The Evolving Role of Immune Checkpoint Inhibitors in Hepatocellular Carcinoma Treatment
Abstract
1. Introduction
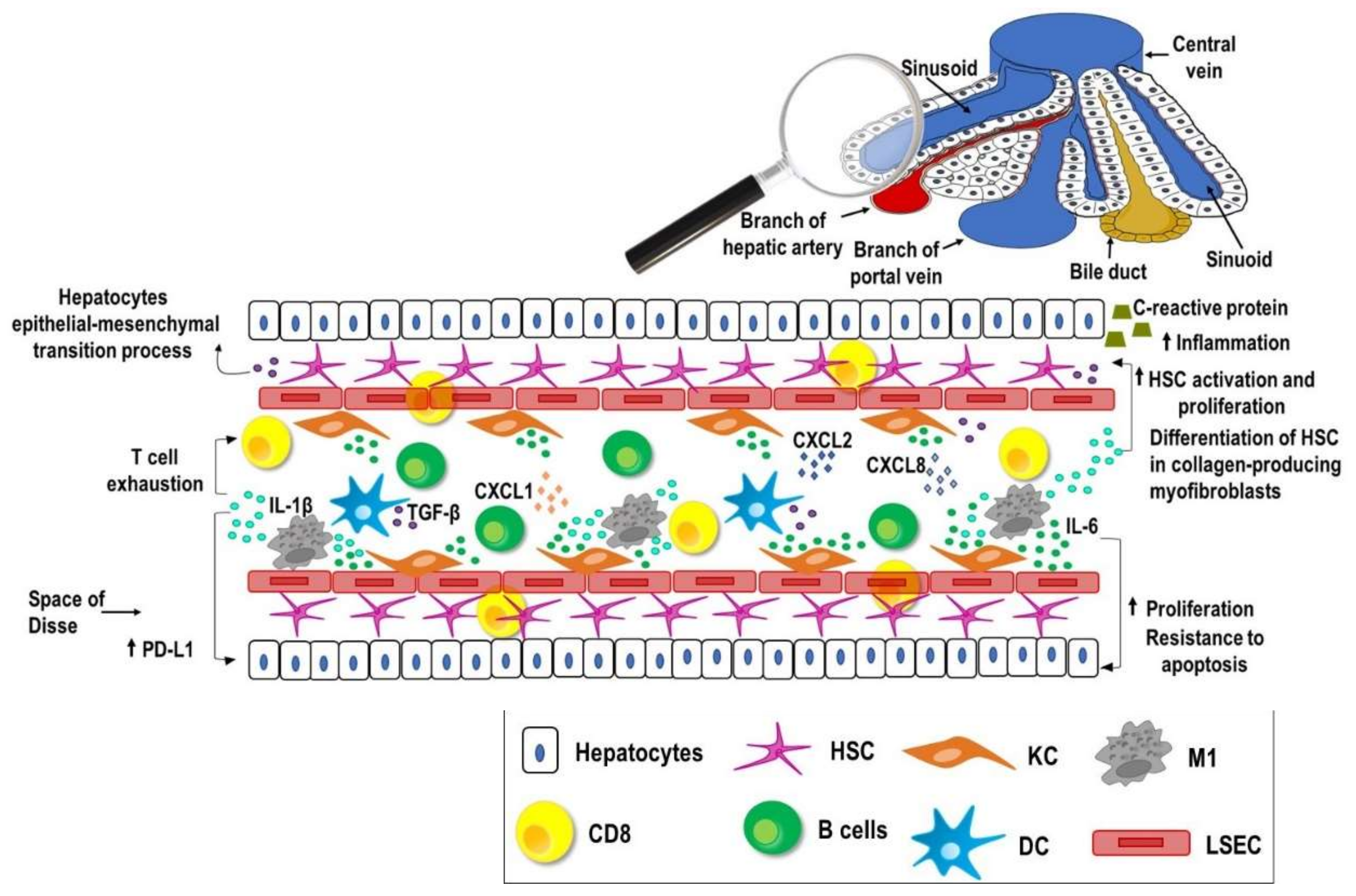
2. Liver, Inflammation and Cancer
3. Immune Checkpoints
4. Immune Checkpoint Inhibitors
4.1. Monotherapy
4.2. Combination Therapy
4.2.1. Combinations of Two Immune Checkpoint Inhibitors
4.2.2. Combination of Immune Checkpoint Inhibitors with Inhibitors of Angiogenesis
4.2.3. Combination of Immune Checkpoint Inhibitors with Locoregional Therapies
4.2.4. Combination of Immune Checkpoint Inhibitors with Chemotherapies
5. Side Effects
6. Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Llovet, J.M.; Zucman-Rossi, J.; Pikarsky, E.; Sangro, B.; Schwartz, M.; Sherman, M.; Gores, G. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2016, 2, 16018. [Google Scholar] [CrossRef] [PubMed]
- Natri, H.M.; Wilson, M.A.; Buetow, K.H. Distinct molecular etiologies of male and female hepatocellular carcinoma. BMC Cancer 2019, 19, 951. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Dimitroulis, D.; Damaskos, C.; Valsami, S.; Davakis, S.; Garmpis, N.; Spartalis, E.; Athanasiou, A.; Moris, D.; Sakellariou, S.; Kykalos, S.; et al. From diagnosis to treatment of hepatocellular carcinoma: An epidemic problem for both developed and developing world. World J. Gastroenterol. 2017, 23, 5282–5294. [Google Scholar] [CrossRef]
- Rossetto, A.; De Re, V.; Steffan, A.; Ravaioli, M.; Miolo, G.; Leone, P.; Racanelli, V.; Uzzau, A.; Baccarani, U.; Cescon, M. Carcinogenesis and Metastasis in Liver: Cell Physiological Basis. Cancers 2019, 11, 1731. [Google Scholar] [CrossRef]
- Sung, P.S.; Racanelli, V.; Shin, E.C. CD8(+) T-Cell Responses in Acute Hepatitis C Virus Infection. Front. Immunol 2014, 5, 266. [Google Scholar] [CrossRef]
- Ramakrishna, G.; Rastogi, A.; Trehanpati, N.; Sen, B.; Khosla, R.; Sarin, S.K. From cirrhosis to hepatocellular carcinoma: New molecular insights on inflammation and cellular senescence. Liver Cancer 2013, 2, 367–383. [Google Scholar] [CrossRef]
- Cardoso, H.; Vale, A.M.; Rodrigues, S.; Goncalves, R.; Albuquerque, A.; Pereira, P.; Lopes, S.; Silva, M.; Andrade, P.; Morais, R.; et al. High incidence of hepatocellular carcinoma following successful interferon-free antiviral therapy for hepatitis C associated cirrhosis. J. Hepatol. 2016, 65, 1070–1071. [Google Scholar] [CrossRef]
- Conti, F.; Buonfiglioli, F.; Scuteri, A.; Crespi, C.; Bolondi, L.; Caraceni, P.; Foschi, F.G.; Lenzi, M.; Mazzella, G.; Verucchi, G.; et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J. Hepatol 2016, 65, 727–733. [Google Scholar] [CrossRef]
- Reig, M.; Marino, Z.; Perello, C.; Inarrairaegui, M.; Ribeiro, A.; Lens, S.; Diaz, A.; Vilana, R.; Darnell, A.; Varela, M.; et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J. Hepatol. 2016, 65, 719–726. [Google Scholar] [CrossRef]
- Meringer, H.; Shibolet, O.; Deutsch, L. Hepatocellular carcinoma in the post-hepatitis C virus era: Should we change the paradigm? World J. Gastroenterol. 2019, 25, 3929–3940. [Google Scholar] [CrossRef]
- Schutte, K.; Schulz, C.; Poranzke, J.; Antweiler, K.; Bornschein, J.; Bretschneider, T.; Arend, J.; Ricke, J.; Malfertheiner, P. Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non-cirrhotic liver. BMC Gastroenterol. 2014, 14, 117. [Google Scholar] [CrossRef]
- Eggert, T.; Greten, T.F. Tumor regulation of the tissue environment in the liver. Pharmacol. Ther. 2017, 173, 47–57. [Google Scholar] [CrossRef]
- Jiang, Y.; Han, Q.J.; Zhang, J. Hepatocellular carcinoma: Mechanisms of progression and immunotherapy. World J. Gastroenterol. 2019, 25, 3151–3167. [Google Scholar] [CrossRef]
- Benson, A.B., 3rd; D’Angelica, M.I.; Abbott, D.E.; Abrams, T.A.; Alberts, S.R.; Saenz, D.A.; Are, C.; Brown, D.B.; Chang, D.T.; Covey, A.M.; et al. NCCN Guidelines Insights: Hepatobiliary Cancers, Version 1.2017. J. Natl. Compr. Canc. Netw. 2017, 15, 563–573. [Google Scholar] [CrossRef]
- Forner, A.; Llovet, J.M.; Bruix, J. Hepatocellular carcinoma. Lancet 2012, 379, 1245–1255. [Google Scholar] [CrossRef]
- Richani, M.; Kolly, P.; Knoepfli, M.; Herrmann, E.; Zweifel, M.; von Tengg-Kobligk, H.; Candinas, D.; Dufour, J.F. Treatment allocation in hepatocellular carcinoma: Assessment of the BCLC algorithm. Ann. Hepatol. 2016, 15, 82–90. [Google Scholar] [CrossRef]
- Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.M.; Meyer, T.; Nault, J.C.; Neumann, U.; Ricke, J.; Sangro, B.; et al. Correction to: "Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up". Ann. Oncol. 2019, 30, 871–873. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Garmpis, N.; Damaskos, C.; Garmpi, A.; Georgakopoulou, V.E.; Sarantis, P.; Antoniou, E.A.; Karamouzis, M.V.; Nonni, A.; Schizas, D.; Diamantis, E.; et al. Histone Deacetylase Inhibitors in the Treatment of Hepatocellular Carcinoma: Current Evidence and Future Opportunities. J. Pers. Med. 2021, 11, 223. [Google Scholar] [CrossRef]
- Bruix, J.; Reig, M.; Sherman, M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology 2016, 150, 835–853. [Google Scholar] [CrossRef] [PubMed]
- Cast, A.E.; Walter, T.J.; Huppert, S.S. Vascular patterning sets the stage for macro and micro hepatic architecture. Dev. Dyn. 2015, 244, 497–506. [Google Scholar] [CrossRef]
- Racanelli, V.; Rehermann, B. The liver as an immunological organ. Hepatology 2006, 43, S54–S62. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.W.; Harmon, C.; O’Farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell Mol. Immunol. 2016, 13, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Maeda, S. Inflammation- and stress-related signaling pathways in hepatocarcinogenesis. World J. Gastroenterol. 2012, 18, 4071–4081. [Google Scholar] [CrossRef] [PubMed]
- Berasain, C.; Castillo, J.; Prieto, J.; Avila, M.A. New molecular targets for hepatocellular carcinoma: The ErbB1 signaling system. Liver Int. 2007, 27, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A. The role of inflammation and liver cancer. Adv. Exp. Med. Biol 2014, 816, 401–435. [Google Scholar] [CrossRef] [PubMed]
- Kitaoka, S.; Shiota, G.; Kawasaki, H. Serum levels of interleukin-10, interleukin-12 and soluble interleukin-2 receptor in chronic liver disease type C. Hepatogastroenterology 2003, 50, 1569–1574. [Google Scholar]
- Nakagawa, H.; Maeda, S.; Yoshida, H.; Tateishi, R.; Masuzaki, R.; Ohki, T.; Hayakawa, Y.; Kinoshita, H.; Yamakado, M.; Kato, N.; et al. Serum IL-6 levels and the risk for hepatocarcinogenesis in chronic hepatitis C patients: An analysis based on gender differences. Int. J. Cancer 2009, 125, 2264–2269. [Google Scholar] [CrossRef]
- Porta, C.; De Amici, M.; Quaglini, S.; Paglino, C.; Tagliani, F.; Boncimino, A.; Moratti, R.; Corazza, G.R. Circulating interleukin-6 as a tumor marker for hepatocellular carcinoma. Ann. Oncol. 2008, 19, 353–358. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, F.; Lu, T.; Duan, Z.; Zhang, Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat. Rev. 2012, 38, 904–910. [Google Scholar] [CrossRef]
- Weinhold, B.; Ruther, U. Interleukin-6-dependent and -independent regulation of the human C-reactive protein gene. Biochem J. 1997, 327 Pt 2, 425–429. [Google Scholar] [CrossRef]
- Han, Y.P.; Zhou, L.; Wang, J.; Xiong, S.; Garner, W.L.; French, S.W.; Tsukamoto, H. Essential role of matrix metalloproteinases in interleukin-1-induced myofibroblastic activation of hepatic stellate cell in collagen. J. Biol. Chem. 2004, 279, 4820–4828. [Google Scholar] [CrossRef]
- Zong, Z.; Zou, J.; Mao, R.; Ma, C.; Li, N.; Wang, J.; Wang, X.; Zhou, H.; Zhang, L.; Shi, Y. M1 Macrophages Induce PD-L1 Expression in Hepatocellular Carcinoma Cells Through IL-1beta Signaling. Front. Immunol. 2019, 10, 1643. [Google Scholar] [CrossRef]
- Dooley, S.; ten Dijke, P. TGF-beta in progression of liver disease. Cell Tissue Res. 2012, 347, 245–256. [Google Scholar] [CrossRef]
- Thorgeirsson, S.S.; Grisham, J.W. Molecular pathogenesis of human hepatocellular carcinoma. Nat. Genet. 2002, 31, 339–346. [Google Scholar] [CrossRef]
- Marra, F.; Tacke, F. Roles for chemokines in liver disease. Gastroenterology 2014, 147, 577–594.e1. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, X.Y.; Qiu, S.J.; Yamato, I.; Sho, M.; Nakajima, Y.; Zhou, J.; Li, B.Z.; Shi, Y.H.; Xiao, Y.S.; et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin. Cancer Res. 2009, 15, 971–979. [Google Scholar] [CrossRef]
- Guo, M.; Yuan, F.; Qi, F.; Sun, J.; Rao, Q.; Zhao, Z.; Huang, P.; Fang, T.; Yang, B.; Xia, J. Expression and clinical significance of LAG-3, FGL1, PD-L1 and CD8(+)T cells in hepatocellular carcinoma using multiplex quantitative analysis. J. Transl. Med. 2020, 18, 306. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Y.; Chen, Z. Tim-3 expression and its role in hepatocellular carcinoma. J. Hematol. Oncol. 2018, 11, 126. [Google Scholar] [CrossRef]
- Shi, F.; Shi, M.; Zeng, Z.; Qi, R.Z.; Liu, Z.W.; Zhang, J.Y.; Yang, Y.P.; Tien, P.; Wang, F.S. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int. J. Cancer 2011, 128, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Lao, X.M.; Chen, M.M.; Liu, R.X.; Wei, Y.; Ouyang, F.Z.; Chen, D.P.; Zhao, X.Y.; Zhao, Q.; Li, X.F.; et al. PD-1hi Identifies a Novel Regulatory B-cell Population in Human Hepatoma That Promotes Disease Progression. Cancer Discov. 2016, 6, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Kuang, D.M.; Zhao, Q.; Peng, C.; Xu, J.; Zhang, J.P.; Wu, C.; Zheng, L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J. Exp. Med. 2009, 206, 1327–1337. [Google Scholar] [CrossRef]
- Li, F.J.; Zhang, Y.; Jin, G.X.; Yao, L.; Wu, D.Q. Expression of LAG-3 is coincident with the impaired effector function of HBV-specific CD8(+) T cell in HCC patients. Immunol. Lett. 2013, 150, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Kryczek, I.; Chen, L.; Zou, W.; Welling, T.H. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res. 2009, 69, 8067–8075. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Sprengers, D.; Boor, P.P.C.; Doukas, M.; Schutz, H.; Mancham, S.; Pedroza-Gonzalez, A.; Polak, W.G.; de Jonge, J.; Gaspersz, M.; et al. Antibodies Against Immune Checkpoint Molecules Restore Functions of Tumor-Infiltrating T Cells in Hepatocellular Carcinomas. Gastroenterology 2017, 153, 1107–1119.e10. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.I.; Jeong, D.; Ji, S.; Ahn, T.S.; Bae, S.H.; Chin, S.; Chung, J.C.; Kim, H.C.; Lee, M.S.; Baek, M.J. Overexpression of PD-L1 and PD-L2 Is Associated with Poor Prognosis in Patients with Hepatocellular Carcinoma. Cancer Res. Treat. 2017, 49, 246–254. [Google Scholar] [CrossRef]
- Li, Y.; Lu, L.; Qian, S.; Fung, J.J.; Lin, F. Hepatic Stellate Cells Directly Inhibit B Cells via Programmed Death-Ligand 1. J. Immunol. 2016, 196, 1617–1625. [Google Scholar] [CrossRef]
- Muhlbauer, M.; Fleck, M.; Schutz, C.; Weiss, T.; Froh, M.; Blank, C.; Scholmerich, J.; Hellerbrand, C. PD-L1 is induced in hepatocytes by viral infection and by interferon-alpha and -gamma and mediates T cell apoptosis. J. Hepatol. 2006, 45, 520–528. [Google Scholar] [CrossRef]
- Guo, C.L.; Yang, X.H.; Cheng, W.; Xu, Y.; Li, J.B.; Sun, Y.X.; Bi, Y.M.; Zhang, L.; Wang, Q.C. Expression of Fas/FasL in CD8+ T and CD3+ Foxp3+ Treg cells--relationship with apoptosis of circulating CD8+ T cells in hepatocellular carcinoma patients. Asian Pac. J. Cancer Prev. 2014, 15, 2613–2618. [Google Scholar] [CrossRef]
- Peggs, K.S.; Quezada, S.A.; Chambers, C.A.; Korman, A.J.; Allison, J.P. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J. Exp. Med. 2009, 206, 1717–1725. [Google Scholar] [CrossRef]
- Moghaddam, Y.; Andalib, A.; Mohammad-Ganji, M.; Homayouni, V.; Sharifi, M.; Ganjalikhani-Hakemi, M. Evaluation of the effect of TIM-3 suppression by miR-498 and its effect on apoptosis and proliferation rate of HL-60 cell line. Pathol. Res. Pract. 2018, 214, 1482–1488. [Google Scholar] [CrossRef]
- Tan, S.; Xu, Y.; Wang, Z.; Wang, T.; Du, X.; Song, X.; Guo, X.; Peng, J.; Zhang, J.; Liang, Y.; et al. Tim-3 Hampers Tumor Surveillance of Liver-Resident and Conventional NK Cells by Disrupting PI3K Signaling. Cancer Res. 2020, 80, 1130–1142. [Google Scholar] [CrossRef]
- Brunetti, O.; Gnoni, A.; Licchetta, A.; Longo, V.; Calabrese, A.; Argentiero, A.; Delcuratolo, S.; Solimando, A.G.; Casadei-Gardini, A.; Silvestris, N. Predictive and Prognostic Factors in HCC Patients Treated with Sorafenib. Medicina 2019, 55, 707. [Google Scholar] [CrossRef]
- Lee, H.W.; Cho, K.J.; Park, J.Y. Current Status and Future Direction of Immunotherapy in Hepatocellular Carcinoma: What Do the Data Suggest? Immune Netw. 2020, 20, e11. [Google Scholar] [CrossRef]
- Zongyi, Y.; Xiaowu, L. Immunotherapy for hepatocellular carcinoma. Cancer Lett 2020, 470, 8–17. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.H.R.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef]
- Qin, S.; Ren, Z.; Meng, Z.; Chen, Z.; Chai, X.; Xiong, J.; Bai, Y.; Yang, L.; Zhu, H.; Fang, W.; et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: A multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 571–580. [Google Scholar] [CrossRef]
- Qin, S.; Finn, R.S.; Kudo, M.; Meyer, T.; Vogel, A.; Ducreux, M.; Macarulla, T.M.; Tomasello, G.; Boisserie, F.; Hou, J.; et al. RATIONALE 301 study: Tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Future Oncol. 2019, 15, 1811–1822. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Segal, N.H.; Jaeger, D.; Lee, K.H.; Marshall, J.; Antonia, S.J.; Butler, M.; Sanborn, R.E.; Nemunaitis, J.J.; Carlson, C.A.; et al. Safety and clinical activity of durvalumab monotherapy in patients with hepatocellular carcinoma (HCC). J. Clin. Oncol. 2017, 35, 4071. [Google Scholar] [CrossRef]
- Sangro, B.; Gomez-Martin, C.; de la Mata, M.; Inarrairaegui, M.; Garralda, E.; Barrera, P.; Riezu-Boj, J.I.; Larrea, E.; Alfaro, C.; Sarobe, P.; et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J. Hepatol. 2013, 59, 81–88. [Google Scholar] [CrossRef]
- Murtaza, A.; Laken, H.; Correia, J.D.S.; McNeeley, P.; Altobell, L.; Zhang, J.; Vancutsem, P.; Wilcoxen, K.; Jenkins, D. Discovery of TSR-022, a novel, potent anti-human TIM-3 therapeutic antibody. Eur. J. Cancer 2016, 69, S102. [Google Scholar] [CrossRef]
- Incyte Biosciences International Sàrl A Safety and Tolerability Study of INCAGN02385 in Select Advanced Malignancies. Available online: https://clinicaltrials.gov/ct2/show/NCT03538028 (accessed on 5 March 2021).
- Abou-Alfa, G.K.; Chan, S.L.; Furuse, J.; Galle, P.R.; Kelley, R.K.; Qin, S.K.; Armstrong, J.; Darilay, A.; Vlahovic, G.; Negro, A.; et al. A randomized, multicenter phase 3 study of durvalumab (D) and tremelimumab (T) as first-line treatment in patients with unresectable hepatocellular carcinoma (HCC): HIMALAYA study. J. Clin. Oncol. 2018, 36, TPS4144. [Google Scholar] [CrossRef]
- Yau, T.; Kang, Y.K.; Kim, T.Y. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The CheckMate 040 randomized clinical trial (October, 10.1001/jamaoncol.2020.4564, 2020). Jama. Oncol. 2021, 7, 140. [Google Scholar] [CrossRef]
- MD Anderson Cancer Center Nivolumab with or Without Ipilimumab in Treating Patients with Resectable Liver Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT03222076 (accessed on 5 March 2021).
- National Health Research Institutes, Taiwan Nivolumab Plus Ipilimumab as Neoadjuvant Therapy for Hepatocellular Carcinoma (HCC). Available online: https://clinicaltrials.gov/ct2/show/NCT03510871 (accessed on 5 March 2021).
- Hoffmann-La Roche A Study of the Safety and Efficacy of Atezolizumab Administered in Combination with Bevacizumab and/or Other Treatments in Participants with Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT02715531 (accessed on 6 March 2021).
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Finn, R.S.; Ikeda, M.; Zhu, A.X.; Sung, M.W.; Baron, A.D.; Kudo, M.; Okusaka, T.; Kobayashi, M.; Kumada, H.; Kaneko, S.; et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J. Clin. Oncol. 2020, 38, 2960–2970. [Google Scholar] [CrossRef]
- Merck Sharp & Dohme Corp. Safety and Efficacy of Lenvatinib (E7080/MK-7902) in Combination with Pembrolizumab (MK-3475) Versus Lenvatinib as First-line Therapy in Participants with Advanced Hepatocellular Carcinoma (MK-7902-002/E7080-G000-311/LEAP-002). Available online: https://clinicaltrials.gov/ct2/show/NCT03713593 (accessed on 6 March 2021).
- Xu, J.M.; Zhang, Y.; Jia, R.; Yue, C.Y.; Chang, L.P.; Liu, R.R.; Zhang, G.R.; Zhao, C.H.; Zhang, Y.Y.; Chen, C.X.; et al. Anti-PD-1 Antibody SHR-1210 Combined with Apatinib for Advanced Hepatocellular Carcinoma, Gastric, or Esophagogastric Junction Cancer: An Open-label, Dose Escalation and Expansion Study. Clin. Cancer Res. 2019, 25, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Innovent Biologics (Suzhou), Co. Ltd. A Study to Evaluate the Efficacy and Safety of Sintilimab in Combination with IBI305 (Anti-VEGF Monoclonal Antibody) Compared to Sorafenib as the First-Line Treatment for Advanced Hepatocellular Carcinoma. Available online: https://clinicaltrials.gov/ct2/show/NCT03794440 (accessed on 1 March 2021).
- Pfizer. A Study Of Avelumab in Combination with Axitinib in Advanced HCC (VEGF Liver 100). Available online: https://clinicaltrials.gov/ct2/show/NCT03289533 (accessed on 6 March 2021).
- Harding, J.J.; Erinjeri, J.P.; Tan, B.R.; Reiss, K.A.; Mody, K.; Khalil, D.; Yarmohammadi, H.; Nadolski, G.; Giardina, J.D.; Capanu, M.; et al. A multicenter pilot study of nivolumab (NIVO) with drug eluting bead transarterial chemoembolization (deb-TACE) in patients (pts) with liver limited hepatocellular carcinoma (HCC). J. Clin. Oncol. 2018, 36, TPS4146. [Google Scholar] [CrossRef]
- AstraZeneca A Global Study to Evaluate Transarterial Chemoembolization (TACE) in Combination with Durvalumab and Bevacizumab Therapy in Patients with Locoregional Hepatocellular Carcinoma (EMERALD-1). Available online: https://clinicaltrials.gov/ct2/show/NCT03778957 (accessed on 1 March 2021).
- Pinato, D.J.; Cole, T.; Bengsch, B.; Tait, P.; Sayed, A.A.; Aborneli, F.; Gramenitskaya, D.; Allara, E.; Thomas, R.; Ward, C.; et al. A phase Ib study of pembrolizumab following trans-arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): PETAL. Ann. Oncol. 2019, 30, 288. [Google Scholar] [CrossRef]
- Institut für Klinische Krebsforschung IKF GmbH at Krankenhaus Nordwest IMMULAB-Immunotherapy with Pembrolizumab in Combination with Local Ablation in Hepatocellular Carcinoma (HCC). Available online: https://clinicaltrials.gov/show/NCT03753659 (accessed on 26 January 2021).
- Autumn McRee, MD, Hoosier Cancer Research Network Pembrolizumab Plus Y90 Radioembolization in HCC Subjects. Available online: https://clinicaltrials.gov/ct2/show/NCT03099564 (accessed on 26 January 2021).
- Longo, V.; Brunetti, O.; Gnoni, A.; Licchetta, A.; Delcuratolo, S.; Memeo, R.; Solimando, A.G.; Argentiero, A. Emerging role of Immune Checkpoint Inhibitors in Hepatocellular Carcinoma. Medicina 2019, 55, 698. [Google Scholar] [CrossRef]
- Jiangsu HengRui Medicine Co. Ltd. A Trial of SHR-1210 (an Anti-PD-1 Inhibitor) in Combination with FOLFOX4 in Subjects with Advanced HCC Who Have Never Received Prior Systemic Treatment. Available online: https://clinicaltrials.gov/ct2/show/NCT03605706 (accessed on 26 January 2021).
- Qin, S.K.; Chen, Z.D.; Liu, Y.; Xiong, J.P.; Ren, Z.G.; Meng, Z.Q.; Gu, S.Z.; Wang, L.N.; Zou, J.J. A phase II study of anti PD-1 antibody camrelizumab plus FOLFOX4 or GEMOX systemic chemotherapy as first-line therapy for advanced hepatocellular carcinoma or biliary tract cancer. Journal of Clinical Oncology 2019, 37, 4074. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef]
- Kelley, R.K.; Abou-Alfa, G.K.; Bendel, J.C.; Kim, T.Y.; Borad, M.J.; Yong, W.P.; Morse, M.; Kang, Y.K.; Rebelatto, M.; Makowsky, M.; et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. J. Clin. Oncol. 2017, 35, 4073. [Google Scholar] [CrossRef]
- Lindsted, T.; Gad, M.; Grandal, M.V.; Frolich, C.; Bhatia, V.K.; Gjetting, T.; Lantto, J.; Horak, I.D.; Kragh, M.; Koefoed, K.; et al. Preclinical characterization of Sym023 a human anti-TIM3 antibody with a novel mechanism of action. Cancer Res. 2018, 78, 5629. [Google Scholar] [CrossRef]
- Qin, S.; Xu, L.; Yi, M.; Yu, S.; Wu, K.; Luo, S. Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer 2019, 18, 155. [Google Scholar] [CrossRef]
- Savitsky, D.; Ward, R.; Riordan, C.; Mundt, C.; Jennings, S.; Connolly, J.; Findeis, M.; Sanicola, M.; Underwood, D.; Nastri, H.; et al. INCAGN02385 is an antagonist antibody targeting the co-inhibitory receptor LAG-3 for the treatment of human malignancies. Cancer Research 2018, 78, 3819. [Google Scholar] [CrossRef]
- Yau, T.; Park, J.W.; Finn, R.S.; Cheng, A.L.; Mathurin, P.; Edeline, J.; Kudo, M.; Han, K.H.; Harding, J.J.; Merle, P.; et al. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann. Oncol. 2019, 30, v874–v875. [Google Scholar] [CrossRef]
- Lee, M.S.; Ryoo, B.Y.; Hsu, C.H.; Numata, K.; Stein, S.; Verret, W.; Hack, S.P.; Spahn, J.; Liu, B.; Abdullah, H.; et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): An open-label, multicentre, phase 1b study. Lancet Oncol. 2020, 21, 808–820. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Kudo, M. Targeted and immune therapies for hepatocellular carcinoma: Predictions for 2019 and beyond. World J. Gastroenterol. 2019, 25, 789–807. [Google Scholar] [CrossRef]
- Duffy, A.G.; Ulahannan, S.V.; Makorova-Rusher, O.; Rahma, O.; Wedemeyer, H.; Pratt, D.; Davis, J.L.; Hughes, M.S.; Heller, T.; ElGindi, M.; et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J. Hepatol. 2017, 66, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Chan, S.L.; Meyer, T.; Reig, M.; El-Khoueiry, A.; Galle, P.R. Diagnosis and management of toxicities of immune checkpoint inhibitors in hepatocellular carcinoma. J. Hepatol. 2020, 72, 320–341. [Google Scholar] [CrossRef]
- Cui, T.M.; Liu, Y.; Wang, J.B.; Liu, L.X. Adverse Effects of Immune-Checkpoint Inhibitors in Hepatocellular Carcinoma. Onco. Targets Ther. 2020, 13, 11725–11740. [Google Scholar] [CrossRef]
- Wang, F.; Qin, S.; Sun, X.; Ren, Z.; Meng, Z.; Chen, Z.; Chai, X.; Xiong, J.; Bai, Y.; Yang, L.; et al. Reactive cutaneous capillary endothelial proliferation in advanced hepatocellular carcinoma patients treated with camrelizumab: Data derived from a multicenter phase 2 trial. J. Hematol. Oncol. 2020, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chavez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suarez-Almazor, M.E. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Primers. 2020, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Jiang, H.; Shi, B.; Zhou, M.; Zhang, H.; Shi, Z.; Du, G.; Luo, H.; Wu, X.; Wang, Y.; et al. Disruption of PD-1 Enhanced the Anti-tumor Activity of Chimeric Antigen Receptor T Cells Against Hepatocellular Carcinoma. Front. Pharmacol. 2018, 9, 1118. [Google Scholar] [CrossRef]
- Batra, S.A.; Rathi, P.; Guo, L.; Courtney, A.N.; Fleurence, J.; Balzeau, J.; Shaik, R.S.; Nguyen, T.P.; Wu, M.F.; Bulsara, S.; et al. Glypican-3-Specific CAR T Cells Coexpressing IL15 and IL21 Have Superior Expansion and Antitumor Activity against Hepatocellular Carcinoma. Cancer Immunol. Res. 2020, 8, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wen, J.; Yi, H.; Hou, X.; Yin, Y.; Ye, G.; Wu, X.; Jiang, X. Split chimeric antigen receptor-modified T cells targeting glypican-3 suppress hepatocellular carcinoma growth with reduced cytokine release. Ther. Adv. Med. Oncol. 2020, 12, 1758835920910347. [Google Scholar] [CrossRef]
- Wang, P.; Qin, W.; Liu, T.; Jiang, D.; Cui, L.; Liu, X.; Fang, Y.; Tang, X.; Jin, H.; Qian, Q. PiggyBac-engineered T cells expressing a glypican-3-specific chimeric antigen receptor show potent activities against hepatocellular carcinoma. Immunobiology 2020, 225, 151850. [Google Scholar] [CrossRef]
- Wu, X.; Luo, H.; Shi, B.; Di, S.; Sun, R.; Su, J.; Liu, Y.; Li, H.; Jiang, H.; Li, Z. Combined Antitumor Effects of Sorafenib and GPC3-CAR T Cells in Mouse Models of Hepatocellular Carcinoma. Mol. Ther. 2019, 27, 1483–1494. [Google Scholar] [CrossRef]
- Kole, C.; Charalampakis, N.; Tsakatikas, S.; Vailas, M.; Moris, D.; Gkotsis, E.; Kykalos, S.; Karamouzis, M.V.; Schizas, D. Immunotherapy for Hepatocellular Carcinoma: A 2021 Update. Cancers 2020, 12, 2859. [Google Scholar] [CrossRef]
- Kleponis, J.; Skelton, R.; Zheng, L. Fueling the engine and releasing the break: Combinational therapy of cancer vaccines and immune checkpoint inhibitors. Cancer Biol. Med. 2015, 12, 201–208. [Google Scholar] [CrossRef]
- Lai, X.; Friedman, A. Combination therapy of cancer with cancer vaccine and immune checkpoint inhibitors: A mathematical model. PLoS ONE 2017, 12, e0178479. [Google Scholar] [CrossRef]



| Trial ID | Treatment | Target | Phase | Patient Number | Lines of Therapy | Status | Ref. |
|---|---|---|---|---|---|---|---|
| NCT 01658878 | Nivolumab | PD-1 | I/II | 262 | First/Second-line | Completed | [57] |
| NCT 02702414 | Pembrolizumab | PD-1 | II | 104 | Second-line | Completed | [58] |
| NCT 02989922 | Camrelizumab | PD-1 | II | 220 | Second-line | Completed | [59] |
| NCT 03412773 | Tislelizumab | PD-1 | III | 674 | First-line | Recruiting | [60] |
| NCT 01693562 | Durvulumab | PD-L1 | I/II | 1022 | First-line | Completed | [61] |
| NCT 01008358 | Tremelimumab | CTLA-4 | II | 21 | First-line | Completed | [62] |
| NCT 02817633 (part 1a) | Cabolimab | Tim-3 | I | 369 | NA | Recruiting | [63] |
| NCT 03538028 | INCAGN02385 | Lag-3 | I | 22 | NA | Completed | [64] |
| Trial ID | Treatment | Target | Phase | Patient Number | Lines of Therapy | Status | Ref. |
|---|---|---|---|---|---|---|---|
| ICI + ICI | |||||||
| NCT 03298451 | Tremelimumab + Durvalumab | CTLA-4 PD-L1 | III | 1504 | First- line | Recruiting | [65] |
| NCT 01658878 | Nivolumab + Ipilimumab | PD-1 CTLA-4 | I/II | 148 | Second-line | Recruiting | [66] |
| NCT 03222076 | Nivolumab + Ipilimumab | PD-1 CTLA-4 | II | 30 | NA | Recruiting | [67] |
| NCT 03510871 | Nivolumab + Ipilimumab | PD-1 CTLA-4 | II | 40 | NA | Recruiting | [68] |
| ICI + angiogenesis inhibitor | |||||||
| NCT 02715531 | Atezolizumab + Bevacizumab | PD-L1 VEGF | Ib | 23 | Second-line | Recruiting | [69] |
| NCT 03434379 | Atezolizumab + Bevacizumab | PD-L1 VEGF | III | 480 | First- line | Recruiting | [70] |
| NCT 03006926 | Lenvatinib + Pembrolizumab | VEGFR PD-1 | Ib | 104 | First- line | Recruiting | [71] |
| NCT 03713593 | Levantinib + pembrolizumab | VEGFR PD-1 | III | 750 | First- line | Recruiting | [72] |
| NCT 02942329 | Camrelizumab + Apatinib | PD-1 VEGFR2 | I | 14 | Second-line | Recruiting | [73] |
| NCT 03794440 | Sintilimab + Bevacizumab | PD-1 VEGF | II/III | 595 | First- line | Recruiting | [74] |
| NCT 03289533 | Avelumab+ Axitinib | PD-L1 VEGF | I | 22 | First- line | Recruiting | [75] |
| ICI + locoregional therapy | |||||||
| NCT 03143270 | Nivolumab + deb-TACE | PD-1 | I | 14 | NA | Recruiting | [76] |
| NCT 03778957 | Bevacizumab + Durvalumab + TACE | VEGF PD-L1 | III | 710 | NA | Recruiting | [77] |
| NCT 03397654 | Pembrolizumab + TACE | PD-1 | Ib | 26 | NA | Recruiting | [78] |
| NCT 03753659 | Pembrolizumab + RFA or MWA or TACE | PD-1 | II | 30 | NA | Recruiting | [79] |
| NCT 03099564 | Pembrolizumab + Yittrium-90 | PD-1 | NA | 30 | NA | Recruiting | [80] |
| NCT 03812562 | Nivolumab + Yittrium-90 | PD-1 | I | 2 | NA | Recruiting | [81] |
| ICI + chemotherapy | |||||||
| NCT 03605706 | Camrelizumab + fluorouracil+ calcium/orfolinate+ oxoliplatin | PD-1 | III | 396 | First-line | Recruiting | [82] |
| NCT 03092895 | Camrelizumab + Apatinib or fluorouracil+ calcium/folinate+ oxoliplatin or gemcitabine + oxoliplatin | PD-1 | II | 152 | NA | Recruiting | [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leone, P.; Solimando, A.G.; Fasano, R.; Argentiero, A.; Malerba, E.; Buonavoglia, A.; Lupo, L.G.; De Re, V.; Silvestris, N.; Racanelli, V. The Evolving Role of Immune Checkpoint Inhibitors in Hepatocellular Carcinoma Treatment. Vaccines 2021, 9, 532. https://doi.org/10.3390/vaccines9050532
Leone P, Solimando AG, Fasano R, Argentiero A, Malerba E, Buonavoglia A, Lupo LG, De Re V, Silvestris N, Racanelli V. The Evolving Role of Immune Checkpoint Inhibitors in Hepatocellular Carcinoma Treatment. Vaccines. 2021; 9(5):532. https://doi.org/10.3390/vaccines9050532
Chicago/Turabian StyleLeone, Patrizia, Antonio Giovanni Solimando, Rossella Fasano, Antonella Argentiero, Eleonora Malerba, Alessio Buonavoglia, Luigi Giovanni Lupo, Valli De Re, Nicola Silvestris, and Vito Racanelli. 2021. "The Evolving Role of Immune Checkpoint Inhibitors in Hepatocellular Carcinoma Treatment" Vaccines 9, no. 5: 532. https://doi.org/10.3390/vaccines9050532
APA StyleLeone, P., Solimando, A. G., Fasano, R., Argentiero, A., Malerba, E., Buonavoglia, A., Lupo, L. G., De Re, V., Silvestris, N., & Racanelli, V. (2021). The Evolving Role of Immune Checkpoint Inhibitors in Hepatocellular Carcinoma Treatment. Vaccines, 9(5), 532. https://doi.org/10.3390/vaccines9050532










