Lymphopenia in COVID-19: γδ T Cells-Based Therapeutic Opportunities
Abstract
:1. Introduction
2. γδ T Cells in the Anti-Viral Host Immune Response
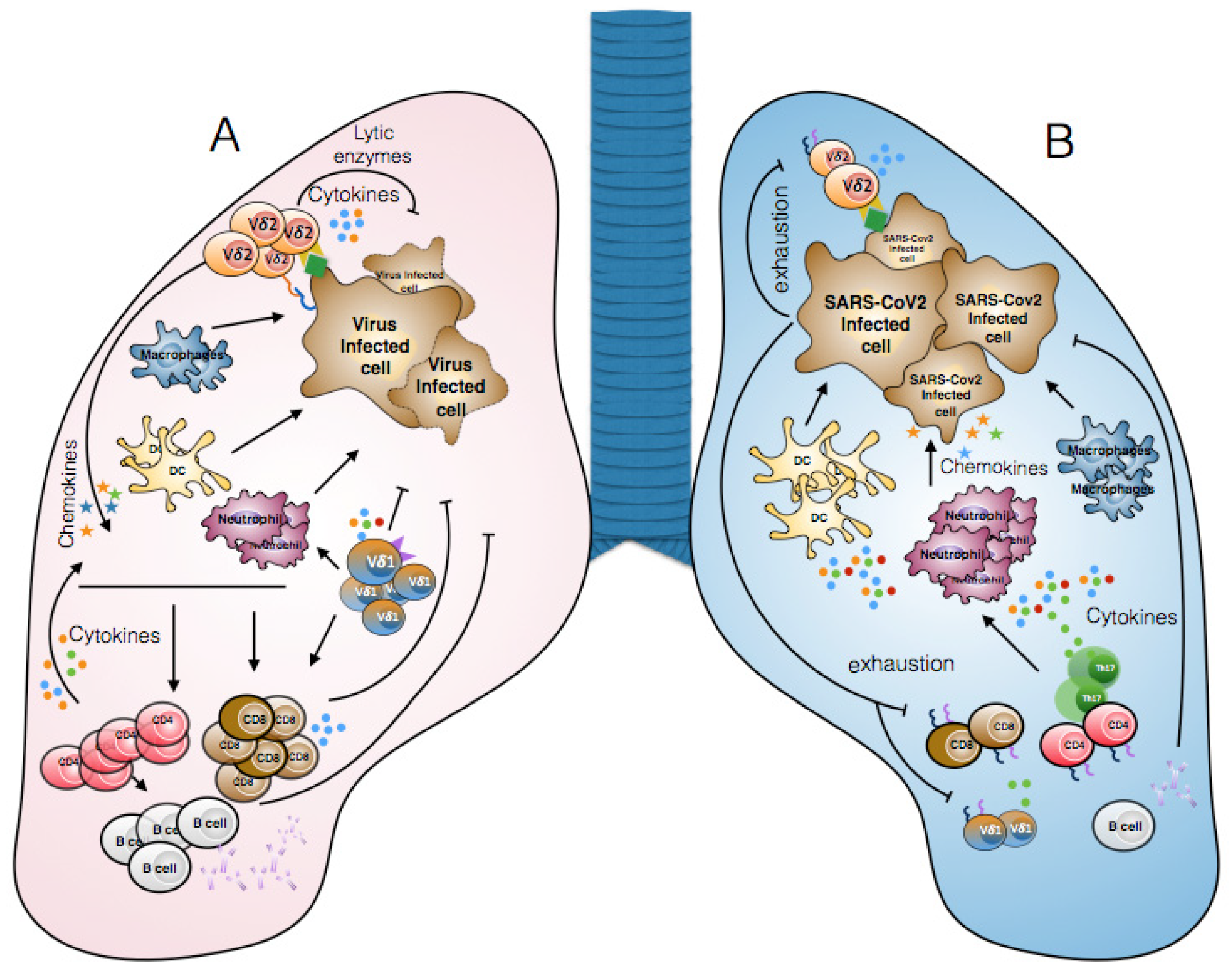
3. Lymphopenia and γδ T Cell Exhaustion in COVID-19
4. Contribution of γδ T Cells to Anti-SARS-CoV-2 Therapy
5. Future Directions and Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Lai, C.C.; Shih, T.P.; Ko, W.C.; Tang, H.J.; Hsueh, P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef] [Green Version]
- Vardhana, S.A.; Wolchok, J.D. The many faces of the anti-COVID immune response. J. Exp. Med. 2020, 217, e20200678. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J.; HLH Across Speciality Collaboration, UK. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Cao, X. COVID-19: Immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020, 20, 269–270. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Zhou, X.; Zhu, C.; Song, Y.; Feng, F.; Qiu, Y.; Feng, J.; Jia, Q.; Song, Q.; Zhu, B.; et al. Immune phenotyping based on neutrophil-to-lymphocyte ratio and IgG predicts disease severity and outcome for patients with COVID-19. Front. Mol. Biosci. 2020, 7, 157. [Google Scholar] [CrossRef] [PubMed]
- Rijkers, G.; Vervenne, T.; van der Pol, P. More bricks in the wall against SARS-CoV-2 infection: Involvement of γ9δ2 T cells. Cell. Mol. Immunol. 2020, 17, 771–772. [Google Scholar] [CrossRef] [PubMed]
- Saghazadeh, A.; Rezaei, N. Immune-epidemiological parameters of the novel coronavirus—A perspective. Expert Rev. Clin. Immunol. 2020, 16, 465–470. [Google Scholar] [CrossRef] [Green Version]
- Lo, M.W.; Kemper, C.; Woodruff, T.M. COVID-19: Complement, Coagulation, and Collateral Damage. J. Immunol. 2020, 205, 1488–1495. [Google Scholar] [CrossRef]
- Wilk, A.J.; Rustagi, A.; Zhao, N.Q.; Roque, J.; Martínez-Colón, G.J.; McKechnie, J.L.; Ivison, G.T.; Ranganath, T.; Vergara, R.; Hollis, T.; et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat. Med. 2020, 26, 1070–1076. [Google Scholar] [CrossRef]
- Carissimo, G.; Xu, W.; Kwok, I.; Abdad, M.Y.; Chan, Y.H.; Fong, S.W.; Puan, K.J.; Lee, C.Y.; Yeo, N.K.; Amrun, S.N.; et al. Whole blood immunophenotyping uncovers immature neutrophil-to-VD2 T-cell ratio as an early marker for severe COVID-19. Nat. Commun. 2020, 11, 5243. [Google Scholar] [CrossRef]
- Jouan, Y.; Guillon, A.; Gonzalez, L.; Perez, Y.; Boisseau, C.; Ehrmann, S.; Ferreira, M.; Daix, T.; Jeannet, R.; François, B.; et al. Phenotypical and functional alteration of unconventional T cells in severe COVID-19 patients. J Exp. Med. 2020, 217, e20200872. [Google Scholar] [CrossRef] [PubMed]
- Laing, A.G.; Lorenc, A.; del Molino del Barrio, I.; Das, A.; Fish, M.; Monin, L.; Muñoz-Ruiz, M.; McKenzie, D.R.; Hayday, T.S.; Francos-Quijorna, I.; et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat. Med. 2020, 26, 1623–1635. [Google Scholar] [CrossRef] [PubMed]
- Lo Presti, E.; Pizzolato, G.; Gulotta, E.; Cocorullo, G.; Gulotta, G.; Dieli, F.; Meraviglia, S. Current Advances in γδ T Cell-Based Tumor Immunotherapy. Front. Immunol. 2017, 8, 1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazdanifar, M.; Mashkour, N.; Bertaina, A. Making a case for using γδ T cells against SARS-CoV-2. Crit. Rev. Microbiol. 2020, 46, 689–702. [Google Scholar] [CrossRef]
- Kenna, T.; Golden-Mason, L.; Norris, S.; Hegarty, J.E.; O’Farrelly, C.; Doherty, D.G. Distinct subpopulations of gamma delta T cells are present in normal and tumor-bearing human liver. Clin. Immunol. 2004, 113, 56–63. [Google Scholar] [CrossRef]
- Knight, A.; Madrigal, A.J.; Grace, S.; Sivakumaran, J.; Kottaridis, P.; Mackinnon, S.; Travers, P.J.; Lowdell, M.W. The role of Vδ2-negative γδ T cells during cytomegalovirus reactivation in recipients of allogeneic stem cell transplantation. Blood 2010, 116, 2164–2172. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y.; Morita, C.T.; Tanaka, Y.; Nieves, E.; Brenner, M.B.; Bloom, B.R. Natural and synthetic non-peptide antigens recognized by human γδT cells. Nature 1995, 75, 155–158. [Google Scholar] [CrossRef]
- Gober, H.J.; Kistowska, M.; Angman, L.; Jeno, P.; Mori, L.; De Libero, G. Human T cell receptor γδ T cells recognize endogenous mevalonate metabolites in tumor cells. J. Exp. Med. 2003, 197, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.S.; Willcox, C.R.; Hunter, S.; Kasatskaya, S.A.; Remmerswaal, E.B.M.; Salim, M.; Mohammed, F.; Bemelman, F.J.; Chudakov, D.M.; Oo, Y.H.; et al. The human Vdelta2(+) T- cell compartment comprises distinct innate-like Vgamma9(+) and adaptive Vgamma9(–) subsets. Nat. Commun. 2018, 9, 1760. [Google Scholar] [CrossRef] [PubMed]
- Hudspeth, K.; Silva-Santos, B.; Mavilio, D. Natural cytotoxicity receptors: Broader expression patterns and functions in innate and adaptive immune cells. Front. Immunol. 2013, 4, 69. [Google Scholar] [CrossRef] [Green Version]
- Mangan, B.A.; Dunne, M.R.; O’Reilly, V.P.; Dunne, P.J.; Exley, M.A.; O’Shea, D.; Scotet, E.; Hogan, A.E.; Doherty, D.G. Cutting edge: CD1d restriction and 1/ 2/ 17 cytokine secretion by human Vδ3 T cells. J. Immunol. 2013, 191, 30–34. [Google Scholar] [CrossRef]
- Poccia, F.; Agrati, C.; Castilletti, C.; Bordi, L.; Gioia, C.; Horejsh, D.; Ippolito, G.; Chan, P.K.; Hui, D.S.; Sung, J.J.; et al. Anti-severe acute respiratory syndrome coronavirus immune responses: The role played by V gamma 9V delta 2 T cells. J. Infect Dis. 2006, 193, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Dandekar, A.A.; O’Malley, K.; Perlman, S. Important roles for gamma interferon and NKG2D in gammadelta T-cell-induced demyelination in T cell receptor beta-deficient mice infected with a coronavirus. J. Virol. 2005, 79, 9388–9396. [Google Scholar] [CrossRef] [Green Version]
- Das, H.; Groh, V.; Kuijl, C.; Sugita, M.; Morita, C.T.; Spies, T.; Bukowski, J.F. MICA engagement by human Vgamma2Vdelta2 T cells enhances their antigen-dependent effector function. Immunity 2001, 15, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Martino, A.; Casetti, R.; D’Alessandri, A.; Sacchi, A.; Poccia, F. Complementary function of gamma delta T-lymphocytes and dendritic cells in the response to isopentenyl-pyrophosphate and lipopolysaccharide antigens. J. Clin. Immunol. 2005, 25, 230–237. [Google Scholar] [CrossRef]
- Brandes, M.; Willimann, K.; Moser, B. Professional antigen-presentation function by human gammadelta T cells. Science 2005, 309, 264–268. [Google Scholar] [CrossRef]
- Kabelitz, D.; Wesch, D. Role of gamma delta T-lymphocytes in HIV infection. Eur. J. Med. Res. 2001, 6, 169–174. [Google Scholar]
- Sant, S.; Jenkins, M.R.; Dash, P.; Watson, K.A.; Wang, Z.; Pizzolla, A.; Koutsakos, M.; Nguyen, T.H.; Lappas, M.; Crowe, J.; et al. Human γδ T-cell receptor repertoire is shaped by influenza viruses, age and tissue compartmentalisation. Clin. Transl. Immunol. 2019, 8, e1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abate, G.; Spencer, C.T.; Hamzabegovic, F.; Blazevic, A.; Xia, M.; Hoft, D.F. Mycobacterium-specific γ9δ2 T cells mediate both pathogen-inhibitory and CD40 ligand-dependent antigen presentation effects important for tuberculosis immunity. Infect. Immun. 2016, 84, 580–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caccamo, N.; Sireci, G.; Meraviglia, S.; Dieli, F.; Ivanyi, J.; Salerno, A. gammadelta T cells condition dendritic cells in vivo for priming pulmonary CD8 T cell responses against Mycobacterium tuberculosis. Eur. J. Immunol. 2006, 36, 2681–2690. [Google Scholar] [CrossRef]
- Lafarge, X.; Merville, P.; Cazin, M.C.; Bergè, F.; Potaux, L.; Moreau, J.F.; Déchanet-Merville, J. Cytomegalovirus infection in transplant recipients resolves when circulating γδ T lymphocytes expand, suggesting a protective antiviral role. J. Infect. Dis. 2001, 184, 533–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biron, C.A.; Byron, K.S.; Sullivan, J.L. Severe herpesvirus infections in an adolescent without natural killer cells. N. Engl. J. Med. 1989, 320, 1731–1735. [Google Scholar] [CrossRef]
- Haljasmägi, L.; Salumets, A.; Rumm, A.P.; Jürgenson, M.; Krassohhina, E.; Remm, A.; Sein, H.; Kareinen, L.; Vapalahti, O.; Sironen, T.; et al. Longitudinal proteomic profiling reveals increased early inflammation and sustained apoptosis proteins in severe COVID-19. Sci. Rep. 2020, 10, 20533. [Google Scholar] [CrossRef] [PubMed]
- Fathi, N.; Nima, R. Lymphopenia in COVID-19: Therapeutic opportunities. Cell Biol. Int. 2020, 44, 1792–1797. [Google Scholar] [CrossRef]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019. Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef] [PubMed]
- De Biasi, S.; Meschiari, M.; Gibellini, L.; Bellinazzi, C.; Borella, R.; Fidanza, L.; Gozzi, L.; Iannone, A.; Lo Tartaro, D.; Mattioli, M.; et al. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat. Commun. 2020, 11, 3434. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Zhu, B.; Liang, H.; Fang, C.; Gong, Y.; Guo, Q.; Sun, X.; Zhao, D.; Shen, J.; et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020, 26, 502–505. [Google Scholar] [CrossRef] [Green Version]
- Bortolotti, D.; Gentili, V.; Rizzo, S.; Rotola, A.; Rizzo, R. SARS-CoV-2 Spike 1 Protein Controls Natural Killer Cell Activation via the HLA-E/NKG2A Pathway. Cells 2020, 9, e1975. [Google Scholar] [CrossRef]
- Zheng, M.; Gao, Y.; Wang, G.; Song, G.; Liu, S.; Sun, D.; Xu, Y.; Tian, Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020, 17, 533–535. [Google Scholar] [CrossRef] [Green Version]
- Antonioli, L.; Fornai, M.; Pellegrini, C.; Blandizzi, C. NKG2A and COVID-19: Another brick in the wall. Cell. Mol. Immunol. 2020, 17, 672–674. [Google Scholar] [CrossRef]
- Bhagat, G.; Naiyer, A.J.; Shah, J.G.; Harper, J.; Jabri, B.; Wang, T.C.; Green, P.H.; Manavalan, J.S. Small intestinal CD8+TCRgammadelta+NKG2A+ intraepithelial lymphocytes have attributes of regulatory cells in patients with celiac disease. J. Clin. Investig. 2008, 118, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Angelini, D.F.; Zambello, R.; Galandrini, R.; Diamantini, A.; Placido, R.; Micucci, F.; Poccia, F.; Semenzato, G.; Borsellino, G.; Santoni, A.; et al. NKG2A inhibits NKG2C effector functions of γδ T cells: Implications in health and disease. J. Leukoc. Biol. 2011, 89, 75–84. [Google Scholar] [CrossRef]
- Zheng, H.Y.; Zhang, M.; Yang, C.X.; Zhang, N.; Wang, X.C.; Yang, X.P.; Dong, X.Q.; Zheng, Y.T. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell. Mol. Immunol. 2020, 17, 541–543. [Google Scholar] [CrossRef]
- Lei, L.; Qian, H.; Yang, X.; Zhang, X.; Zhang, D.; Dai, T.; Guo, R.; Shi, L.; Cheng, Y.; Zhang, B.; et al. The phenotypic changes of γδ T cells in COVID-19 patients. J. Cell Mol. Med. 2020, 24, 11603–11606. [Google Scholar] [CrossRef]
- Youm, Y.H.; Kanneganti, T.D.; Vandanmagsar, B.; Zhu, X.; Ravussin, A.; Adijiang, A.; Owen, J.S.; Thomas, M.J.; Francis, J.; Parks, J.S.; et al. The Nlrp3 inflammasome promotes age-related thymic demise and immunosenescence. Cell Rep. 2012, 1, 56–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, L.; Wang, Q.; Zhang, D.; Ding, J.; Huang, Q.; Tang, Y.Q.; Wang, Q.; Miao, H. Lymphopenia predicts disease severity of COVID-19: A descriptive and predictive study. Signal Transduct. Target. Ther. 2020, 5, 33. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Liu, J.; Liang, B.; Wang, X.; Wang, H.; Li, W.; Tong, Q.; Yi, J.; Zhao, L.; et al. Longitudinal characteristics of lymphocyte responses and cytokine pro les in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020, 55, 102763. [Google Scholar] [CrossRef]
- Bai, Y.; Huang, W.; Li, Y.; Lai, C.; Huang, S.; Wang, G.; He, Y.; Hu, L.; Chen, C. Lipidomic alteration of plasma in cured COVID-19 patients using ultra high-performance liquid chromatography with high-resolution mass spectrometry. Biosci. Rep. 2021, 41. [Google Scholar]
- Meoni, G.; Ghini, V.; Maggi, L.; Vignoli, A.; Mazzoni, A.; Salvati, L.; Capone, M.; Vanni, A.; Tenori, L.; Fontanari, P.; et al. Metabolomic/lipidomic profiling of COVID-19 and individual response to tocilizumab. PLoS Pathog. 2021, 17, e1009243. [Google Scholar] [CrossRef]
- Lopes, N.; McIntyre, C.; Martin, S.; Raverdeau, M.; Sumaria, N.; Kohlgruber, A.C.; Fiala, G.J.; Agudelo, L.Z.; Dyck, L.; Kane, H.; et al. Distinct metabolic programs established in the thymus control effector functions of γδ T cell subsets in tumor microenvironments. Nat. Immunol. 2021, 22, 179–192. [Google Scholar] [CrossRef]
- Rodrigues, N.V.; Correia, D.V.; Mensurado, S.; Nóbrega-Pereira, S.; deBarros, A.; Kyle- Cezar, F.; Tutt, A.; Hayday, A.C.; Norell, H.; Silva-Santos, B.; et al. Low-Density Lipoprotein Uptake Inhibits the Activation and Antitumor Functions of Human Vγ9Vδ2 T Cells. Cancer Immunol. Res. 2018, 6, 448–457. [Google Scholar] [CrossRef] [Green Version]
- Gattinoni, L.; Powell, D.J., Jr.; Rosenberg, S.A.; Restifo, N.P. Adoptive immuno- therapy for cancer: Building on success. Nat. Rev. Immunol. 2006, 6, 383–393. [Google Scholar] [CrossRef] [Green Version]
- Silva-Santos, B.; Serre, K.; Norell, H. γδ T cells in cancer. Nat. Rev. Immunol. 2015, 15, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Garber, K. γδ T cells bring unconventional cancer-targeting to the clinic-again. Nat. Biotechnol. 2020, 38, 389–391. [Google Scholar] [CrossRef] [Green Version]
- Fournié, J.J.; Sicard, H.; Poupot, M.; Bezombes, C.; Blanc, A.; Romagné, F.; Ysebaert, L.; Laurent, G. What lessons can be learned from γδ T cell-based cancer immunotherapy trials? Cell. Mol. Immunol. 2013, 10, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caccamo, N.; Sullivan, L.C.; Brooks, A.G.; Dieli, F. Harnessing HLA-E-restricted CD8 T lymphocytes for adoptive cell therapy of patients with severe COVID-19. Br. J. Haematol. 2020, 190, e185–e187. [Google Scholar] [CrossRef]
- Brufsky, A.; Marti, J.L.G.; Nasrazadani, A.; Lotze, M.T. Boning up: Amino- bisphophonates as immunostimulants and endosomal disruptors of dendritic cell in SARS-CoV-2 infection. J. Transl. Med. 2020, 18, 261. [Google Scholar] [CrossRef] [PubMed]
- Polito, V.A.; Cristantielli, R.; Weber, G.; Del Bufalo, F.; Belardinilli, T.; Arnone, C.M.; Petretto, A.; Antonucci, L.; Giorda, E.; Tumino, N.; et al. Universal Ready-to-Use Immunotherapeutic Approach for the Treatment of Cancer: Expanded and Activated Polyclonal γδ Memory T Cells. Front. Immunol. 2019, 10, 2717. [Google Scholar] [CrossRef] [Green Version]
- Caccamo, N.; Battistini, L.; Bonneville, M.; Poccia, F.; Fournié, J.J.; Meraviglia, S.; Borsellino, G.; Kroczek, R.A.; La Mendola, C.; Scotet, E.; et al. CXCR5 identifies a subset of Vgamma9Vdelta2 T cells which secrete IL-4 and IL-10 and help B cells for antibody production. J. Immunol. 2006, 177, 5290–5295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rampoldi, F.; Ullrich, L.; Prinz, I. Revisiting the Interaction of γδ T-Cells and B-Cells. Cells 2020, 9, 743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Sousa, T.R.; Victor, J.R. Natural Self-Ligand Gamma Delta T Cell Receptors (γδTCRs) Insight: The Potential of Induced IgG. Vaccines 2020, 8, 436. [Google Scholar] [CrossRef] [PubMed]
| Ref. | Author | Patients Enrolled | Early Infection | Hospitalized ICU | Died | Non-COVID-19 | Healthy Subject | ||
|---|---|---|---|---|---|---|---|---|---|
| n°8 | Rijkers et al. | 24 | - | 18 | 6 | - | _ | ||
| n°11 | Wilk et al. | 7 | 6 | 1 | - | 6 | |||
| n°12 | Carissimo et al. | 54 | - | 28 | - | - | 19 | ||
| n°13 | Jouan et al. | 30 | - | 30 | - | 17 | 20 | ||
| n°46 | Lei et al. | 38 | - | - | _ | - | 18 | ||
| Patients Enrolled | Low | Moderate | Severe | Non-COVID-19 | Healthy Subject | ||||
| n°14 | Laing et al. | 63 | - | 6 | 26 | 31 | 42 | 23 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Presti, E.; Dieli, F.; Meraviglia, S. Lymphopenia in COVID-19: γδ T Cells-Based Therapeutic Opportunities. Vaccines 2021, 9, 562. https://doi.org/10.3390/vaccines9060562
Lo Presti E, Dieli F, Meraviglia S. Lymphopenia in COVID-19: γδ T Cells-Based Therapeutic Opportunities. Vaccines. 2021; 9(6):562. https://doi.org/10.3390/vaccines9060562
Chicago/Turabian StyleLo Presti, Elena, Francesco Dieli, and Serena Meraviglia. 2021. "Lymphopenia in COVID-19: γδ T Cells-Based Therapeutic Opportunities" Vaccines 9, no. 6: 562. https://doi.org/10.3390/vaccines9060562
APA StyleLo Presti, E., Dieli, F., & Meraviglia, S. (2021). Lymphopenia in COVID-19: γδ T Cells-Based Therapeutic Opportunities. Vaccines, 9(6), 562. https://doi.org/10.3390/vaccines9060562







