes associated with RSV lower respiratory tract infection and asthma: The application of genetic epidemiological methods to understand causality. Future Virol. 2015, 10, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Johnston, D.; Earley, B.; McCabe, M.S.; Lemon, K.; Duffy, C.; McMenamy, M.; Cosby, S.L.; Kim, J.; Blackshields, G.; Taylor, J.F.; et al. Experimental challenge with bovine respiratory syncytial virus in dairy calves: Bronchial lymph node transcriptome response. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Johnston, D.; Kim, J.; Taylor, J.F.; Earley, B.; McCabe, M.S.; Lemon, K.; Duffy, C.; McMenamy, M.; Cosby, S.L.; Waters, S.M. ATAC-Seq identifies regions of open chromatin in the bronchial lymph nodes of dairy calves experimentally challenged with bovine respiratory syncytial virus. BMC Genom. 2021, 22, 14. [Google Scholar] [CrossRef] [PubMed]
- Lahti, M.; Löfgren, J.; Marttila, R.; Renko, M.; Klaavuniemi, T.; Haataja, R.; Rämet, M.; Hallman, M. Surfactant protein D gene polymorphism associated with severe respiratory syncytial virus infection. Pediatr. Res. 2002, 51, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Griese, M. Respiratory syncytial virus and pulmonary surfactant. Viral Immunol. 2002, 15, 357–363. [Google Scholar] [CrossRef]
- Sano, H.; Kuroki, Y. The lung collectins, SP-A and SP-D, modulate pulmonary innate immunity. Mol. Immunol. 2005, 42, 279–287. [Google Scholar] [CrossRef]
- Neibergs, H.L.; Seabury, C.M.; Wojtowicz, A.J.; Wang, Z.; Scraggs, E.; Kiser, J.N.; Neupane, M.; Womack, J.E.; van Eenennaam, A.; Hagevoort, G.R. Susceptibility loci revealed for bovine respiratory disease complex in pre-weaned holstein calves. BMC Genom. 2014, 15, 1164. [Google Scholar] [CrossRef]
- Theurer, M.E.; Larson, R.L.; White, B.J. Systematic review and meta-analysis of the effectiveness of commercially available vaccines against bovine herpesvirus, Bovine viral diarrhea virus, Bovine respiratory syncytial virus, And parainfluenza type 3 virus for mitigation of bovine respiratory di. J. Am. Vet. Med. Assoc. 2015, 246, 126–142. [Google Scholar] [CrossRef]
- Chamorro, M.F.; Palomares, R.A. Bovine Respiratory Disease Vaccination Against Viral Pathogens: Modified-Live Versus Inactivated Antigen Vaccines, Intranasal Versus Parenteral, What Is the Evidence? Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 461–472. [Google Scholar] [CrossRef]
- West, K.; Petrie, L.; Konoby, C.; Haines, D.M.; Cortese, V.; Ellis, J.A. The efficacy of modified-live bovine respiratory syncytial virus vaccines in experimentally infected calves. Vaccine 1999, 18, 907–919. [Google Scholar] [CrossRef]
- Endsley, J.J.; Ridpath, J.F.; Neill, J.D.; Sandbulte, M.R.; Roth, J.A. Induction of T Lymphocytes Specific for Bovine Viral Diarrhea Virus in Calves with Maternal Antibody. Viral Immunol. 2004, 17, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Stevens, E.T.; Brown, M.S.; Burdett, W.W.; Bolton, M.W.; Nordstrom, S.T.; Chase, C.C.L. Efficacy of a Non-adjuvanted, Modified-live Virus Vaccine in Calves with Maternal Antibodies against a Virulent Bovine Viral Diarrhea Virus Type 2a Challenge Seven Months following Vaccination. Bov. Pract. 2011, 45, 23–31. [Google Scholar]
- Step, D.L.; Krehbiel, C.R.; Burciaga-Robles, L.O.; Holland, B.P.; Fulton, R.W.; Confer, A.W.; Bechtol, D.T.; Brister, D.L.; Hutcheson, J.P.; Newcomb, H.L. Comparison of single vaccination versus revaccination with a modified-live virus vaccine containing bovine herpesvirus-1, bovine viral diarrhea virus (types la and 2a), parainfluenza type 3 virus, and bovine respiratory syncytial virus in the prevention o. J. Am. Vet. Med. Assoc. 2009, 235, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Ridpath, J.F.; Dominowski, P.; Mannan, R.; Yancey, R.; Jackson, J.A.; Taylor, L.; Mediratta, S.; Eversole, R.; Mackenzie, C.D.; Neill, J.D. Evaluation of three experimental bovine viral diarrhea virus killed vaccines adjuvanted with combinations of Quil A cholesterol and dimethyldioctadecylammonium (DDA) bromide. Vet. Res. Commun. 2010, 34, 691–702. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mori, K.; Kato, T.; Yokota, O.; Ohtsuka, H. Field trial of primary and booster dose of inactivated vaccine against bovine respiratory bacteria in young Holstein calves. J. Vet. Res. 2020, 64, 223–230. [Google Scholar] [CrossRef]
- Agriculture and Horticulture Development Board. Use of Vaccines in Dairy and Beef Cattle Production (2011–2017); Agriculture and Horticulture Development Board: Warwick, UK, 2018. [Google Scholar]
- Stilwell, G.; Matos, M.; Carolino, N.; Lima, M.S. Effect of a quadrivalent vaccine against respiratory virus on the incidence of respiratory disease in weaned beef calves. Prev. Vet. Med. 2008, 85, 151–157. [Google Scholar] [CrossRef]
- Makoschey, B.; Muñoz Bielsa, J.; Oliviero, L.; Roy, O.; Pillet, F.; Valla, G.; Cavirani, S. Field efficacy of combination vaccines against bovine respiratory pathogens in calves. Acta Vet. Hung. 2008, 56, 485–493. [Google Scholar] [CrossRef]
- Nagai, K.; Otomaru, K.; Ogawa, R.; Oishi, S.; Wataya, K.; Honkawa, Y.; Iwamoto, Y.; Ando, T.; Hyakutake, K.; Shirahama, H.; et al. Effect of combined vaccination for pasteurella multocida, mannheimia haemolytica, and Histophilus somni to prevent respiratory diseases in young Japanese black calves in the field. J. Vet. Med. Sci. 2019, 81, 1355–1358. [Google Scholar] [CrossRef]
- Mahan, S.M.; Sobecki, B.; Johnson, J.; Oien, N.L.; Meinert, T.R.; Verhelle, S.; Mattern, S.J.; Bowersock, T.L.; Leyh, R.D. Efficacy of intranasal vaccination with a multivalent vaccine containing temperature-sensitive modified-live bovine herpesvirus type I for protection of seronegative and seropositive calves against respiratory disease. J. Am. Vet. Med. Assoc. 2016, 248, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.; Gow, S.; West, K.; Waldner, C.; Rhodes, C.; Mutwiri, G.; Rosenberg, H. Response of calves to challenge exposure with virulent bovine respiratory syncytial virus following intranasal administration of vaccines formulated for parenteral administration. J. Am. Vet. Med. Assoc. 2007, 230, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Wildman, B.K.; Perrett, T.; Abutarbush, S.M.; Guichon, P.T.; Pittman, T.J.; Booker, C.W.; Schunicht, O.C.; Fenton, R.K.; Jim, G.K. A comparison of 2 vaccination programs in feedlot calves at ultra-high risk of developing undifferentiated fever/bovine respiratory disease. Can. Vet. J. 2008, 49, 463–472. [Google Scholar]
- Bryant, T.C.; Rogers, K.C.; Stone, N.D.; Miles, D.G. Effect of viral respiratory vaccine treatment on performance, health and carcass traits of auction-origin feeder steers. Bov. Pract. 2008, 42, 98–103. [Google Scholar]
- Potgieter, L.N.; McCracken, M.D.; Hopkins, F.M.; Walker, R.D.; Guy, J.S. Experimental production of bovine respiratory tract disease with bovine viral diarrhea virus. Am. J. Vet. Res. 1984, 45, 1582–1585. [Google Scholar] [PubMed]
- Agnes, J.T.; Zekarias, B.; Shao, M.; Anderson, M.L.; Gershwin, L.J.; Corbeil, L.B. Bovine respiratory syncytial virus and Histophilus somni interaction at the alveolar barrier. Infect. Immun. 2013, 81, 2592–2597. [Google Scholar] [CrossRef] [PubMed]
- Sudaryatma, P.E.; Nakamura, K.; Mekata, H.; Sekiguchi, S.; Kubo, M.; Kobayashi, I.; Subangkit, M.; Goto, Y.; Okabayashi, T. Bovine respiratory syncytial virus infection enhances Pasteurella multocida adherence on respiratory epithelial cells. Vet. Microbiol. 2018, 220, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Fulton, R.W.; Purdy, C.W.; Confer, A.W.; Saliki, J.T.; Loan, R.W.; Briggs, R.E.; Burge, L.J. Bovine viral diarrhea viral infections in feeder calves with respiratory disease: Interactions with Pasteurella spp., parainfluenza-3 virus, and bovine respiratory syncytial virus. Can. J. Vet. Res. 2000, 64, 151–159. [Google Scholar]
- Martin, S.W.; Bohac, J.G. The association between serological titers in infectious bovine rhinotracheitis virus, bovine virus diarrhea virus, parainfluenza-3 virus, respiratory syncytial virus and treatment for respiratory disease in Ontario feedlot calves. Can. J. Vet. Res. 1986, 50, 351–358. [Google Scholar]
- Richer, L.; Marois, P.; Lamontagne, L. Association of bovine viral diarrhea virus with multiple viral infections in bovine respiratory disease outbreaks. Can. Vet. J. Rev. Vet. Can. 1988, 29, 713–717. [Google Scholar]
- Fent, G.M.; Fulton, R.W.; Saliki, J.T.; Caseltine, S.L.; Lehmkuhl, H.D.; Confer, A.W.; Purdy, C.W.; Briggs, R.E.; Loan, R.W.; Duff, G.C. Bovine adenovirus serotype 7 infections in postweaning calves. Am. J. Vet. Res. 2002, 63, 976–978. [Google Scholar] [CrossRef] [PubMed]
- Härtel, H.; Nikunen, S.; Neuvonen, E.; Tanskanen, R.; Kivelä, S.L.; Aho, P.; Soveri, T.; Saloniemi, H. Viral and bacterial pathogens in bovine respiratory disease in Finland. Acta Vet. Scand. 2004, 45, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Storz, J.; Lin, X.; Purdy, C.W.; Chouljenko, V.N.; Kousoulas, K.G.; Enright, F.M.; Gilmore, W.C.; Briggs, R.E.; Loan, R.W. Coronavirus and Pasteurella infections in bovine shipping fever pneumonia and Evans’ criteria for causation. J. Clin. Microbiol. 2000, 38, 3291–3298. [Google Scholar] [CrossRef]
- Storz, J.; Purdy, C.W.; Lin, X.; Burrell, M.; Truax, R.E.; Briggs, R.E.; Frank, G.H.; Loan, R.W. Isolation of respiratory bovine coronavirus, other cytocidal viruses, and Pasteurella spp from cattle involved in two natural outbreaks of shipping fever. J. Am. Vet. Med. Assoc. 2000, 216, 1599–1604. [Google Scholar] [CrossRef]
- Lathrop, S.L.; Wittum, T.E.; Brock, K.V.; Loerch, S.C.; Perino, L.J.; Bingham, H.R.; McCollum, F.T.; Saif, L.J. Association between infection of the respiratory tract attributable to bovine coronavirus and health and growth performance of cattle in feedlots. Am. J. Vet. Res. 2000, 61, 1062–1066. [Google Scholar] [CrossRef]
- Zhang, M.; Hill, J.E.; Fernando, C.; Alexander, T.W.; Timsit, E.; van der Meer, F.; Huang, Y. Respiratory viruses identified in western Canadian beef cattle by metagenomic sequencing and their association with bovine respiratory disease. Transbound Emerg. Dis. 2019, 66, 1379–1386. [Google Scholar] [CrossRef]
- Hause, B.M.; Collin, E.A.; Anderson, J.; Hesse, R.A.; Anderson, G. Bovine rhinitis viruses are common in U.S. cattle with bovine respiratory disease. PLoS ONE 2015, 10, 1998. [Google Scholar] [CrossRef]
- Nissly, R.H.; Zaman, N.; Ibrahim, P.A.S.; McDaniel, K.; Lim, L.; Kiser, J.N.; Bird, I.; Chothe, S.K.; Bhushan, G.L.; Vandegrift, K.; et al. Influenza C and D viral load in cattle correlates with bovine respiratory disease (BRD): Emerging role of orthomyxoviruses in the pathogenesis of BRD. Virology 2020, 551, 10–15. [Google Scholar] [CrossRef]
- Ferguson, L.; Olivier, A.K.; Genova, S.; Epperson, W.B.; Smith, D.R.; Schneider, L.; Barton, K.; McCuan, K.; Webby, R.J.; Wan, X.; et al. Pathogenesis of Influenza D Virus in Cattle. J. Virol. 2016, 90, 5636–5642. [Google Scholar] [CrossRef]
- Hause, B.M.; Huntimer, L.; Falkenberg, S.; Henningson, J.; Lechtenberg, K.; Halbur, T. An inactivated influenza D virus vaccine partially protects cattle from respiratory disease caused by homologous challenge. Vet. Microbiol. 2017, 199, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Niewiesk, S. Maternal antibodies: Clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front. Immunol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.A.; Gow, S.P.; Goji, N. Response to experimentally induced infection with bovine respiratory syncytial virus following intranasal vaccination of seropositive and seronegative calves. J. Am. Vet. Med. Assoc. 2010, 236, 991–999. [Google Scholar] [CrossRef]
- Menanteau-Horta, A.M.; Ames, T.R.; Johnson, D.W.; Meiske, J.C. Effect of maternal antibody upon vaccination with infectious bovine rhinotracheitis and bovine virus diarrhea vaccines. Can. J. Comp. Med. 1985, 49, 10–14. [Google Scholar] [PubMed]
- Ellis, J.; Gow, S.; Bolton, M.; Burdett, W.; Nordstrom, S. Inhibition of priming for bovine respiratory syncytial virus-specific protective immune responses following parenteral vaccination of passively immune calves. Can. Vet. J. 2014, 55, 1180–1185. [Google Scholar] [PubMed]
- Zimmerman, A.; Buterbaugh, R.; Schnackel, J.A.; Chase, C.C.L. Efficacy of a modified-live virus vaccine administered to calves with maternal antibodies and challenged seven months later with a virulent bovine viral diarrhea type 2. Bov. Pract. 2009, 43, 35–43. [Google Scholar]
- Erickson, N.; Ellis, J.; Waldner, C.; Lardner, H.; Gow, S.; Campbell, J.; Berenik, A. A field comparison of heterologous and homologous routes of administration of modified live vaccines for the control of bovine respiratory disease in recently weaned beef calves. Can. Vet. J. 2020, 61, 530–533. [Google Scholar]
- Ellis, J.; Gow, S.; Berenik, A.; Lacoste, S.; Erickson, N. Comparative efficacy of modified-live and inactivated vaccines in boosting responses to bovine respiratory syncytial virus following neonatal mucosal priming of beef calves. Can. Vet. J. 2018, 59, 1311–1319. [Google Scholar]
- Griffin, C.M.; Scott, J.A.; Karisch, B.B.; Woolums, A.R.; Blanton, J.R.; Kaplan, R.M.; Epperson, W.B.; Smith, D.R. A randomized controlled trial to test the effect of on-arrival vaccination and deworming on stocker cattle health and growth performance. Bov. Pract. 2018, 52, 26–33. [Google Scholar]
- Richeson, J.T.; Falkner, T.R. Bovine Respiratory Disease Vaccination: What Is the Effect of Timing? Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 473–485. [Google Scholar] [CrossRef]
- Richeson, J.T.; Kegley, E.B.; Gadberry, M.S.; Beck, P.A.; Powell, J.G.; Jones, C.A. Effects of on-arrival versus delayed clostridial or modified live respiratory vaccinations on health, performance, bovine viral diarrhea virus type I titers, and stress and immune measures of newly received beef calves. J. Anim. Sci. 2009, 87, 2409–2418. [Google Scholar] [CrossRef]
- Poe, K.D.; Beck, P.A.; Richeson, J.T.; Gadberry, M.S.; Kegley, E.B.; Hess, T.W.; Hubbell, D.S. Effects of respiratory vaccination timing and growth-promoting implant on health, performance, and immunity of high-risk, newly received stocker cattle. Prof. Anim. Sci. 2013, 29, 413–419. [Google Scholar] [CrossRef]
- Schumaher, T.F.; Cooke, R.F.; Brandão, A.P.; Schubach, K.M.; de Sousa, O.A.; Bohnert, D.W.; Marques, R.S. Effects of vaccination timing against respiratory pathogens on performance, antibody response, and health in feedlot cattle. J. Anim. Sci. 2019, 97, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Richeson, J.T.; Carroll, J.A.; Burdick Sanchez, N.C.; May, N.D.; Hughes, H.D.; Roberts, S.L.; Broadway, P.R.; Sharon, K.P.; Ballou, M.A. Dexamethasone treatment differentially alters viral shedding and the antibody and acute phase protein response after multivalent respiratory vaccination in beef steers. J. Anim. Sci. 2016, 94, 3501–3509. [Google Scholar] [CrossRef]
- Cresswell, E.; Brennan, M.L.; Barkema, H.W.; Wapenaar, W. A questionnaire-based survey on the uptake and use of cattle vaccines in the UK. Vet. Rec. Open. 2014, 1, e000042. [Google Scholar] [CrossRef]
- Williams, P.D.; Paixão, G. On-farm storage of livestock vaccines may be a risk to vaccine efficacy: A study of the performance of on-farm refrigerators to maintain the correct storage temperature. BMC Vet. Res. 2018, 14, 136. [Google Scholar] [CrossRef]

| Vaccine | Type | Component(s) |
|---|---|---|
| Bovalto Pastobov | Type A1 antigen | M. haemolytica |
| Rispoval Pasteurella | Inactivated | M. haemolytica |
| Rispoval RS | MLV | BRSV |
| Bovalto Respi Intranasal | MLV | BRSV PIV3 |
| Imuresp RP | MLV | BoHV-1, PIV3 |
| Rispoval 3 | MLV | BRSV, PIV3, BVDV |
| Rispoval 4 | MLV | BRSV, PIV3, BVDV, BoHV-1 |
| Rispoval RS + PI3 Intranasal | MLV | BRSV, PIV3 |
| Hiprabovis SOMNI/Lkt | Inactivated | M. haemolytica and H. somni |
| Bovalto Respi 3 | Inactivated | M. haemolytica and PIV3 |
| Bovilis Bovipast RSP | Inactivated | M. haemolytica and BRSV |
| Bovalto Respi 4 | Inactivated | BRSV, PIV3, BVDV, M. haemolytica |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bell, R.L.; Turkington, H.L.; Cosby, S.L. The Bacterial and Viral Agents of BRDC: Immune Evasion and Vaccine Developments. Vaccines 2021, 9, 337. https://doi.org/10.3390/vaccines9040337
Bell RL, Turkington HL, Cosby SL. The Bacterial and Viral Agents of BRDC: Immune Evasion and Vaccine Developments. Vaccines. 2021; 9(4):337. https://doi.org/10.3390/vaccines9040337
Chicago/Turabian StyleBell, Rachael Lynda, Hannah Louise Turkington, and Sara Louise Cosby. 2021. "The Bacterial and Viral Agents of BRDC: Immune Evasion and Vaccine Developments" Vaccines 9, no. 4: 337. https://doi.org/10.3390/vaccines9040337
APA StyleBell, R. L., Turkington, H. L., & Cosby, S. L. (2021). The Bacterial and Viral Agents of BRDC: Immune Evasion and Vaccine Developments. Vaccines, 9(4), 337. https://doi.org/10.3390/vaccines9040337







