A Synthetic Peptide CTL Vaccine Targeting Nucleocapsid Confers Protection from SARS-CoV-2 Challenge in Rhesus Macaques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Macaque MHC Class I Typing
2.2. Vaccine Design/Peptide Selection and Manufacture
2.2.1. In Silico Design and Selection of SARS-CoV-2 CTL Epitopes
2.2.2. Microsphere Preparation and Adjuvant Formulation
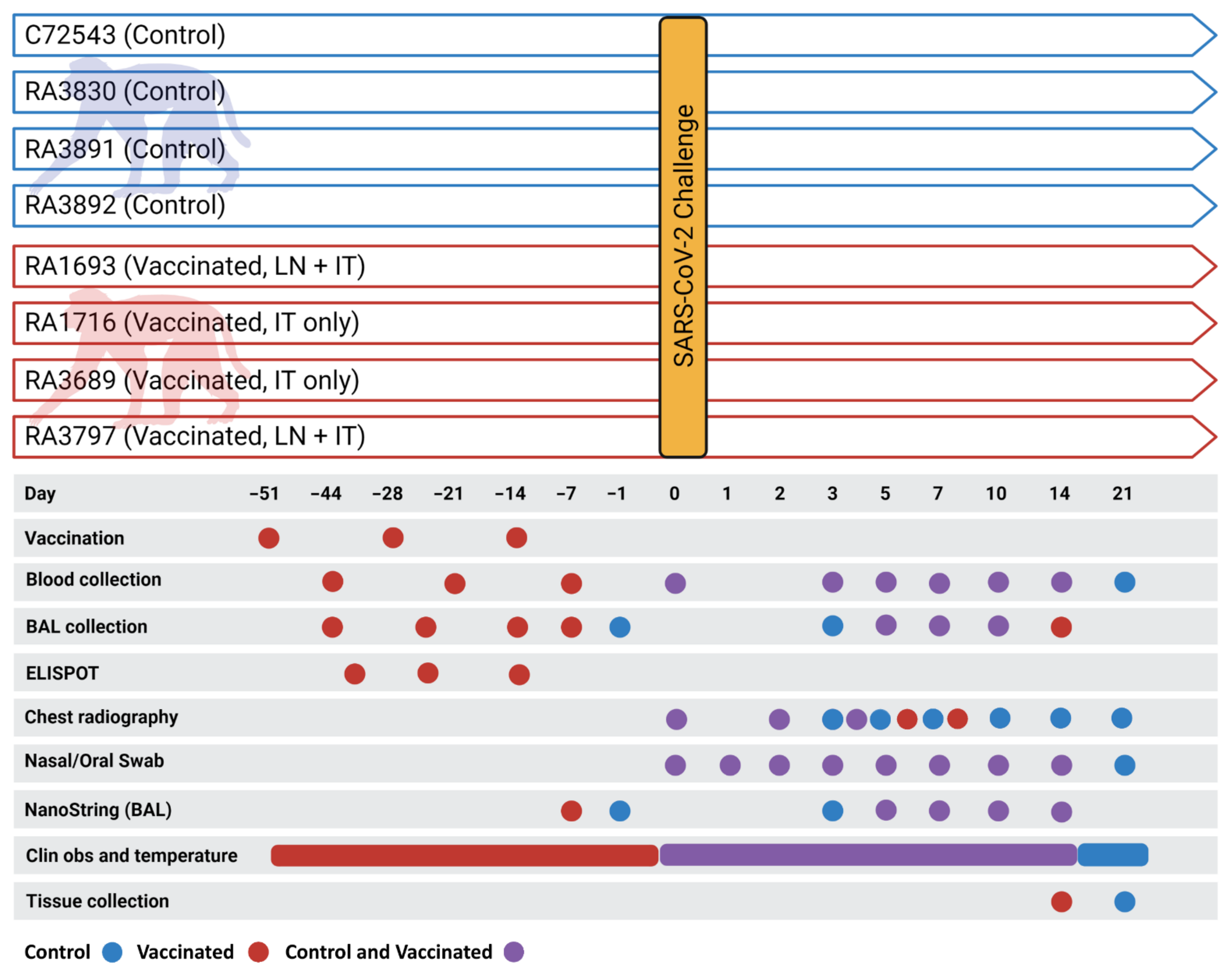
2.3. Animal Studies
2.3.1. Ethics Statement
2.3.2. Macaques
2.4. Immunization, Virus Challenge, Post-Challenge Monitoring and Biosampling
2.4.1. Immunization and ELISPOT Analysis
2.4.2. Virus Challenge
2.4.3. Post-Challenge Monitoring and Chest Radiography
2.4.4. Biosampling
2.5. Viral Load Analysis
2.5.1. Infectious Viral Load (TCID50)
2.5.2. qRT-PCR
2.6. Gene Expression Profiling
2.7. Study Termination
2.8. Statistical Analysis
3. Results
3.1. Primary Clinical Outcome
3.1.1. Clinical Signs, Body Temperature Alterations, and Hematology
3.1.2. Viral Load
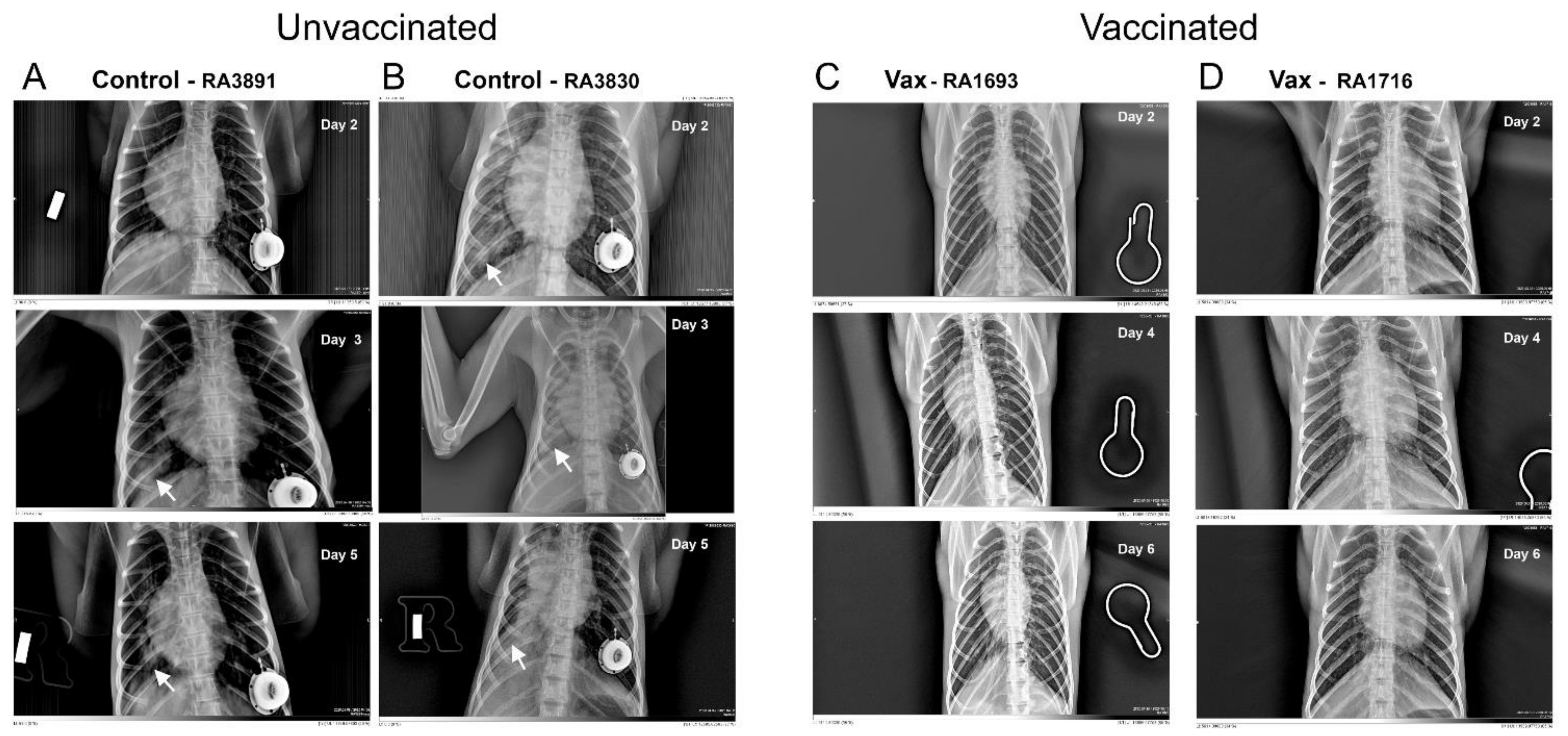
3.1.3. Macaque Chest Radiography
3.2. Secondary Outcomes
Analysis of Gene Expression Patterns in BAL Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADE | antibody mediated disease enhancement |
| APC | antigen-presenting cell |
| BAL | bronchoalveolar lavage |
| CGM | complete growth medium |
| cGMP | current good manufacturing practice |
| CpG | cytosine triphosphate deoxynucleotide-guanine triphosphate deoxynucleotide oligodeoxynucleotide |
| CTL | cytotoxic T lymphocyte |
| DICOM | digital imaging and communications in medicine |
| DMSO | dimethyl sulfoxide |
| EDTA | ethylenediaminetetraacetic acid |
| ELISPOT | enzyme-linked immune absorbent spot assay |
| HLA | human leucocyte antigen |
| IFNγ | interferon gamma |
| IT | intratracheal |
| LLOD | lower limit of detection |
| LN | lymph node |
| MHC | major histocompatibility complex |
| MPLA | monophosphoryl lipid A |
| NHP | non-human primate |
| PBMC | peripheral blood mononuclear cells |
| PLGA | poly-L-lactide-co-glycolide |
| qRT-PCR | real-time quantitative reverse transcription polymerase chain reaction |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| TCID50 | fifty-percent tissue culture infective dose |
| Th1 | T helper cell type 1 |
| Th2 | T helper cell type 2 |
| TLR | toll-like receptor |
| VAERD | vaccine-associated enhanced respiratory disease |
References
- Ritchie, H.; Ortiz-Ospina, E.; Diana, B.; Mathieu, E.; Joe, H.; Bobbie, M.; Charlie, G.; Max, R. Coronavirus (COVID-19) Vaccinations. Available online: https://ourworldindata.org/covid-vaccinations (accessed on 31 January 2021).
- Hoffmann, M.; Arora, P.; Gross, R.; Seidel, A.; Hornich, B.F.; Hahn, A.S.; Kruger, N.; Graichen, L.; Hofmann-Winkler, H.; Kempf, A.; et al. SARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell 2021, 184, 2384–2393.e12. [Google Scholar] [CrossRef]
- Rubsamen, R.; Herst, C.; Lloyd, P.; Heckerman, D. Eliciting cytotoxic T-lymphocyte responses from synthetic vectors containing one or two epitopes in a C57BL/6 mouse model using peptide-containing biodegradable microspheres and adjuvants. Vaccine 2014, 32, 4111–4116. [Google Scholar] [CrossRef] [PubMed]
- Herst, C.; Burkholz, S.; Sidney, J.; Sette, A.; Harris, P.; Massey, S.; Brasel, T.; Cunha-Neto, E.; Rosa, D.; Chao, W.; et al. An effective CTL peptide vaccine for Ebola Zaire Based on Survivors’ CD8+ targeting of a particular nucleocapsid protein epitope with potential implications for COVID-19 vaccine design. Vaccine 2020, 38, 4464–4475. [Google Scholar] [CrossRef] [PubMed]
- Aid, M.; Busman-Sahay, K.; Vidal, S.J.; Maliga, Z.; Bondoc, S.; Starke, C.; Terry, M.; Jacobson, C.A.; Wrijil, L.; Ducat, S.; et al. Vascular Disease and Thrombosis in SARS-CoV-2-Infected Rhesus Macaques. Cell 2020, 183, 1354–1366.e13. [Google Scholar] [CrossRef]
- Blair, R.V.; Vaccari, M.; Doyle-Meyers, L.A.; Roy, C.J.; Russell-Lodrigue, K.; Fahlberg, M.; Monjure, C.J.; Beddingfield, B.; Plante, K.S.; Plante, J.A.; et al. Acute Respiratory Distress in Aged, SARS-CoV-2-Infected African Green Monkeys but Not Rhesus Macaques. Am. J. Pathol. 2020, 191, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Bray, M.; Hatfill, S.; Hensley, L.; Huggins, J. Haematological, Biochemical and Coagulation Changes in Mice, Guinea-pigs and Monkeys Infected with a Mouse-adapted Variant of Ebola Zaire Virus. J. Comp. Pathol. 2001, 125, 243–253. [Google Scholar] [CrossRef] [Green Version]
- Chandrashekar, A.; Liu, J.; Martinot, A.J.; Mcmahan, K.; Mercado, N.B.; Peter, L.; Tostanoski, L.H.; Yu, J.; Maliga, Z.; Nekorchuk, M.; et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science 2020, 369, 812–817. [Google Scholar] [CrossRef]
- Deng, W.; Bao, L.; Liu, J.; Xiao, C.; Liu, J.; Xue, J.; Lv, Q.; Qi, F.; Gao, H.; Yu, P.; et al. Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science 2020, 369, 818–823. [Google Scholar] [CrossRef]
- Feng, L.; Wang, Q.; Shan, C.; Yang, C.; Feng, Y.; Wu, J.; Liu, X.; Zhou, Y.; Jiang, R.; Hu, P.; et al. An adenovirus-vectored COVID-19 vaccine confers protection from SARS-COV-2 challenge in rhesus macaques. Nat. Commun. 2020, 11, 4207. [Google Scholar] [CrossRef]
- McAuliffe, J.; Vogel, L.; Subbarao, K.; Roberts, A.; Fahle, G.; Fischer, S.; Shieh, W.-J.; Butler, E.; Zaki, S.; Claire, M.S.; et al. Replication of SARS coronavirus administered into the respiratory tract of African Green, rhesus and cynomolgus monkeys. Virology 2004, 330, 8–15. [Google Scholar] [CrossRef] [Green Version]
- McMahan, K.; Yu, J.; Mercado, N.B.; Loos, C.; Tostanoski, L.H.; Chandrashekar, A.; Liu, J.; Peter, L.; Atyeo, C.; Zhu, A.; et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2020, 590, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Mercado, N.B.; Zahn, R.; Wegmann, F.; Loos, C.; Chandrashekar, A.; Yu, J.; Liu, J.; Peter, L.; Mcmahan, K.; Tostanoski, L.H.; et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, L.; Yadav, P.D.; Gupta, N.; Mohandas, S.; Patil, D.Y.; Shete-Aich, A.; Panda, S.; Bhargava, B. Comparison of the immunogenicity & protective efficacy of various SARS-CoV-2 vaccine candidates in non-human primates. Indian J. Med. Res. 2020, 153, 93–114. [Google Scholar] [CrossRef]
- Munster, V.J.; Feldmann, F.; Williamson, B.N.; van Doremalen, N.; Perez-Perez, L.; Schulz, J.; Meade-White, K.; Okumura, A.; Callison, J.; Brumbaugh, B.; et al. Respiratory disease and virus shedding in rhesus macaques inoculated with SARS-CoV-2. bioRxiv 2020, 585, 268–272. [Google Scholar] [CrossRef] [Green Version]
- Shan, C.; Yao, Y.-F.; Yang, X.-L.; Zhou, Y.-W.; Gao, G.; Peng, Y.; Yang, L.; Hu, X.; Xiong, J.; Jiang, R.-D.; et al. Infection with novel coronavirus (SARS-CoV-2) causes pneumonia in Rhesus macaques. Cell Res. 2020, 30, 670–677. [Google Scholar] [CrossRef]
- Singh, D.K.; Singh, B.; Ganatra, S.R.; Gazi, M.; Cole, J.; Thippeshappa, R.; Alfson, K.J.; Clemmons, E.; Gonzalez, O.; Escobedo, R.; et al. Responses to acute infection with SARS-CoV-2 in the lungs of rhesus macaques, baboons and marmosets. Nat. Microbiol. 2021, 6, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Song, T.-Z.; Zheng, H.-Y.; Han, J.-B.; Jin, L.; Yang, X.; Liu, F.-L.; Luo, R.-H.; Tian, R.-R.; Cai, H.-R.; Feng, X.-L.; et al. Delayed severe cytokine storm and immune cell infiltration in SARS-CoV-2-infected aged Chinese rhesus macaques. Zool. Res. 2020, 41, 503–516. [Google Scholar] [CrossRef]
- van Doremalen, N.; Lambe, T.; Spencer, A.; Belij-Rammerstorfer, S.; Purushotham, J.N.; Port, J.R.; Avanzato, V.; Bushmaker, T.; Flaxman, A.; Ulaszewska, M.; et al. ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques. bioRxiv 2020, 586, 578–582. [Google Scholar] [CrossRef]
- Yu, P.; Qi, F.; Xu, Y.; Li, F.; Liu, P.; Liu, J.; Bao, L.; Deng, W.; Gao, H.; Xiang, Z.; et al. Age-related rhesus macaque models of COVID-19. Anim. Model. Exp. Med. 2020, 3, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Li, H.; Guo, L.; Liang, Y.; Li, J.; Wang, X.; Hu, Y.; Wang, L.; Liao, Y.; Yang, F.; et al. Virulence and pathogenesis of SARS-CoV-2 infection in rhesus macaques: A nonhuman primate model of COVID-19 progression. PLoS Pathog. 2020, 16, e1008949. [Google Scholar] [CrossRef]
- Corbett, K.S.; Flynn, B.; Foulds, K.E.; Francica, J.R.; Boyoglu-Barnum, S.; Werner, A.P.; Flach, B.; O’Connell, S.; Bock, K.W.; Minai, M.; et al. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N. Engl. J. Med. 2020, 383, 1544–1555. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Bao, L.; Mao, H.; Wang, L.; Xu, K.; Yang, M.; Li, Y.; Zhu, L.; Wang, N.; Lv, Z.; et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020, 369, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Guebre-Xabier, M.; Patel, N.; Tian, J.-H.; Zhou, B.; Maciejewski, S.; Lam, K.; Portnoff, A.D.; Massare, M.J.; Frieman, M.B.; Piedra, P.A.; et al. NVX-CoV2373 vaccine protects cynomolgus macaque upper and lower airways against SARS-CoV-2 challenge. Vaccine 2020, 38, 7892–7896. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.-N.; Li, X.-F.; Deng, Y.-Q.; Zhao, H.; Huang, Y.-J.; Yang, G.; Huang, W.-J.; Gao, P.; Zhou, C.; Zhang, R.-R.; et al. A Thermostable mRNA Vaccine against COVID-19. Cell 2020, 182, 1271–1283.e16. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Huang, B.; Deng, W.; Quan, Y.; Wang, W.; Xu, W.; Zhao, Y.; Li, N.; Zhang, J.; et al. Development of an Inactivated Vaccine Candidate, BBIBP-CorV, with Potent Protection against SARS-CoV-2. Cell 2020, 182, 713–721.e9. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Tostanoski, L.H.; Peter, L.; Mercado, N.B.; Mcmahan, K.; Mahrokhian, S.H.; Nkolola, J.P.; Liu, J.; Li, Z.; Chandrashekar, A.; et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science 2020, 369, 806–811. [Google Scholar] [CrossRef]
- Peng, H.; Yang, L.-T.; Wang, L.-Y.; Li, J.; Huang, J.; Lu, Z.-Q.; Koup, R.A.; Bailer, R.T.; Wu, C.-Y. Long-lived memory T lymphocyte responses against SARS coronavirus nucleocapsid protein in SARS-recovered patients. Virology 2006, 351, 466–475. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Mentzer, A.J.; Liu, G.; Yao, X.; Yin, Z.; Dong, D.; Dejnirattisai, W.; Rostron, T.; Supasa, P.; Liu, C.; et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat. Immunol. 2020, 21, 1336–1345. [Google Scholar] [CrossRef]
- Kared, H.; Redd, A.D.; Bloch, E.M.; Bonny, T.S.; Sumatoh, H.R.; Kairi, F.; Carbajo, D.; Abel, B.; Newell, E.W.; Bettinotti, M.; et al. SARS-CoV-2-specific CD8+ T cell responses in convalescent COVID-19 individuals. J. Clin. Investig. 2021, 131, e145476. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef]
- Ravi, R.K.; Walton, K.; Khosroheidari, M. MiSeq: A Next Generation Sequencing Platform for Genomic Analysis. Methods Mol. Biol. 2018, 1706, 223–232. [Google Scholar] [CrossRef]
- Grifoni, A.; Sidney, J.; Zhang, Y.; Scheuermann, R.H.; Peters, B.; Sette, A. A Sequence Homology and Bioinformatic Approach Can Predict Candidate Targets for Immune Responses to SARS-CoV-2. Cell Host Microbe 2020, 27, 671–680.e2. [Google Scholar] [CrossRef] [PubMed]
- Dimonte, S.; Babakir-Mina, M.; Hama-Soor, T.; Ali, S. Genetic Variation and Evolution of the 2019 Novel Coronavirus. Public Health Genom. 2021, 10, 1–13. [Google Scholar] [CrossRef]
- Almazάn, F.; Galάn, C.; Enjuanes, L. The Nucleoprotein Is Required for Efficient Coronavirus Genome Replication. J. Virol. 2004, 78, 12683–12688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vita, R.; Mahajan, S.; Overton, J.; Dhanda, S.K.; Martini, S.; Cantrell, J.R.; Wheeler, D.K.; Sette, A.; Peters, B. The Immune Epitope Database (IEDB): 2018 update. Nucleic Acids Res. 2019, 47, D339–D343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurtz, V.I.; Paul, S.; Andreatta, M.; Marcatili, P.; Peters, B.; Nielsen, M. NetMHCpan-4.0: Improved Peptide–MHC Class I Interaction Predictions Integrating Eluted Ligand and Peptide Binding Affinity Data. J. Immunol. 2017, 199, 3360–3368. [Google Scholar] [CrossRef]
- Qin, E.; Zhu, Q.; Yu, M.; Fan, B.; Chang, G.; Si, B.; Yang, B.; Peng, W.; Jiang, T.; Liu, B.; et al. A complete sequence and comparative analysis of a SARS-associated virus (Isolate BJ01). Chin. Sci. Bull. 2003, 48, 941–948. [Google Scholar] [CrossRef]
- Poran, A.; Harjanto, D.; Gaynor, R.B.; Malloy, M.; Arieta, C.M.; Rothenberg, D.A.; Lenkala, D.; Van Buuren, M.M.; Addona, T.A.; Rooney, M.S.; et al. Sequence-based prediction of SARS-CoV-2 vaccine targets using a mass spectrometry-based bioinformatics predictor identifies immunogenic T cell epitopes. Genome Med. 2020, 12, 70. [Google Scholar] [CrossRef]
- Mothé, B.R.; Southwood, S.; Sidney, J.; English, A.M.; Wriston, A.; Hoof, I.; Shabanowitz, J.; Hunt, D.F.; Sette, A. Peptide-binding motifs associated with MHC molecules common in Chinese rhesus macaques are analogous to those of human HLA supertypes and include HLA-B27-like alleles. Immunogenetics 2013, 65, 371–386. [Google Scholar] [CrossRef]
- Cunha-Neto, E.; Rosa, D.S.; Harris, P.E.; Olson, T.; Morrow, A.; Ciotlos, S.; Herst, C.V.; Rubsamen, R.M. An Approach for a Synthetic CTL Vaccine Design against Zika Flavivirus Using Class I and Class II Epitopes Identified by Computer Modeling. Front. Immunol. 2017, 8, 640. [Google Scholar] [CrossRef]
- Brining, D.L.; Mattoon, J.S.; Kercher, L.; LaCasse, R.; Safronetz, D.; Feldmann, H.; Parnell, M.J. Thoracic Radiography as a Refinement Methodology for the Study of H1N1 Influenza in Cynomologus Macaques (Macaca fascicularis). Comp. Med. 2010, 60, 389–395. [Google Scholar] [PubMed]
- Ramakrishnan, M.A. Determination of 50% endpoint titer using a simple formula. World J. Virol. 2016, 5, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, Y.; Benjamini, Y. More powerful procedures for multiple significance testing. Stat. Med. 1990, 9, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Finch, C.L.; Crozier, I.; Lee, J.H.; Byrum, R.; Cooper, T.K.; Liang, J.; Sharer, K.; Solomon, J.; Sayre, P.J.; Kocher, G.; et al. Characteristic and quantifiable COVID-19-like abnormalities in CT- and PET/CT-imaged lungs of SARS-CoV-2-infected crab-eating macaques (Macaca fascicularis). bioRxiv 2020, 14. [Google Scholar] [CrossRef]
- Lu, S.; Zhao, Y.; Yu, W.; Yang, Y.; Gao, J.; Wang, J.; Kuang, D.; Yang, M.; Yang, J.; Ma, C.; et al. Comparison of nonhuman primates identified the suitable model for COVID-19. Signal Transduct. Target. Ther. 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Xie, L.; Zhou, Q.; Liu, S.; Wu, Q.; Ji, Y.; Zhang, L.; Xu, F.; Gong, W.; Melgiri, N.D.; Xie, P. Normal Thoracic Radiographic Appearance of the Cynomolgus Monkey (Macaca fascicularis). PLoS ONE 2014, 9, e84599. [Google Scholar] [CrossRef]
- Silverman, S.; Morgan, J.P. Thoracic radiography of the normal rhesus macaque (Macaca mulatta). Am. J. Veter. Res. 1980, 41, 1704–1719. [Google Scholar]
- Singh, D.K.; Ganatra, S.R.; Singh, B.; Cole, J.; Alfson, K.J.; Clemmons, E.; Gazi, M.; Gonzalez, O.; Escobedo, R.; Lee, T.-H.; et al. SARS-CoV-2 infection leads to acute infection with dynamic cellular and inflammatory flux in the lung that varies across nonhuman primate species. bioRxiv 2020, 136481. [Google Scholar] [CrossRef]
- Maaskant, A.; Meijer, L.; Bakker, J.; van Geest, L.; Zijlmans, D.G.; Langermans, J.A.; Stammes, M.A. Bronchoalveolar lavage affects thorax computed tomography of healthy and SARS-CoV-2 infected rhesus macaques (Macaca mulatta). bioRxiv 2021. Preprint. [Google Scholar] [CrossRef]
- Rabenstein, H.; Behrendt, A.C.; Ellwart, J.W.; Naumann, R.; Horsch, M.; Beckers, J.; Obst, R. Differential Kinetics of Antigen Dependency of CD4+ and CD8+ T Cells. J. Immunol. 2014, 192, 3507–3517. [Google Scholar] [CrossRef] [Green Version]
- Trobaugh, D.W.; Yang, L.; Ennis, F.A.; Green, S. Altered effector functions of virus-specific and virus cross-reactive CD8 + T cells in mice immunized with related flaviviruses. Eur. J. Immunol. 2010, 40, 1315–1327. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Spoor, M.S.; Gerth, A.J.; Brody, S.L.; Peng, S.L. Modulation of Th1 Activation and Inflammation by the NF-B Repressor Foxj1. Science 2004, 303, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, A.M.; Higgins, M.; Holguin, F.; Brown, L.A.S.; Teague, W.G.; National Institutes of Health/National Heart; Blood Institute’s Severe Asthma Research. The molecular phenotype of severe asthma in children. J. Allergy Clin. Immunol. 2010, 125, 851–857.e18. [Google Scholar] [CrossRef] [Green Version]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Karlsson, M.J.; Zhong, W.; Tebani, A.; Pou, C.; Mikes, J.; Lakshmikanth, T.; Forsström, B.; Edfors, F.; Odeberg, J.; et al. A genome-wide transcriptomic analysis of protein-coding genes in human blood cells. Science 2019, 366, eaax9198. [Google Scholar] [CrossRef] [PubMed]
- Amati, F.; Vancheri, C.; Latini, A.; Colona, V.L.; Grelli, S.; D’Apice, M.R.; Balestrieri, E.; Passarelli, C.; Minutolo, A.; Loddo, S.; et al. Expression profiles of the SARS-CoV-2 host invasion genes in nasopharyngeal and oropharyngeal swabs of COVID-19 patients. Heliyon 2020, 6, e05143. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.S.; Mou, H.; Smits, S.L.; Dekkers, D.H.W.; Müller, M.A.; Dijkman, R.; Muth, D.; Demmers, J.A.A.; Zaki, A.; Fouchier, R.A.M.; et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 2013, 495, 251–254. [Google Scholar] [CrossRef] [Green Version]
- Salyer, A.C.D.; David, S.A. Transcriptomal signatures of vaccine adjuvants and accessory immunostimulation of sentinel cells by toll-like receptor 2/6 agonists. Hum. Vaccines Immunother. 2018, 14, 1686–1696. [Google Scholar] [CrossRef] [Green Version]
- Olafsdottir, T.; Lindqvist, M.; Harandi, A.M. Molecular signatures of vaccine adjuvants. Vaccine 2015, 33, 5302–5307. [Google Scholar] [CrossRef] [Green Version]
- Mosca, F.; Tritto, E.; Muzzi, A.; Monaci, E.; Bagnoli, F.; Iavarone, C.; O’Hagan, D.; Rappuoli, R.; De Gregorio, E. Molecular and cellular signatures of human vaccine adjuvants. Proc. Natl. Acad. Sci. USA 2008, 105, 10501–10506. [Google Scholar] [CrossRef] [Green Version]
- Lampe, A.T.; Puniya, B.L.; Pannier, A.K.; Helikar, T.; Brown, D.M. Combined TLR4 and TLR9 agonists induce distinct phenotypic changes in innate immunity in vitro and in vivo. Cell. Immunol. 2020, 355, 104149. [Google Scholar] [CrossRef]
- An, H.; Xu, H.; Yu, Y.; Zhang, M.; Qi, R.; Yan, X.; Liu, S.; Wang, W.; Guo, Z.; Qin, Z.; et al. Up-regulation of TLR9 gene expression by LPS in mouse macrophages via activation of NF-κB, ERK and p38 MAPK signal pathways. Immunol. Lett. 2002, 81, 165–169. [Google Scholar] [CrossRef]
- Fahlberg, M.D.; Blair, R.V.; Threeton, B.M.; Golden, N.; Datta, P.K.; Roy, C.J.; Bohm, R.P.; Maness, N.J.; Fischer, T.; Rappaport, J.; et al. Cellular events of acute, resolving or progressive COVID-19 in SARS-CoV-2 infected non-human primates. Nat. Commun. 2020, 11, 6078. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, N.; Bonilla, R.G.; Jacobson, R.M.; O’Kane, D.; Poland, G.A. Differential HLA Gene Expression in Measles Vaccine Seropositive and Seronegative Subjects: A Pilot Study. Scand. J. Infect. Dis. 2003, 35, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Tjan, L.H.; Furukawa, K.; Nagano, T.; Kiriu, T.; Nishimura, M.; Arii, J.; Hino, Y.; Iwata, S.; Nishimura, Y.; Mori, Y. Early differences in cytokine production distinguish severity of COVID-19. J. Infect. Dis. 2021, 223, 1145–1149. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Gresh, L.; Halloran, M.E.; Mercado, J.C.; Kuan, G.; Gordon, A.; Balmaseda, A.; Harris, E. Antibody-dependent enhancement of severe dengue disease in humans. Science 2017, 358, 929–932. [Google Scholar] [CrossRef] [Green Version]
- Chin, J.; Magoffin, R.L.; Shearer, L.A.; Schieble, J.H.; Lennette, E.H. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am. J. Epidemiol. 1969, 89, 449–463. [Google Scholar] [CrossRef]
- Kim, H.W.; Canchola, J.G.; Brandt, C.D.; Pyles, G.; Chanock, R.M.; Jensen, K.; Parrott, R.H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 1969, 89, 422–434. [Google Scholar] [CrossRef]
- Rauh, L.W.; Schmidt, R. Measles Immunization with Killed Virus Vaccine. Serum Antibody Titers and Experience with Exposure to Measles Epidemic. Am. J. Dis. Child. 1965, 109, 232–237. [Google Scholar] [CrossRef]
- Georas, S.N.; Guo, J.; De Fanis, U.; Casolaro, V. T-helper cell type-2 regulation in allergic disease. Eur. Respir. J. 2005, 26, 1119–1137. [Google Scholar] [CrossRef]
- Salguero, F.J.; White, A.D.; Slack, G.S.; Fotheringham, S.A.; Bewley, K.R.; Gooch, K.E.; Longet, S.; Humphries, H.E.; Watson, R.J.; Hunter, L.; et al. Comparison of rhesus and cynomolgus macaques as an infection model for COVID-19. Nat. Commun. 2021, 12, 1260. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatullin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G.; et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. Lancet 2020, 396, 887–897. [Google Scholar] [CrossRef]
- Zhu, F.-C.; Guan, X.-H.; Li, Y.-H.; Huang, J.-Y.; Jiang, T.; Hou, L.-H.; Li, J.-X.; Yang, B.-F.; Wang, L.; Wang, W.-J.; et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Rakhra, K.; Abraham, W.; Wang, C.; Moynihan, K.D.; Li, N.; Donahue, N.; Baldeon, A.D.; Irvine, D.J. Exploiting albumin as a mucosal vaccine chaperone for robust generation of lung-resident memory T cells. Sci. Immunol. 2021, 6, eabd8003. [Google Scholar] [CrossRef] [PubMed]
- van Doremalen, N.; Purushotham, J.; Schulz, J.; Holbrook, M.; Bushmaker, T.; Carmody, A.; Port, J.; Yinda, K.C.; Okumura, A.; Saturday, G.; et al. Intranasal ChAdOx1 nCoV-19/AZD1222 vaccination reduces shedding of SARS-CoV-2 D614G in rhesus macaques. bioRxiv 2021, 426058. [Google Scholar] [CrossRef]
- Bui, H.-H.; Sidney, J.; Dinh, K.; Southwood, S.; Newman, M.J.; Sette, A. Predicting population coverage of T-cell epitope-based diagnostics and vaccines. BMC Bioinform. 2006, 7, 153. [Google Scholar] [CrossRef] [Green Version]
- Fukami-Kobayashi, K.; Shiina, T.; Anzai, T.; Sano, K.; Yamazaki, M.; Inoko, H.; Tateno, Y. Genomic evolution of MHC class I region in primates. Proc. Natl. Acad. Sci. USA 2005, 102, 9230–9234. [Google Scholar] [CrossRef] [Green Version]




| Upregulated Transcripts on Days 5 and 7 Post-Challenge 1 | |||||
|---|---|---|---|---|---|
| Gene ID | Function | Distribution | Gene ID | Function | Distribution |
| Mamu MHC1 A | Antigen presentation to CD8+ T cells1 | Low cell-type specificity 1 | CD8 | Coreceptor for TCR binding to MHC C1 | T cells |
| Mamu MHC1 B | Antigen presentation to CD8+ T cells | Low cell-type specificity | IL2 | Differentiation/maturation of T cells | CD4+ and CD8+ T cells |
| TAPBP | MHC class I antigen presentation | Low cell-type specificity | CD81 | Costimulatory signal with CD3 | Low cell-type specificity |
| HLA-DRA 2 | Antigen presentation to CD4+ T cells | Professional APC | CD9 | Cell adhesion, recognized by CD81 | Low cell-type specificity |
| HLA-DQA1 2 | Antigen presentation to CD4+ T cells | Professional APC | CD59 | Inhibitor of the complement membrane attack complex | Low cell-type specificity |
| HLA-DQB1 2 | Antigen presentation to CD4+ T cells | Professional APC | CD24 | Cell adhesion molecule | Eosinophils and B cells |
| CD74 | MHC class II antigen presentation | Professional APC | CD47 | High affinity receptor for thrombospondin-1 | Low cell-type specificity |
| HLA-DMA | MHC class II antigen presentation | Professional APC | CD58 | Ligand of the T-lymphocyte CD2 glycoprotein | Low cell-type specificity |
| HLA-DMB | MHC class II antigen presentation | Professional APC | CD164 | Facilitates adhesion of CD34+ cells | Low cell-type specificity |
| Upregulated Transcripts on Days 5 or 7 Post-Challenge | Upregulated Transcripts on Day 10 Post-Challenge | ||||
| IL17 B | Proinflamatory cytokine | Low cell-type specificity | IL6R | Low affinity receptor for Interleukin 6 | Neutrophil |
| CX3CL1 | Chemotactic for T cells and monocytes | Low cell-type specificity | ABL1 | Tyrosine-protein kinase, role cell growth and survival | Low cell-type specificity |
| CD99 | Facilitates T-cell adhesion | Low cell-type specificity | TYK2 | Tyrosine-protein kinase, initiation of type I IFN signaling | Low cell-type specificity |
| Downregulated Transcripts on Days 5 and 7 Post-Challenge 1 | |||||
|---|---|---|---|---|---|
| Gene ID | Function | Distribution | Gene ID | Function | Distribution |
| IFNA2 | Inhibition of viral replication | Macrophages, eosinophils | CD28 | Provides costimulatory signals required for T-cell activation Receptor for CD80 | T cell |
| CCR1 | C-C chemokine receptor, recruitment of immune effector cells | Macrophages | IL1RAP | Coreceptor with IL1R1 in the IL-1 signaling system | Neutrophil |
| CD274 | Ligand of PD-1 (PDL1), inhibits expansion of antigen-specific CD8+ T cells and CD4+ helper cells | Monocytes, granulocytes | IL1R2 | Decoy receptor for IL1α and IL1β (IL1B) inhibiting signaling | Macrophage, neutrophils |
| Downregulated Transcripts on Days 5 or 7 Post-Challenge | Downregulated Transcripts on Day 10 Post-Challenge | ||||
| CD80 | Receptor for CD28 and CTLA-4 on T cells | B cells and monocytes, APCs | HLA-DRA2 | Antigen presentation to CD4+ T cells | Professional APC |
| IFNGR2 | β chain of the gamma interferon receptor | B cells, APCs and neutrophils | HLA-DMA | Antigen presentation to CD4+ T cells | Professional APC |
| IL8 | C-X-C chemokine for recruitment of neutrophils | Macrophages, epithelial and endothelial cells | LY96 | Confers responsiveness to LPS | Macrophages |
| IL21 | Regulates proliferation of mature B and T cells in response to activating stimuli | Activated CD4+ T cells, NKT cells | CTSC | Cathepsin protease | Macrophages |
| DPP4 | Protease upregulated in SARS-CoV-2 [57] Possible viral entry receptor [58] | T-cell CD2 | TyroBP | Mediates NK cell activation | Macrophages, monocytes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris, P.E.; Brasel, T.; Massey, C.; Herst, C.V.; Burkholz, S.; Lloyd, P.; Blankenberg, T.; Bey, T.M.; Carback, R.; Hodge, T.; et al. A Synthetic Peptide CTL Vaccine Targeting Nucleocapsid Confers Protection from SARS-CoV-2 Challenge in Rhesus Macaques. Vaccines 2021, 9, 520. https://doi.org/10.3390/vaccines9050520
Harris PE, Brasel T, Massey C, Herst CV, Burkholz S, Lloyd P, Blankenberg T, Bey TM, Carback R, Hodge T, et al. A Synthetic Peptide CTL Vaccine Targeting Nucleocapsid Confers Protection from SARS-CoV-2 Challenge in Rhesus Macaques. Vaccines. 2021; 9(5):520. https://doi.org/10.3390/vaccines9050520
Chicago/Turabian StyleHarris, Paul E., Trevor Brasel, Christopher Massey, C. V. Herst, Scott Burkholz, Peter Lloyd, Tikoes Blankenberg, Thomas M. Bey, Richard Carback, Thomas Hodge, and et al. 2021. "A Synthetic Peptide CTL Vaccine Targeting Nucleocapsid Confers Protection from SARS-CoV-2 Challenge in Rhesus Macaques" Vaccines 9, no. 5: 520. https://doi.org/10.3390/vaccines9050520
APA StyleHarris, P. E., Brasel, T., Massey, C., Herst, C. V., Burkholz, S., Lloyd, P., Blankenberg, T., Bey, T. M., Carback, R., Hodge, T., Ciotlos, S., Wang, L., Comer, J. E., & Rubsamen, R. M. (2021). A Synthetic Peptide CTL Vaccine Targeting Nucleocapsid Confers Protection from SARS-CoV-2 Challenge in Rhesus Macaques. Vaccines, 9(5), 520. https://doi.org/10.3390/vaccines9050520






