Vaccine Efficacy of a Newly Developed Feed-Based Whole-Cell Polyvalent Vaccine against Vibriosis, Streptococcosis and Motile Aeromonad Septicemia in Asian Seabass, Lates calcarifer
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Reviving the Virulence and Preparation of Pathogenic Bacterial Strains
PCR Amplification Using 16S rRNA
2.3. Feed-Based Vaccine Preparation
2.4. Feed Quality Analysis
2.4.1. Proximate Analysis
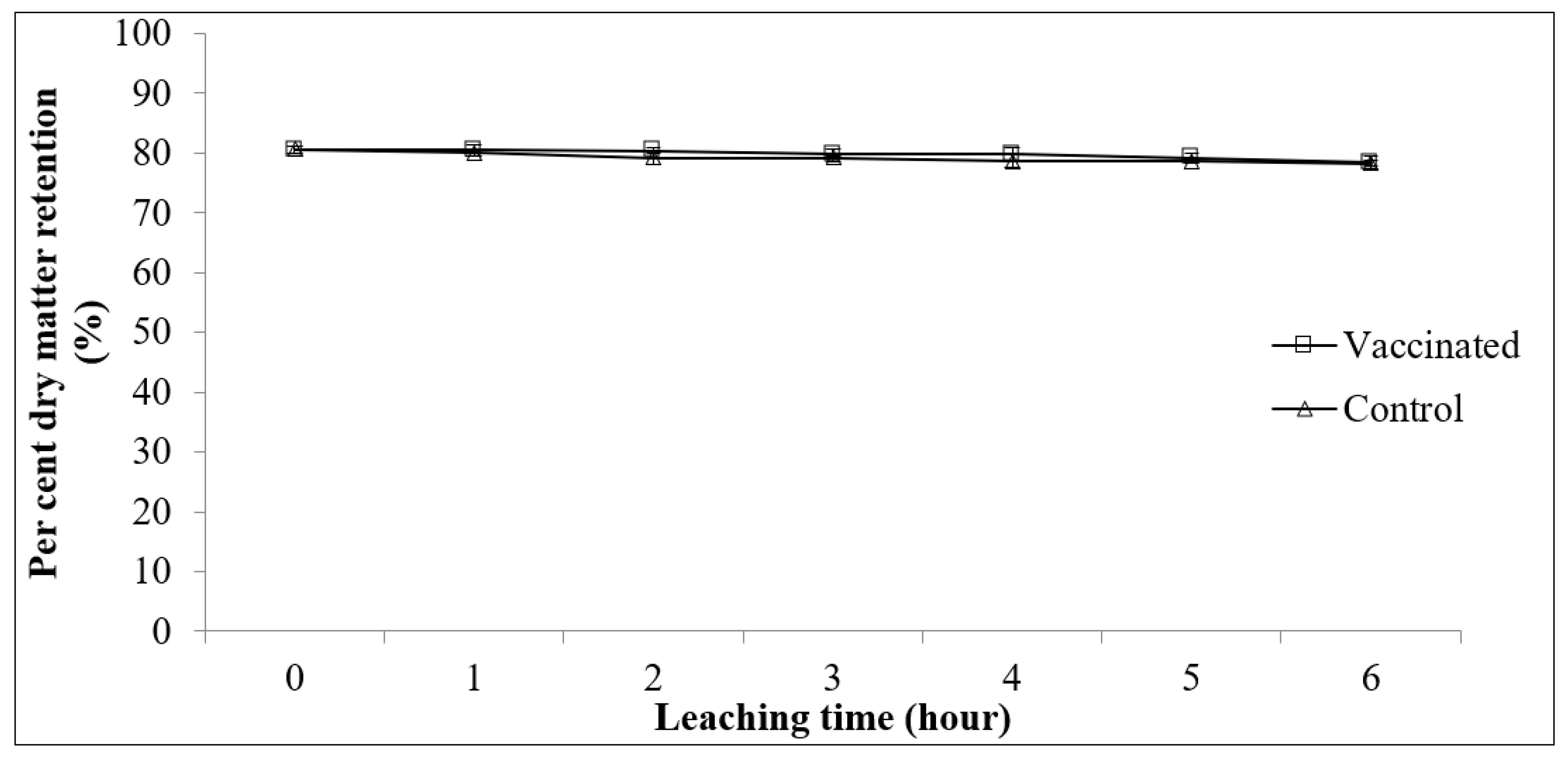
2.4.2. Physical Stability of Feed in Water
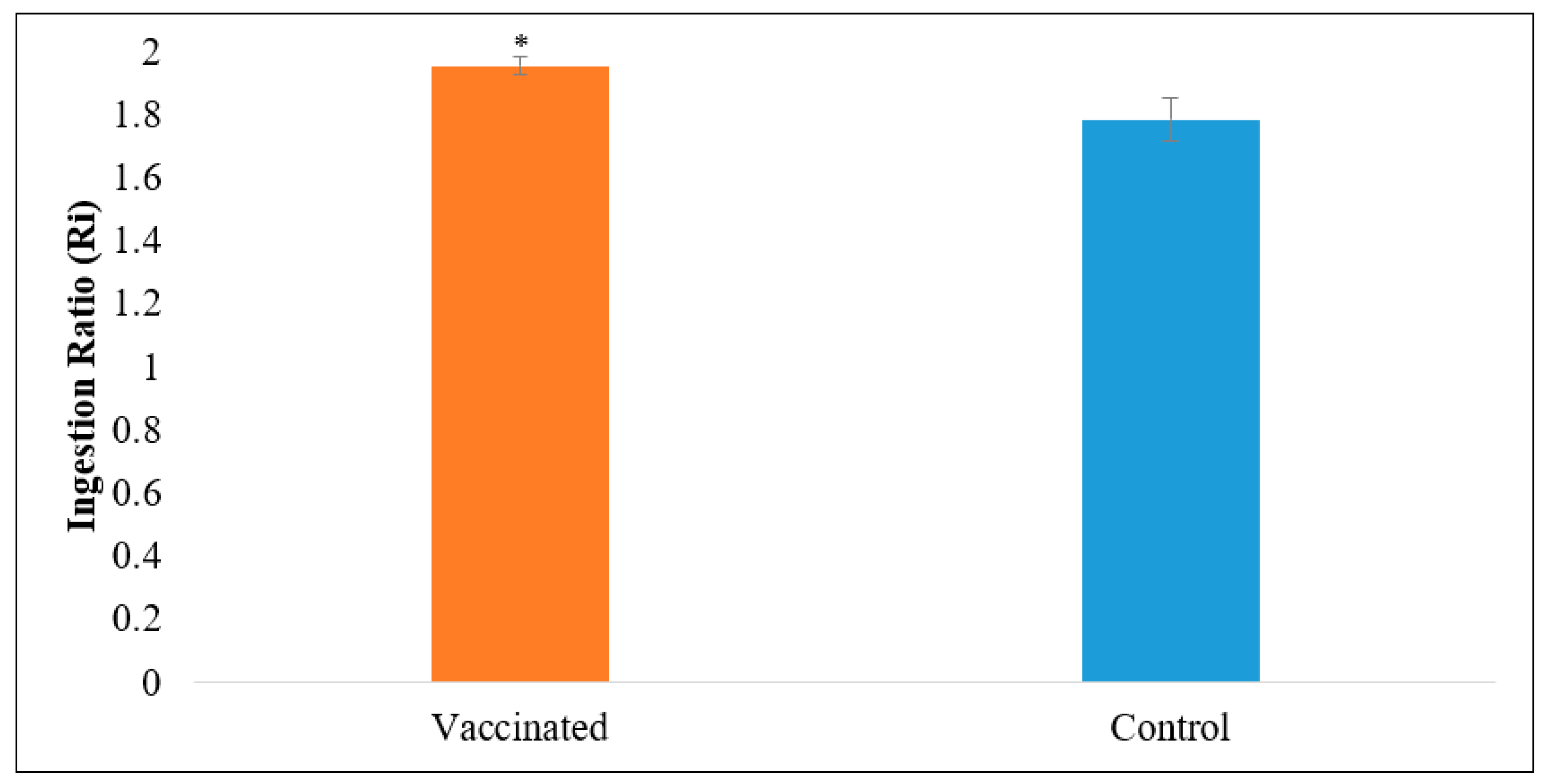
2.4.3. Palatability Test
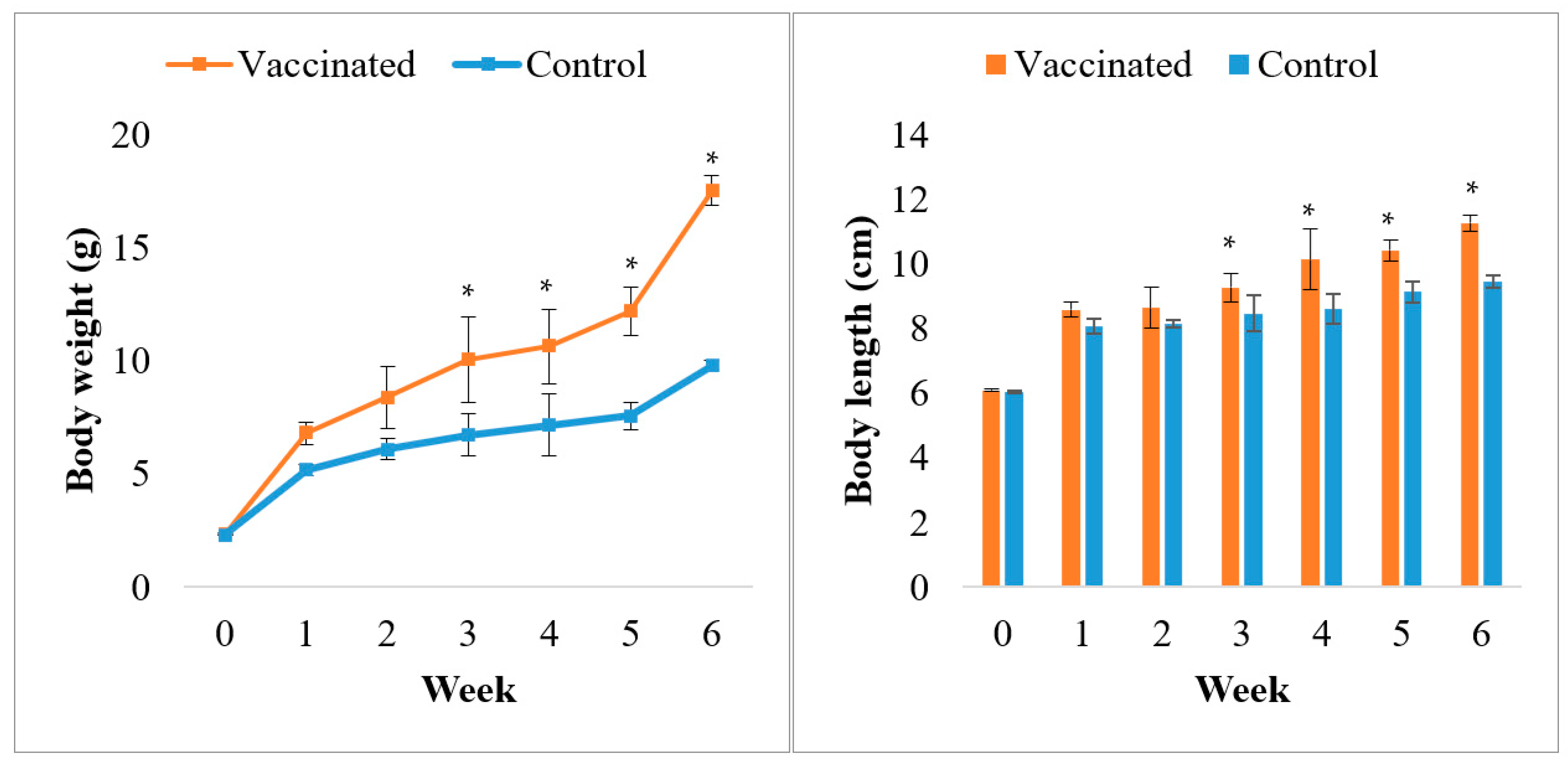
2.4.4. Growth Performance
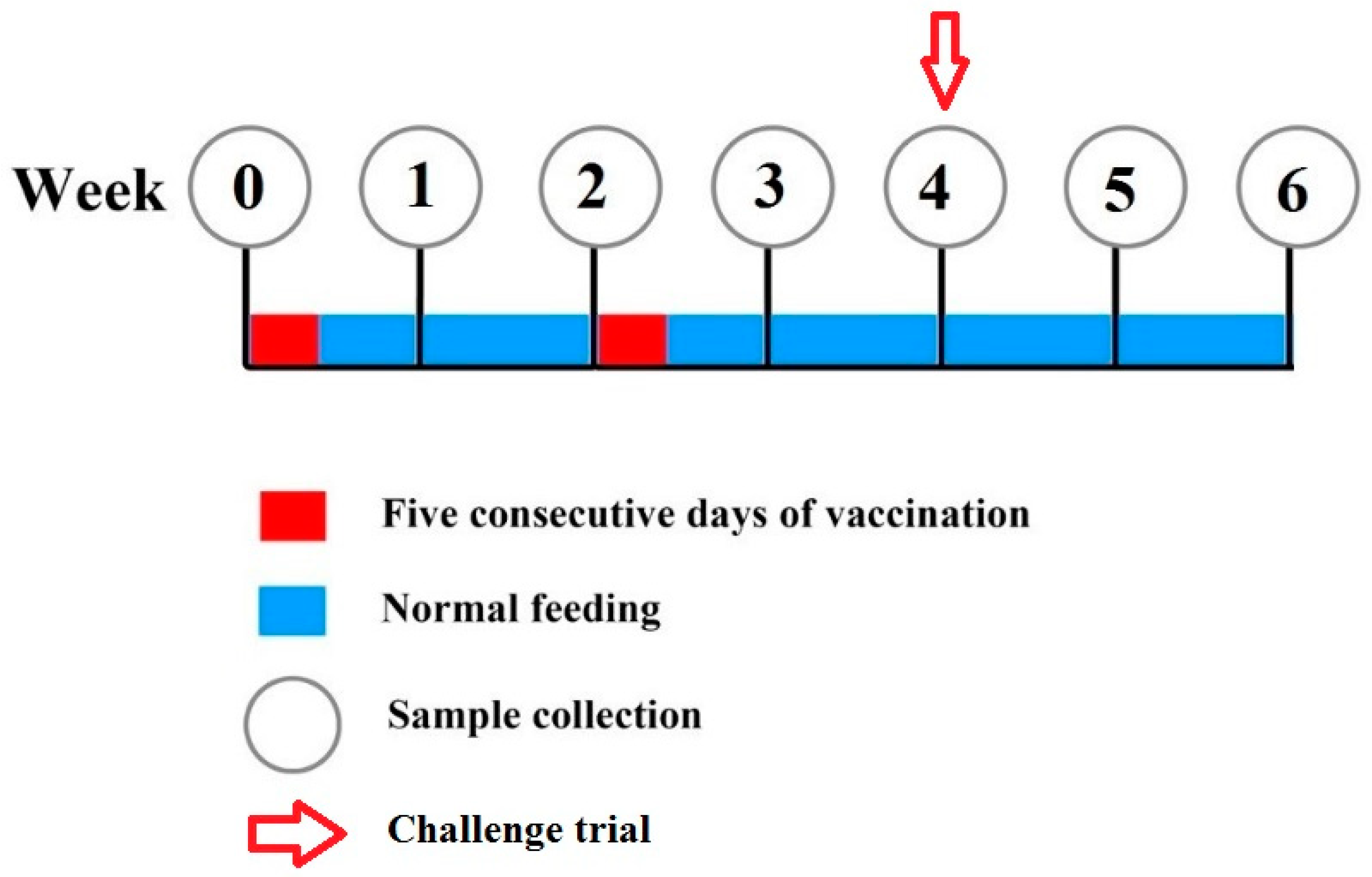
2.5. Vaccination Trial
2.5.1. The Fish
2.5.2. Experimental Design
2.5.3. Challenge Test
2.6. Sample Processing
2.6.1. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6.2. Serum Lysozyme Activity
2.6.3. Quantitative Real-Time PCR (qPCR)
2.7. Statistical Analysis
3. Results
3.1. Feed Quality Analysis and Growth Performance
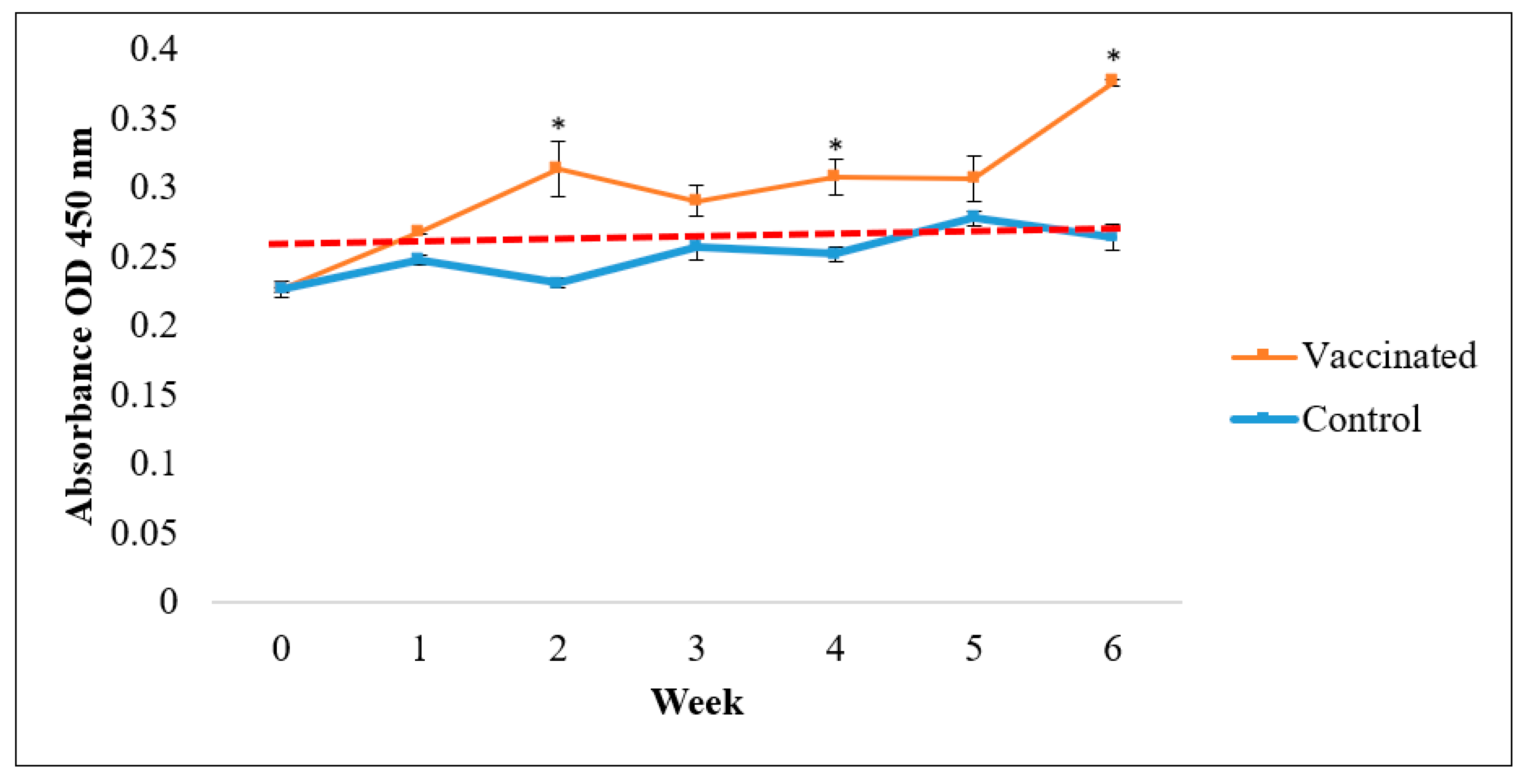
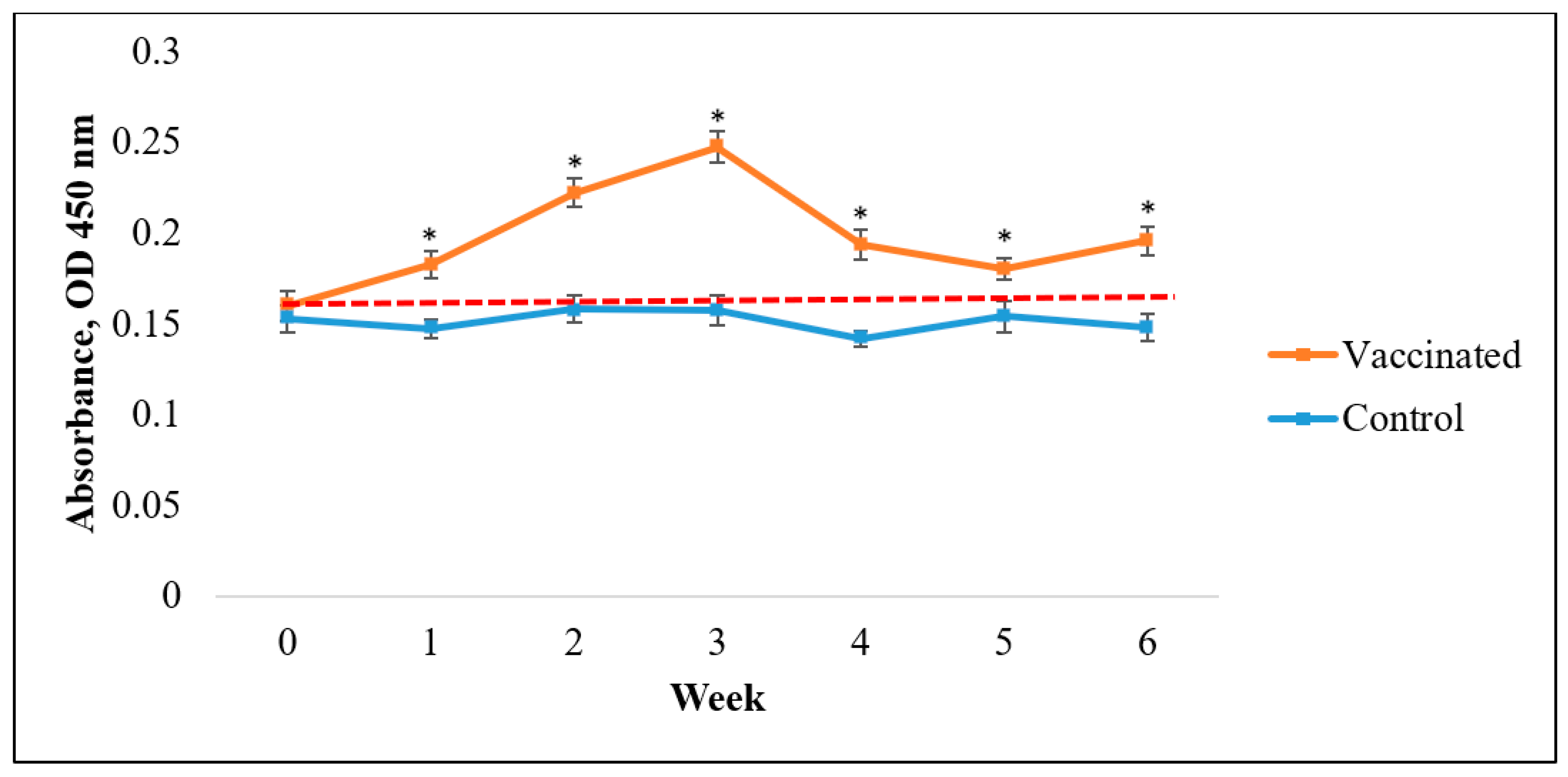
3.2. IgM Antibody Responses
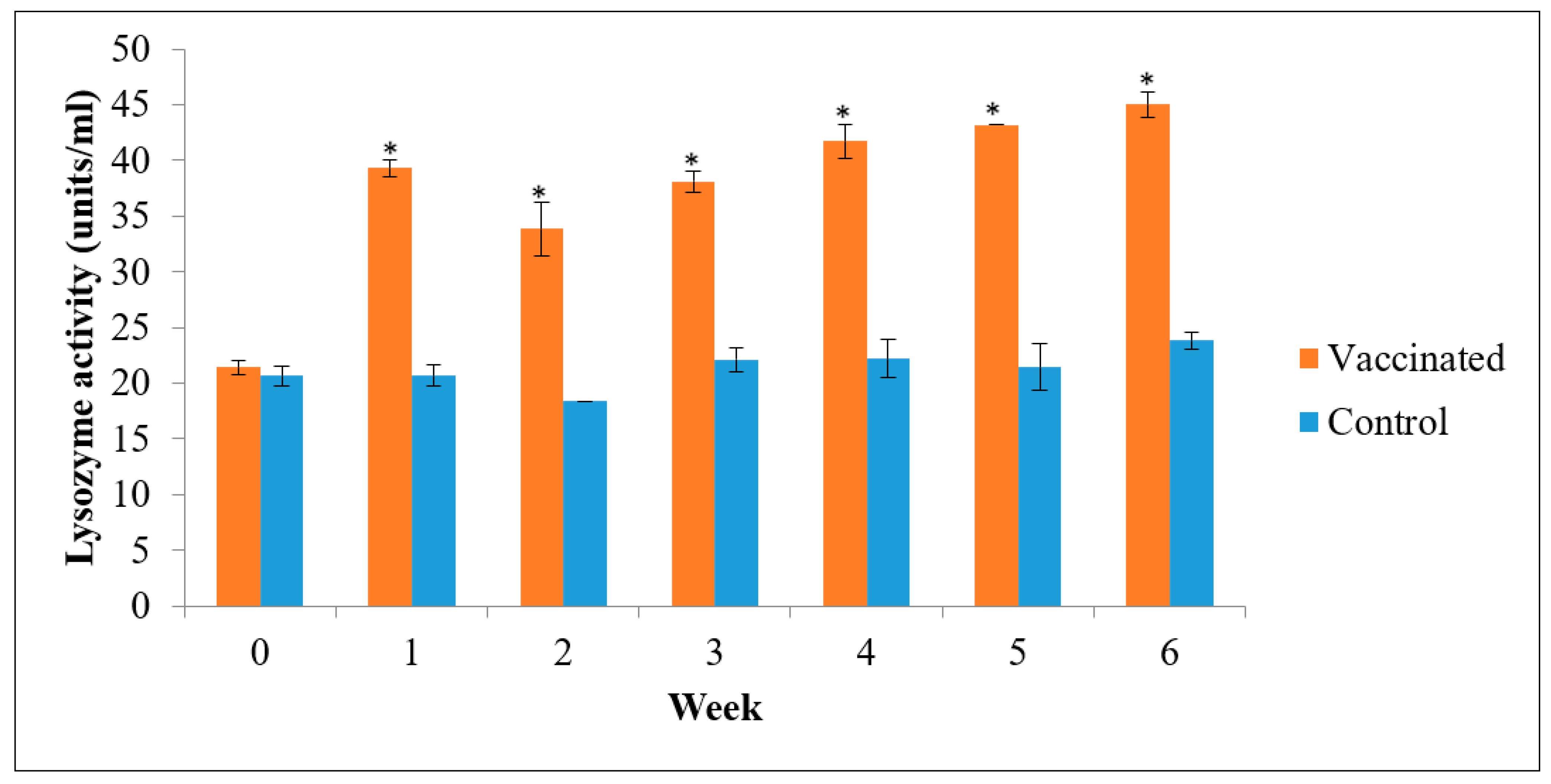
3.3. Lysozyme Activity
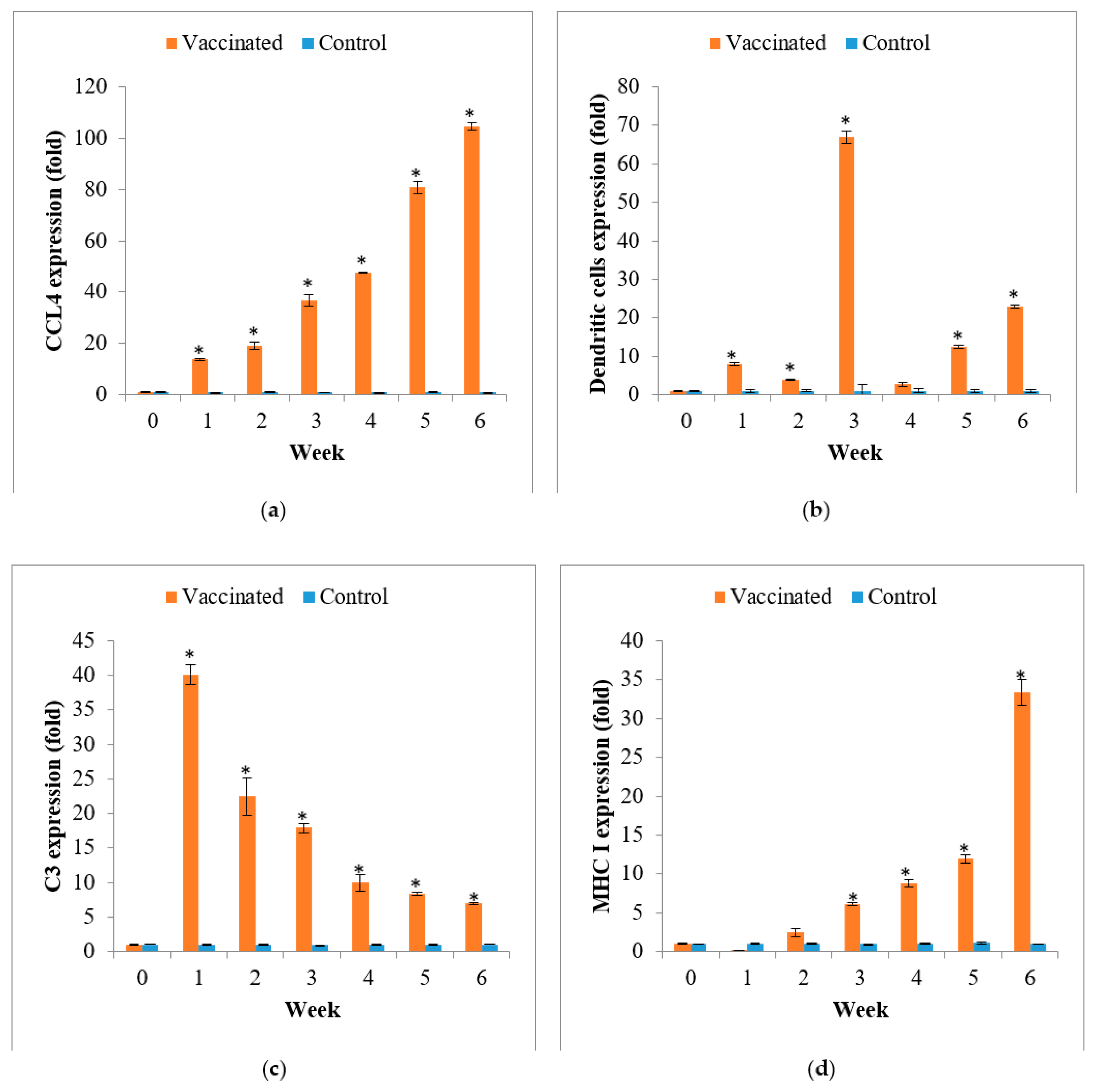
3.4. Gene Expression
3.5. Efficacy of the Feed-Based Polyvalent Vaccine
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Defoirdt, T.; Sorgeloos, P.; Bossier, P. Alternatives to Antibiotics for the Control of Bacterial Disease in Aquaculture. Curr. Opin. Microbiol. 2011, 14, 251–258. [Google Scholar] [CrossRef]
- Hounmanou, Y.M.G.; Mdegela, R.H.; Dougnon, T.V.; Achoh, M.E.; Mhongole, O.J.; Agadjihouèdé, H.; Gangbè, L.; Dalsgaard, A. Tilapia Lake Virus Threatens Tilapiines Farming and Food Security: Socio-Economic Challenges and Preventive Measures in Sub-Saharan Africa. Aquaculture 2018, 493, 123–129. [Google Scholar] [CrossRef]
- López, J.R.; Navas, J.I.; Thanantong, N.; de la Herran, R.; Sparagano, O.A.E. Simultaneous Identification of Five Marine Fish Pathogens Belonging to the Genera Tenacibaculum, Vibrio, Photobacterium and Pseudomonas by Reverse Line Blot Hybridization. Aquaculture 2012, 324–325, 33–38. [Google Scholar] [CrossRef]
- Lima, P.C.; Harris, J.O.; Cook, M. Exploring RNAi as a Therapeutic Strategy for Controlling Disease in Aquaculture. Fish Shellfish Immunol. 2013, 34, 729–743. [Google Scholar] [CrossRef]
- Ismail, M.S.; Syafiq, M.R.; Siti-Zahrah, A.; Fahmi, S.; Shahidan, H.; Hanan, Y.; Amal, M.N.A.; Zamri Saad, M. The Effect of Feed-Based Vaccination on Tilapia Farm Endemic for Streptococcosis. Fish Shellfish Immunol. 2017, 60, 21–24. [Google Scholar] [CrossRef]
- Pasaribu, W.; Sukenda, S.; Nuryati, S. The Efficacy of Nile Tilapia (Oreochromis Niloticus) Broodstock and Larval Immunization against Streptococcus Agalactiae and Aeromonas Hydrophila. Fishes 2018, 3, 16. [Google Scholar] [CrossRef]
- Amal, M.N.A.; Zamri-Saad, M. Streptococcosis in Tilapia (Oreochromis Niloticus): A Review. Pertanika J. Trop. Agric. Sci. 2011, 34, 195–206. [Google Scholar]
- Ransangan, J.; Manin, B.O. Mass Mortality of Hatchery-Produced Larvae of Asian Seabass, Lates Calcarifer (Bloch), Associated with Viral Nervous Necrosis in Sabah, Malaysia. Vet. Microbiol. 2010, 145, 153–157. [Google Scholar] [CrossRef]
- Abdolnabi, S.; Ina-Salwany, M.Y.; Daud, H.M.; Mariana, N.S.; Abdelhadi, Y.M. Pathogenicity of Aeromonas Hydrophila in Giant Freshwater Prawn; Macrobrachium Rosenbergii, Cultured in East Malaysia. Iran. J. Fish. Sci. 2015, 14, 232–245. [Google Scholar]
- Tumbol, R.A.; Baiano, J.C.F.; Barnes, A.C. Differing Cell Population Structure Reflects Differing Activity of Percoll-Separated Pronephros and Peritoneal Leucocytes from Barramundi (Lates Calcarifer). Aquaculture 2009, 292, 180–188. [Google Scholar] [CrossRef]
- Thu Lan, N.G.; Salin, K.R.; Longyant, S.; Senapin, S.; Dong, H.T. Systemic and Mucosal Antibody Response of Freshwater Cultured Asian Seabass (Lates Calcarifer) to Monovalent and Bivalent Vaccines against Streptococcus Agalactiae and Streptococcus Iniae. Fish Shellfish Immunol. 2020, 108, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.T.; Taengphu, S.; Sangsuriya, P.; Charoensapsri, W.; Phiwsaiya, K.; Sornwatana, T.; Khunrae, P.; Rattanarojpong, T.; Senapin, S. Recovery of Vibrio Harveyi from Scale Drop and Muscle Necrosis Disease in Farmed Barramundi, Lates Calcarifer in Vietnam. Aquaculture 2017, 473, 89–96. [Google Scholar] [CrossRef]
- Lin, H.L.; Shiu, Y.L.; Chiu, C.S.; Huang, S.L.; Liu, C.H. Screening Probiotic Candidates for a Mixture of Probiotics to Enhance the Growth Performance, Immunity, and Disease Resistance of Asian Seabass, Lates Calcarifer (Bloch), against Aeromonas Hydrophila. Fish Shellfish Immunol. 2017, 60, 474–482. [Google Scholar] [CrossRef]
- Galindo-Villegas, J.; Mulero, I.; García-Alcazar, A.; Muñoz, I.; Peñalver-Mellado, M.; Streitenberger, S.; Scapigliati, G.; Meseguer, J.; Mulero, V. Recombinant TNFα as Oral Vaccine Adjuvant Protects European Sea Bass against Vibriosis: Insights into the Role of the CCL25/CCR9 Axis. Fish Shellfish Immunol. 2013, 35, 1260–1271. [Google Scholar] [CrossRef]
- Cruz-Lacierda, E.R.; Erazo-pagador, G.E. Diseases of Cultured Groupers—Parasitic Diseases; Southeast Asian Fisheries Development Center: Loilo, Philippines, 2004. [Google Scholar]
- Zamri-Saad, M.; Amal, M.N.A.; Siti-Zahrah, A.; Zulkafli, A.R. Control and Prevention of Streptococcosis in Cultured Tilapia in Malaysia: A Review. Pertanika J. Trop. Agric. Sci. 2014, 37, 389–410. [Google Scholar]
- Le, T.S.; Nguyen, T.H.; Vo, H.P.; Doan, V.C.; Nguyen, H.L.; Tran, M.T.; Tran, T.T.; Southgate, P.C.; İpek Kurtböke, D. Protective Effects of Bacteriophages against Aeromonas Hydrophila Species Causing Motile Aeromonas Septicemia (MAS) in Striped Catfish. Antibiotics 2018, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Awad, E.; Awaad, A. Role of Medicinal Plants on Growth Performance and Immune Status in Fish. Fish Shellfish Immunol. 2017, 67, 40–54. [Google Scholar] [CrossRef]
- Romero, J.; Gloria, C.; Navarrete, P. Antibiotics in Aquaculture—Use, Abuse and Alternatives. In Health and Environment in Aquaculture; InTech: Rijeka, Croatia, 2012; pp. 159–198. [Google Scholar] [CrossRef]
- Sapkota, A.; Sapkota, A.R.; Kucharski, M.; Burke, J.; McKenzie, S.; Walker, P.; Lawrence, R. Aquaculture Practices and Potential Human Health Risks: Current Knowledge and Future Priorities. Environ. Int. 2008, 34, 1215–1226. [Google Scholar] [CrossRef]
- Cheng, Z.-X.; Ma, Y.-M.; Li, H.; Peng, X.-X. N-Acetylglucosamine Enhances Survival Ability of Tilapias Infected by Streptococcus Iniae. Fish Shellfish Immunol. 2014, 40, 524–530. [Google Scholar] [CrossRef]
- Ina-Salwany, M.Y.; Al-saari, N.; Mohamad, A.; Mursidi, F.; Mohd-Aris, A.; Amal, M.N.A.; Kasai, H.; Mino, S.; Sawabe, T.; Zamri-Saad, M. Vibriosis in Fish: A Review on Disease Development and Prevention. J. Aquat. Anim. Health 2019, 31, 3–22. [Google Scholar] [CrossRef]
- Huang, L.Y.; Wang, K.Y.; Xiao, D.; Chen, D.F.; Geng, Y.; Wang, J.; He, Y.; Wang, E.L.; Huang, J.L.; Xiao, G.Y. Safety and Immunogenicity of an Oral DNA Vaccine Encoding Sip of Streptococcus Agalactiae from Nile Tilapia Oreochromis Niloticus Delivered by Live Attenuated Salmonella Typhimurium. Fish Shellfish Immunol. 2014, 38, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Nehlah, R.; Firdaus-Nawi, M.; Nik-Haiha, N.Y.; Karim, M.; Zamri-Saad, M.; Ina-Salwany, M.Y. Recombinant Vaccine Protects Juvenile Hybrid Grouper, Epinephelus Fuscoguttatus × Epinephelus Lanceolatus, against Infection by Vibrio Alginolyticus. Aquac. Int. 2017, 25, 2047–2059. [Google Scholar] [CrossRef]
- Ghosh, B.; Bridle, A.R.; Nowak, B.F.; Cain, K.D. Assessment of Immune Response and Protection against Bacterial Coldwater Disease Induced by a Live-Attenuated Vaccine Delivered Orally or Intraperitoneally to Rainbow Trout, Oncorhynchus Mykiss (Walbaum). Aquaculture 2015, 446, 242–249. [Google Scholar] [CrossRef]
- Wei, G.; Cai, S.; Wu, Y.; Ma, S.; Huang, Y. Immune Effect of Vibrio Harveyi Formalin-Killed Cells Vaccine Combined with Chitosan Oligosaccharide and Astragalus Polysaccharides in ♀Epinephelus Fuscoguttatus × ♂Epinephelus Lanceolatus. Fish Shellfish Immunol. 2020, 98, 186–192. [Google Scholar] [CrossRef]
- Adams, A. Progress, Challenges and Opportunities in Fish Vaccine Development. Fish Shellfish Immunol. 2019, 90, 210–214. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Quillet, E.; Boudinot, P.; Fischer, U. What Could Be the Mechanisms of Immunological Memory in Fish? Fish Shellfish Immunol. 2019, 85, 3–8. [Google Scholar] [CrossRef]
- Gudding, R.; Van Muiswinkel, W.B. A History of Fish Vaccination: Science-Based Disease Prevention in Aquaculture. Fish Shellfish Immunol. 2013, 35, 1683–1688. [Google Scholar] [CrossRef]
- Firdaus-Nawi, M.; Yusoff, S.M.; Yusof, H.; Abdullah, S.Z.; Zamri-Saad, M. Efficacy of Feed-Based Adjuvant Vaccine against Streptococcus Agalactiae in Oreochromis Spp. in Malaysia. Aquac. Res. 2013, 45, 87–96. [Google Scholar] [CrossRef]
- Plant, K.P.; LaPatra, S.E. Advances in Fish Vaccine Delivery. Dev. Comp. Immunol. 2011, 35, 1256–1262. [Google Scholar] [CrossRef]
- Mohd-Aris, A.; Saad, M.Z.; Daud, H.M.; Yusof, M.T.; Ina-Salwany, M.Y. Vibrio Harveyi Protease Deletion Mutant as a Live Attenuated Vaccine Candidate against Vibriosis and Transcriptome Profiling Following Vaccination for Epinephelus Fuscoguttatus. Aquac. Int. 2019, 27, 125–140. [Google Scholar] [CrossRef]
- Matusin, S. Molecular Characterization of Aromonas Hydrophila and Development of Recombinant Cells Vaccine Expressing Outer Membrane Proteins against ITS in African Catfish (Clarias Gariepinus Burchell); Universiti Putra Malaysia: Kuala Lumpur, Malaysia, 2015. [Google Scholar]
- Yin, B.; Liu, H.; Tan, B.; Dong, X.; Chi, S.; Yang, Q.; Zhang, S. Preliminary Study of Mechanisms of Intestinal Inflammation Induced by Plant Proteins in Juvenile Hybrid Groupers (♀Epinephelus Fuscoguttatus × ♂E. Lanceolatu). Fish Shellfish Immunol. 2020, 106, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Mursidi, F. Antigenic Analysis of Outer Membrane Protein of Vibrio Species and Development of Versatile Recombinant VhDnaJ Vaccine against Vibriosis; Universiti Putra Malaysia: Kuala Lumpur, Malaysia, 2018. [Google Scholar]
- Chin, Y.K.; Al-saari, N.; Zulperi, Z.; Mohd-Aris, A.; Salleh, A.; Silvaraj, S.; Mohamad, A.; Lee, J.Y.; Zamri-Saad, M.; Ina-Salwany, M.Y. Efficacy of Bath Vaccination with a Live Attenuated Vibrio Harveyi against Vibriosis in Asian Seabass Fingerling, Lates calcarifer. Aquac. Res. 2020, 51, 389–399. [Google Scholar] [CrossRef]
- Chin, Y.K.; Al-saari, N.; Mohd-Aris, A.; Zamri-Saad, M.; Md Yasin, I.-S. Administration of Live-Attenuated Vaccine of Vibrio Harveyi to Improve Survival of Gnotobiotic Brine Shrimp (Artemia Salina) Model against MultipleVibrio Infection. Int. J. Biosci. 2019, 14, 1–14. [Google Scholar] [CrossRef]
- Sulaiman, M.A.; Kamarudin, M.S.; Romano, N.; Syukri, F. Effects of Increasing Dietary Carbohydrate Level on Feed Utilisation, Body Composition, Liver Glycogen, and Intestinal Short Chain Fatty Acids of Hybrid Lemon Fin Barb (Barbonymus Gonionotus ♀ X Hypsibarbus Wetmorei Male ♂). Aquac. Rep. 2020, 16, 100250. [Google Scholar] [CrossRef]
- Obaldo, L.G.; Divakaran, S.; Tacon, A.G. Method for Determining the Physical Stability of Shrimp Feeds in Water. Aquac. Res. 2002, 33, 369–377. [Google Scholar] [CrossRef]
- Dong, C.; He, G.; Mai, K.; Zhou, H.; Xu, W. Palatability of Water-Soluble Extracts of Protein Sources and Replacement of Fishmeal by a Selected Mixture of Protein Sources for Juvenile Turbot (Scophthalmus Maximus). J. Ocean Univ. China 2016, 15, 561–567. [Google Scholar] [CrossRef]
- Mao, Z.; He, C.; Qiu, Y.; Chen, J. Expression of Vibrio Harveyi OmpK in the Yeast Pichia Pastoris: The First Step in Developing an Oral Vaccine against Vibriosis? Aquaculture 2011, 318, 268–272. [Google Scholar] [CrossRef]
- Samuelsen, O.B. Pharmacokinetics of Quinolones in Fish: A. Review. Aquaculture 2006, 255, 55–75. [Google Scholar] [CrossRef]
- Monir, M.S.; Yusoff, S.B.M.; Zulperi, Z.B.M.; Hassim, H.B.A.; Mohamad, A.; Ngoo, M.S.B.M.H.; Ina-Salwany, M.Y. Haemato-Immunological Responses and Effectiveness of Feed-Based Bivalent Vaccine against Streptococcus Iniae and Aeromonas Hydrophila Infections in Hybrid Red Tilapia (Oreochromis Mossambicus × O. Niloticus). BMC Vet. Res. 2020, 16, 1–14. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, W.-W.; Hou, J.-H.; Sun, L. Immunoprotective Analysis of VhhP2, a Vibrio Harveyi Vaccine Candidate. Vaccine 2009, 27, 2733–2740. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, C.-S.; Sun, L. Isolation and Analysis of the Vaccine Potential of an Attenuated Edwardsiella Tarda Strain. Vaccine 2010, 28, 6344–6350. [Google Scholar] [CrossRef]
- Tu, F.P.; Chu, W.H.; Zhuang, X.Y.; Lu, C.P. Effect of Oral Immunization with Aeromonas Hydrophila Ghosts on Protection against Experimental Fish Infection. Lett. Appl. Microbiol. 2010, 50, 13–17. [Google Scholar] [CrossRef]
- Wang, H.-R.; Hu, Y.-H.; Zhang, W.-W.; Sun, L. Construction of an Attenuated Pseudomonas Fluorescens Strain and Evaluation of Its Potential as a Cross-Protective Vaccine. Vaccine 2009, 27, 4047–4055. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.S.; Siti-Zahrah, A.; Syafiq, M.R.M.; Amal, M.N.A.; Firdaus-Nawi, M.; Zamri-Saad, M. Feed-Based Vaccination Regime against Streptococcosis in Red Tilapia, Oreochromis Niloticus x Oreochromis Mossambicus. BMC Vet. Res. 2016, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Byadgi, O.; Uyen, N.H.N.; Chou, R.L.; Guo, J.J.; Lee, Y.H.; Lee, J.W.; Cheng, T.C. Immunogenicity of Inactivated Formalin-Killed Photobacterium Damselae Subsp. Piscicida Combined with Toll-like Receptor 9 Agonist in Cobia Rachycentron Canadum. Aquaculture 2018, 492, 369–378. [Google Scholar] [CrossRef]
- Zoccola, E.; Delamare-Deboutteville, J.; Barnes, A.C. Identification of Barramundi (Lates Calcarifer) DC-SCRIPT, a Specific Molecular Marker for Dendritic Cells in Fish. PLoS ONE 2015, 10, e0132687. [Google Scholar] [CrossRef]
- Mohd-Shaharuddin, N.; Mohd-Adnan, A.; Kua, B.C.; Nathan, S. Expression Profile of Immune-Related Genes in Lates Calcarifer Infected by Cryptocaryon Irritans. Fish Shellfish Immunol. 2013, 34, 762–769. [Google Scholar] [CrossRef]
- Bondad-Reantaso, M.G.; Subasinghe, R.P.; Arthur, J.R.; Ogawa, K.; Chinabut, S.; Adlard, R.; Tan, Z.; Shariff, M. Disease and Health Management in Asian Aquaculture. Vet. Parasitol. 2005, 132, 249–272. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Lacierda, E.R.; De la Peña, L.; Lumanlan-Mayo, S. The use of chemicals in aquaculture in the Philippines. InUse of Chemicals in Aquaculture in Asia: Proceedings of the Meeting on the Use of Chemicals in Aquaculture in Asia 20–22 May 1996, Tigbauan, Iloilo, Philippines 2000 (pp. 155–184). Aquaculture Department, Southeast Asian Fisheries Development Center Talpur, A.D.; Ikhwanuddin, M.; Ambok Bolong, A.M. Nutritional Effects of Ginger (Zingiber Officinale Roscoe) on Immune Response of Asian Sea Bass, Lates Calcarifer (Bloch) and Disease Resistance against Vibrio Harveyi. Aquaculture 2013, 400–401, 46–52. [Google Scholar] [CrossRef]
- Park, S.B.; Nho, S.W.; Jang, H.B.; Cha, I.S.; Kim, M.S.; Lee, W.J.; Jung, T.S. Development of Three-Valent Vaccine against Streptococcal Infections in Olive Flounder, Paralichthys Olivaceus. Aquaculture 2016, 461, 25–31. [Google Scholar] [CrossRef]
- Sommerset, I.; Krossoy, B.; Biering, E.; Frost, P. Vaccines for Fish Aquaculture Vaccines for Fish in Aquaculture. Expert Rev. Vaccines 2005, 4, 89–101. [Google Scholar] [CrossRef]
- Cheng, S.; Hu, Y.-H.; Zhang, M.; Sun, L. Analysis of the Vaccine Potential of a Natural Avirulent Edwardsiella Tarda Isolate. Vaccine 2010, 28, 2716–2721. [Google Scholar] [CrossRef]
- Cheng, Z.-X.; Chu, X.; Wang, S.-N.; Peng, X.-X.; Li, H. Six Genes of OmpA Family Shuffling for Development of Polyvalent Vaccines against Vibrio Alginolyticus and Edwardsiella Tarda. Fish Shellfish Immunol. 2018, 75, 308–315. [Google Scholar] [CrossRef]
- Liu, L.; Gong, Y.X.; Liu, G.L.; Zhu, B.; Wang, G.X. Protective Immunity of Grass Carp Immunized with DNA Vaccine against Aeromonas Hydrophila by Using Carbon Nanotubes as a Carrier Molecule. Fish Shellfish Immunol. 2016, 55, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B.; Nguyen, T.D.; Crosbie, P.B.B.; Nowak, B.F.; Bridle, A.R. Oral Vaccination of First-Feeding Atlantic Salmon, Salmo Salar L. Confers Greater Protection against Yersiniosis than Immersion Vaccination. Vaccine 2016, 34, 599–608. [Google Scholar] [CrossRef]
- Mayo, C.; Lee, J.; Kopanke, J.; MacLachlan, N.J. A Review of Potential Bluetongue Virus Vaccine Strategies. Vet. Microbiol. 2017, 206, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Terpstra, A.H.M. The Composition and Production of Fish Feeds; Universitate Vadensi: Orando, The Netherlands, 2015. [Google Scholar]
- Ayisi, C.L.; Zhao, J.; Rupia, E.J. Growth Performance, Feed Utilization, Body and Fatty Acid Composition of Nile Tilapia (Oreochromis Niloticus)Fed Diets Containing Elevated Levels of Palm Oil. Aquac. Fish. 2017, 2, 67–77. [Google Scholar] [CrossRef]
- Thompson, B.; Subasinghe, R. Aquaculture’s Role in Improving Food and Nutrition Security. In Combating Micronutrient Deficiencies: Food-Based Approaches; Thompson, B., Amoroso, L., Eds.; Food and Agriculture Organization of the United Nations; CABI: Rome, Italy, 2010; pp. 150–162. [Google Scholar] [CrossRef]
- Fraser, T.W.K.; Hansen, T.; Mayer, I.; Skjæraasen, J.E.; Glover, K.A.; Sambraus, F.; Fjelldal, P.G. The Effect of Triploidy on Vaccine Side-Effects in Atlantic Salmon. Aquaculture 2014, 433, 481–490. [Google Scholar] [CrossRef]
- Chalmers, L.; Thompson, K.D.; Taylor, J.F.; Black, S.; Migaud, H.; North, B.; Adams, A. A Comparison of the Response of Diploid and Triploid Atlantic Salmon (Salmo Salar) Siblings to a Commercial Furunculosis Vaccine and Subsequent Experimental Infection with Aeromonas Salmonicida. Fish Shellfish Immunol. 2016, 57, 301–308. [Google Scholar] [CrossRef][Green Version]
- Reyes, M.; Ramírez, C.; Ñancucheo, I.; Villegas, R.; Schaffeld, G.; Kriman, L.; Gonzalez, J.; Oyarzun, P. A Novel “in-Feed” Delivery Platform Applied for Oral DNA Vaccination against IPNV Enables High Protection in Atlantic Salmon (Salmon Salar). Vaccine 2017, 35, 626–632. [Google Scholar] [CrossRef]
- Beck, B.R.; Lee, S.H.; Kim, D.; Park, J.H.; Lee, H.K.; Kwon, S.S.; Lee, K.H.; Lee, J.I.; Song, S.K. A Lactococcus Lactis BFE920 Feed Vaccine Expressing a Fusion Protein Composed of the OmpA and FlgD Antigens from Edwardsiella Tarda Was Significantly Better at Protecting Olive Flounder (Paralichthys Olivaceus) from Edwardsiellosis than Single Antigen Vac. Fish Shellfish Immunol. 2017, 68, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Bahurmiz, O.M.; Ng, W.K. Effects of Dietary Palm Oil Source on Growth, Tissue Fatty Acid Composition and Nutrient Digestibility of Red Hybrid Tilapia, Oreochromis Sp., Raised from Stocking to Marketable Size. Aquaculture 2007, 262, 382–392. [Google Scholar] [CrossRef]
- Ismail, N.I.A.; Amal, M.N.A.; Shohaimi, S.; Saad, M.Z.; Abdullah, S.Z. Associations of Water Quality and Bacteria Presence in Cage Cultured Red Hybrid Tilapia, Oreochromis Niloticus × O. Mossambicus. Aquac. Rep. 2016, 4, 57–65. [Google Scholar] [CrossRef]
- Do Vale Pereira, G.; da Silva, B.C.; do Nascimento Vieira, F.; Seiffert, W.Q.; Ushizima, T.T.; Mouriño, J.L.P.; Martins, M.L. Vaccination Strategies with Oral Booster for Surubim Hybrid (Pseudoplatystoma Corruscans × P. Reticulatum) against Haemorrhagic Septicaemia. Aquac. Res. 2015, 46, 1831–1841. [Google Scholar] [CrossRef]
- Sompayrac, L. How the Immune System Works; Wiley: Hoboken, NJ, USA, 1999; Volume 108. [Google Scholar] [CrossRef]
- Martínez, D.; Díaz-Ibarrola, D.; Vargas-Lagos, C.; Oyarzún, R.; Pontigo, J.P.; Muñoz, J.L.P.; Yáñez, A.J.; Vargas-Chacoff, L. Immunological Response of the Sub-Antarctic Notothenioid Fish Eleginops Maclovinus Injected with Two Strains of Piscirickettsia Salmonis. Fish Shellfish Immunol. 2018, 75, 139–148. [Google Scholar] [CrossRef]
- Adelmann, M.; Köllner, B.; Bergmann, S.M.; Fischer, U.; Lange, B.; Weitschies, W.; Enzmann, P.J.; Fichtner, D. Development of an Oral Vaccine for Immunisation of Rainbow Trout (Oncorhynchus Mykiss) against Viral Haemorrhagic Septicaemia. Vaccine 2008, 26, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Chideroli, R.T.; Amoroso, N.; Mainardi, R.M.; Suphoronski, S.A.; de Padua, S.B.; Alfieri, A.F.; Alfieri, A.A.; Mosela, M.; Moralez, A.T.P.; de Oliveira, A.G.; et al. Emergence of a New Multidrug-Resistant and Highly Virulent Serotype of Streptococcus Agalactiae in Fish Farms from Brazil. Aquaculture 2017, 479, 45–51. [Google Scholar] [CrossRef]
- Giri, S.S.; Sen, S.S.; Jun, J.W.; Sukumaran, V.; Park, S.C. Role of Bacillus Subtilis VSG4-Derived Biosurfactant in Mediating Immune Responses in Labeo Rohita. Fish Shellfish Immunol. 2016, 54, 220–229. [Google Scholar] [CrossRef]
- Secombes, C.J.; Wang, T. The Innate and Adaptive Immune System of Fish; Woodhead Publishing Limited: Cambridge, UK, 2012. [Google Scholar] [CrossRef]
- Chen, W.; Yi, L.; Feng, S.; Zhao, L.; Li, J.; Zhou, M.; Liang, R.; Gu, N.; Wu, Z.; Tu, J.; et al. Characterization of MicroRNAs in Orange-Spotted Grouper (Epinephelus Coioides) Fin Cells upon Red-Spotted Grouper Nervous Necrosis Virus Infection. Fish Shellfish Immunol. 2017, 63, 228–236. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Takizawa, F.; Furihata, M.; Soto-Lampe, V.; Dijkstra, J.M.; Fischer, U. Teleost Cytotoxic T Cells. Fish Shellfish Immunol. 2019, 95, 422–439. [Google Scholar] [CrossRef]
- Sun, Y.; Ding, S.; He, M.; Liu, A.; Long, H.; Guo, W.; Cao, Z.; Xie, Z.; Zhou, Y. Construction and Analysis of the Immune Effect of Vibrio Harveyi Subunit Vaccine and DNA Vaccine Encoding TssJ Antigen. Fish Shellfish Immunol. 2020, 98, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Bao, P.; Sun, X.; Liu, Q.; Zhang, Y.; Liu, X. Synergistic Effect of a Combined Live Vibrio Anguillarum and Edwardsiella Piscicida Vaccine in Turbot. Fish. Shellfish Immunol. 2019, 88, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Chettri, J.K.; Jaafar, R.M.; Skov, J.; Kania, P.W.; Dalsgaard, I.; Buchmann, K. Booster Immersion Vaccination Using Diluted Yersinia Ruckeri Bacterin Confers Protection against ERM in Rainbow Trout. Aquaculture 2015, 440, 1–5. [Google Scholar] [CrossRef]
- Gravningen, K.; Sakai, M.; Mishiba, T.; Fujimoto, T. The Efficacy and Safety of an Oil-Based Vaccine against Photobacterium Damsela Subsp. Piscicida in Yellowtail (Seriola Quinqueradiata): A Field Study. Fish Shellfish Immunol. 2008, 24, 523–529. [Google Scholar] [CrossRef]
- Rømer-Villumsen, K.; Koppang, E.O.; Raida, M.K. Adverse and Long-Term Protective Effects Following Oil-Adjuvanted Vaccination against Aeromonas Salmonicida in Rainbow Trout. Fish Shellfish Immunol. 2015, 42, 193–203. [Google Scholar] [CrossRef]
- Fredriksen, B.N.; Olsen, R.H.; Furevik, A.; Souhoka, R.A.; Gauthier, D.; Brudeseth, B. Efficacy of a Divalent and a Multivalent Water-in-Oil Formulated Vaccine against a Highly Virulent Strain of Flavobacterium Psychrophilum after Intramuscular Challenge of Rainbow Trout (Oncorhynchus Mykiss). Vaccine 2013, 31, 1994–1998. [Google Scholar] [CrossRef]
- SongLin, G.; PanPan, L.; JianJun, F.; JinPing, Z.; Peng, L.; LiHua, D. A Novel Recombinant Bivalent Outer Membrane Protein of Vibrio Vulnificus and Aeromonas Hydrophila as a Vaccine Antigen of American Eel (Anguilla Rostrata). Fish Shellfish Immunol. 2015, 43, 477–484. [Google Scholar] [CrossRef]
- Xing, J.; Li, P.; Tang, X.; Zhan, W. Recombinant Hsp33 and OmpC Protein Can Serve as Promising Divalent Vaccine with Protection against Vibrio Anguillarum and Edwardsiella Tarda in Flounder (Paralichthys Olivaceus). Fish Shellfish Immunol. 2018, 74, 341–348. [Google Scholar] [CrossRef]
- Shoemaker, C.A.; LaFrentz, B.R.; Klesius, P.H. Bivalent Vaccination of Sex Reversed Hybrid Tilapia against Streptococcus Iniae and Vibrio Vulnificus. Aquaculture 2012, 354–355, 45–49. [Google Scholar] [CrossRef]
- Liu, X.; Xu, J.; Zhang, H.; Liu, Q.; Xiao, J.; Zhang, Y. Design and Evaluation of an Edwardsiella Tarda DNA Vaccine Co-Encoding Antigenic and Adjuvant Peptide. Fish Shellfish Immunol. 2016, 59, 189–195. [Google Scholar] [CrossRef]
- Bastardo, A.; Ravelo, C.; Castro, N.; Calheiros, J.; Romalde, J.L. Effectiveness of Bivalent Vaccines against Aeromonas Hydrophila and Lactococcus Garvieae Infections in Rainbow Trout Oncorhynchus Mykiss (Walbaum). Fish. Shellfish Immunol. 2012, 32, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Toranzo, A.E.; Romalde, J.L.; Magariños, B.; Barja, J.L. Present and Future of Aquaculture Vaccines against Fish Bacterial Diseases. Options Méditerr. Sér. A 2009, 86, 155–176. [Google Scholar]


| Bacterial Strain | Description/Genotype | Source | Ref. |
|---|---|---|---|
| Pathogenic V. harveyi strain Vh1 | Wild type, local isolate | Lab collection | [32] |
| Pathogenic A. hydrophila strain Ah1sa5 | Wild type, local isolate | Lab collection | [33] |
| Pathogenic S. agalactiae strain Sa2k | Wild type, local isolate | Lab collection | [30] |
| Primers | Primer Sequence (5’-3’) | Tm (°C) | Expected Size (bp) | Reference |
|---|---|---|---|---|
| 16S rRNA_R | AGAGTTTGATCCTGGCTCAG | 54.0 | 1541 | [34] |
| 16S rRNA_F | GGTTACCTTGTTACGACTT |
| Target | Sequences (5’-3’) | Product Size | Annealing Temperature (˚C) | References | Note |
|---|---|---|---|---|---|
| Dendritic cells | F:AACAGCACACGCTCACTCAC R:CGATCATGTGAGCCTTGAGA | 153 | 60 | Zoccola et al. [50] | Initiate an adaptive immune response |
| C3 | F:GCAATCCTCCACAACTACAG R:ACTCTGACCTCCTGACGATAC | 93 | 59 | Mohd-Shaharuddin et al. [51] | Innate defense against common pathogens |
| CCL4 | F:TCCTCGTCTCACTCTGTCTGT R:GACCTGCCACTGTCTTCAGC | 301 | 60 | Chin et al. [36] | Chemokine attracts innate immune cells |
| MHC I | F:GGCTGTTTTTGCCGCTCTG R:GTGGACAGGTCTGGATAAAG | 112 | 60 | Chin et al. [36] | Molecule-presenting antigen for CD8+ |
| β-actin (control) | F:TACCACCGGTATCGTCATGGA R:CCACGCTCTGTCAGGATCTTC | 126 | 60 | Chin et al. [36] | Reference gene |
| Composition | Feed | |
|---|---|---|
| Control | Vaccinated | |
| Crude protein (%) | 32 ± 7.2 | 33.1 ± 6.0 |
| Crude lipid (%) | 3 ± 1.0 | 4 ± 0.5 |
| Crude fiber (%) | 6.8 ± 2 | 6.2 ± 0.0 |
| Ash (%) | 11.6 ± 1.8 | 10.8 ± 2.11 |
| Moisture (%) | 12 ± 3.3 | 13 ± 1.4 * |
| Carbohydrate (%) | 34.6 ± 7.65 | 32.9 ± 5.0 |
| Parameters | Treatment Group | |
|---|---|---|
| Control Group | Vaccinated Group | |
| Initial body weight (g) | 2.31 ± 0.08 | 2.31 ± 0.08 |
| Final body weight (g) | 9.78 ± 0.05 | 17.5 ± 2.23 * |
| Weight gain (g) | 7.63 ± 1.11 | 14.35 ± 1.09 * |
| SGR (%/day) | 0.36 ± 0.03 | 0.71 ± 0.05 * |
| FCR (g/g) | 0.28 ± 0.06 | 0.16 ± 0.01 * |
| Group | Number of Fish | Primary Vaccination (Day 0–5, Oral, 5% Fish Body Weight) | Booster Dose (Day 14–18, Oral, 5% Fish Body Weight | Challenge Group (10 Fish/Tank in Duplicates) | Challenge Dose/Fish (Day 28, IP, 0.1 mL/Fish) | RPS (%) |
|---|---|---|---|---|---|---|
| Control | 80 | PBS + POA | PBS + POA | Control (+PBS) | PBS | - |
| Control (+Vh) | 107 CFU Vh | - | ||||
| Control (+Sa) | 107 CFU Sa | - | ||||
| Control (+Ah) | 107 CFU Ah | - | ||||
| Vaccinated | 80 | 106 cells/kg of feed + POA | 106 cells/kg of feed + POA | Vaccinated (+PBS) | PBS | - |
| Vaccinated (+Vh) | 107 CFU Vh | 75 ± 7.07 | ||||
| Vaccinated (+Sa) | 107 CFU Sa | 80 ± 0 | ||||
| Vaccinated (+Ah) | 107 CFU Ah | 80 ± 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamad, A.; Zamri-Saad, M.; Amal, M.N.A.; Al-saari, N.; Monir, M.S.; Chin, Y.K.; Md Yasin, I.-S. Vaccine Efficacy of a Newly Developed Feed-Based Whole-Cell Polyvalent Vaccine against Vibriosis, Streptococcosis and Motile Aeromonad Septicemia in Asian Seabass, Lates calcarifer. Vaccines 2021, 9, 368. https://doi.org/10.3390/vaccines9040368
Mohamad A, Zamri-Saad M, Amal MNA, Al-saari N, Monir MS, Chin YK, Md Yasin I-S. Vaccine Efficacy of a Newly Developed Feed-Based Whole-Cell Polyvalent Vaccine against Vibriosis, Streptococcosis and Motile Aeromonad Septicemia in Asian Seabass, Lates calcarifer. Vaccines. 2021; 9(4):368. https://doi.org/10.3390/vaccines9040368
Chicago/Turabian StyleMohamad, Aslah, Mohd Zamri-Saad, Mohammad Noor Azmai Amal, Nurhidayu Al-saari, Md. Shirajum Monir, Yong Kit Chin, and Ina-Salwany Md Yasin. 2021. "Vaccine Efficacy of a Newly Developed Feed-Based Whole-Cell Polyvalent Vaccine against Vibriosis, Streptococcosis and Motile Aeromonad Septicemia in Asian Seabass, Lates calcarifer" Vaccines 9, no. 4: 368. https://doi.org/10.3390/vaccines9040368
APA StyleMohamad, A., Zamri-Saad, M., Amal, M. N. A., Al-saari, N., Monir, M. S., Chin, Y. K., & Md Yasin, I.-S. (2021). Vaccine Efficacy of a Newly Developed Feed-Based Whole-Cell Polyvalent Vaccine against Vibriosis, Streptococcosis and Motile Aeromonad Septicemia in Asian Seabass, Lates calcarifer. Vaccines, 9(4), 368. https://doi.org/10.3390/vaccines9040368







