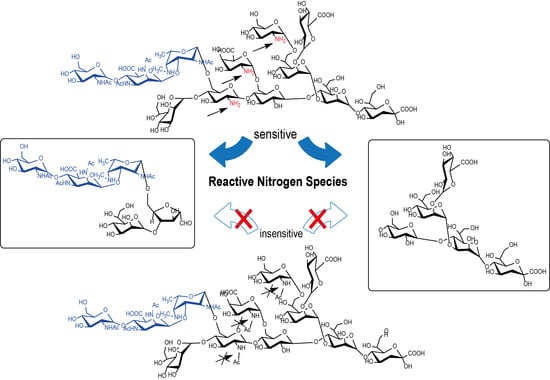
Structure and Immunogenicity of the Bordetella pertussis LOS-Derived Oligosaccharides in the Endosomal-Like Pre-Processing Mice Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria
2.2. Preparation of B. pertussis Lipooligosaccharides
2.3. Deamination of B. pertussis 186 LOS
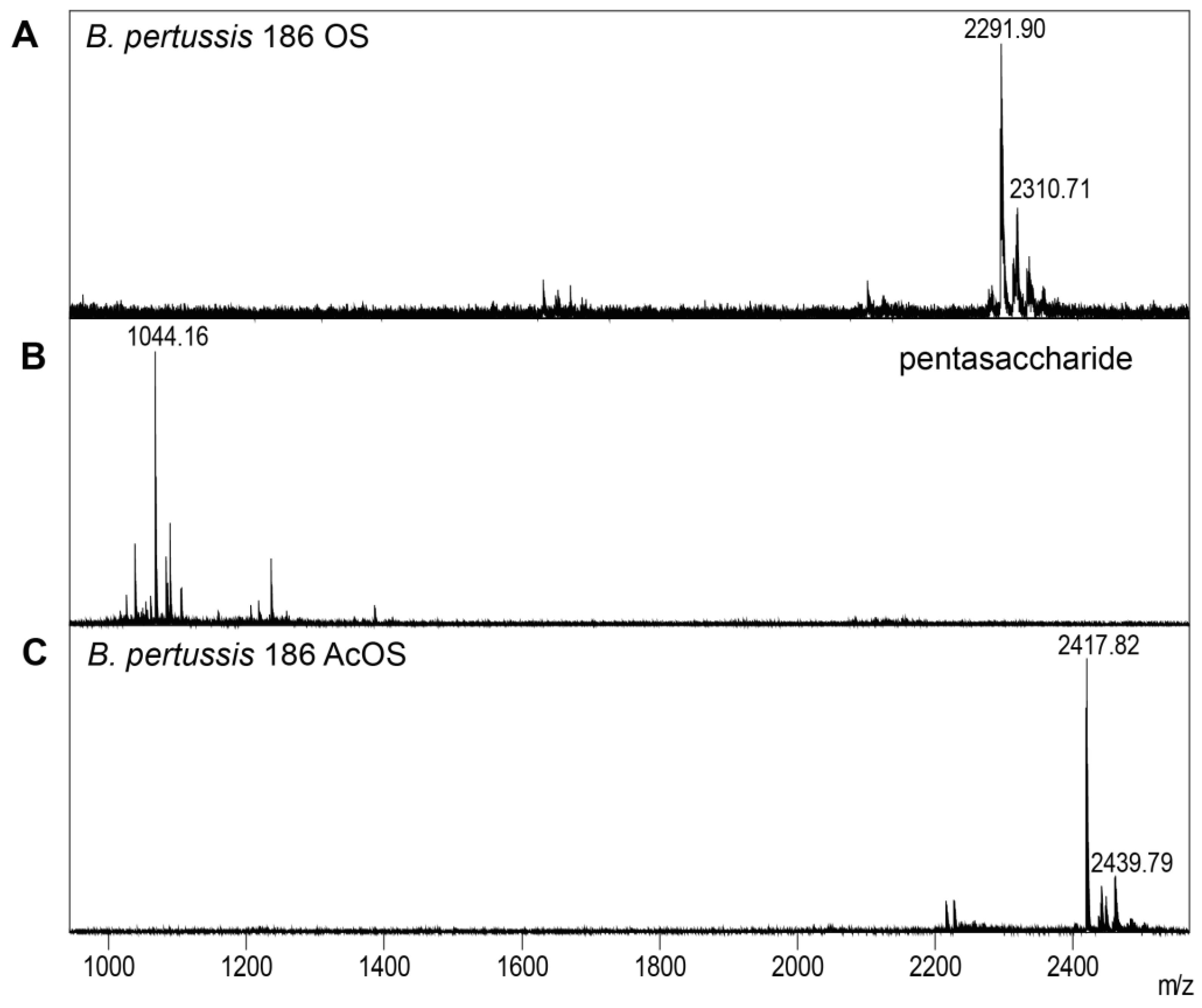
2.4. Isolation of B. pertussis 186 OS
2.5. N-Acetylation of B. pertussis 186 OS
2.6. Selective Oxidation of B. pertussis 186 Oligosaccharide
2.7. Conjugation of the Oligosaccharides with the Ovalbumin Peptide (OVAp)
2.8. Conjugation of the Oligosaccharides with the Pertussis Toxin (PT)
2.9. Immunizations
2.10. Analytical Methods
2.11. Isolation of IgG-Enriched Fraction from Sera
2.12. Mass Spectrometry
2.13. NMR Spectroscopy
2.14. Simulation of B. pertussis 186 OS Deamination Traced by Kinetic NMR Measurement
3. Results
3.1. Isolation of Oligosaccharides with the Defined Structures from B. pertussis 186 Lipooligosaccharide
3.2. Synthesis of B. pertussis 186 Oligosaccharide-Carrier Neoglycoconjugates. Structural Analysis and Immunochemical Properties of Conjugates
3.3. Immunizations and Analysis of Immunogenicity of B. pertussis 186 LOS-Derived OS Neoglycoconjugates
3.4. Immunochemical Properties of Antibodies Recognizing the Pre-Processed B. pertussis 186 Oligosaccharides
3.5. In Vitro Chemical Simulation of Deamination of B. pertussis 186 LOS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Pertussis Chapter—Epidemiology of Vaccine-Preventable Diseases. In The Pink Book: Course Textbook, 13th ed.; Public Health Foundation: Washington, DC, USA, 2015. [Google Scholar]
- Sadkowska-Todys, M.; Zieliński, A.; Czarkowski, M.P. Infectious diseases in Poland in 2016. Prz. Epidemiol. 2018, 72, 129–141. [Google Scholar]
- Morton, T.; Birtwistle, C.; Fumerton, R.; Allison, S. Large pertussis outbreak in rural Canada: Lessons learned from Haida Gwaii. Can. Fam. Physician 2018, 64, e317–e324. [Google Scholar] [PubMed]
- Mbayei, S.A.; Faulkner, A.; Miner, C.; Edge, K.; Cruz, V.; Peña, S.A.; Kudish, K.; Coleman, J.; Pradhan, E.; Thomas, S.; et al. Severe Pertussis Infections in the United States, 2011–2015. Clin. Infect. Dis. 2018, 69, 218–226. [Google Scholar] [CrossRef]
- Skoff, T.H.; Hadler, S.; Hariri, S. The Epidemiology of Nationally Reported Pertussis in the United States, 2000–2016. Clin. Infect. Dis. 2019, 68, 1634–1640. [Google Scholar] [CrossRef] [PubMed]
- Rumik, A.; Aradowska-Stankiewicz, I.; Rudowska, J.; Chrześcijańska, I.; Paradowska-Stankiewicz, I. Pertussis in Poland in 2016. Prz. Epidemiol. 2018, 72, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Tefon, B.E.; Maaß, S.; Özcengiz, E.; Becher, D.; Hecker, M.; Özcengiz, G. A comprehensive analysis of Bordetella pertussis surface proteome and identification of new immunogenic proteins. Vaccine 2011, 29, 3583–3595. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S. Prevention of pertussis: from clinical trials to Real World Evidence. J. Prev. Med. Hyg. 2018, 59, E177–E186. [Google Scholar]
- Esposito, S.; Principi, N. Prevention of pertussis: An unresolved problem. Hum. Vaccines Immunother. 2018, 14, 2452–2459. [Google Scholar] [CrossRef] [PubMed]
- Di Mattia, G.; Nicolai, A.; Frassanito, A.; Petrarca, L.; Nenna, R.; Midulla, F. Pertussis: New preventive strategies for an old disease. Paediatr. Respir. Rev. 2018, 29, 68–73. [Google Scholar] [CrossRef]
- Klein, N.P.; Abu-Elyazeed, R.; Cheuvart, B.; Janssens, W.; Mesaros, N. Immunogenicity and safety following primary and booster vaccination with a hexavalent diphtheria, tetanus, acellular pertussis, hepatitis B, inactivated poliovirus and Hae-mophilus influenzae type b vaccine: A randomized trial in the United States. Hum. Vaccines Immunother. 2018, 15, 809–821. [Google Scholar] [CrossRef]
- Klein, N.P.; Bartlett, J.; Fireman, B.; Rowhani-Rahbar, A.; Baxter, R. Comparative Effectiveness of Acellular Versus Whole-Cell Pertussis Vaccines in Teenagers. Pediatrics 2013, 131, e1716–e1722. [Google Scholar] [CrossRef]
- Cherry, J.D. Why Do Pertussis Vaccines Fail? Pediatrics 2012, 129, 968–970. [Google Scholar] [CrossRef]
- de Gouw, D.; Diavatopoulos, D.A.; Bootsma, H.J.; Hermans, P.W.M.; Mooi, F.R. Pertussis: A matter of immune modulation. FEMS Microbiol. Rev. 2011, 35, 441–474. [Google Scholar] [CrossRef]
- Ayme, G.; Caroff, M.; Chaby, R.; Haeffner-Cavaillon, N.; Le Dur, A.; Moreau, M.; Muset, M.; Mynard, M.C.; Roumiantzeff, M.; Schulz, D.; et al. Biological activities of fragments derived from Bordetella pertussis endotoxin: isolation of a nontoxic, Shwartzman-negative lipid A possessing high adjuvant properties. Infect. Immun. 1980, 27, 739–745. [Google Scholar] [CrossRef]
- Watanabe, M.; Takimoto, H.; Kumazawa, Y.; Amano, K. Biological properties of lipopolysaccharides from Bordetella species. Microbiology 1990, 136, 489–493. [Google Scholar] [CrossRef]
- Caroff, M.; Chaby, R.; Karibian, D.; Perry, J.; Deprun, C.; Szabó, L. Variations in the carbohydrate regions of Bordetella pertussis lipopolysaccharides: Electrophoretic, serological, and structural features. J. Bacteriol. 1990, 172, 1121–1128. [Google Scholar] [CrossRef]
- Flak, T.A.; Goldman, W.E. Signalling and cellular specificity of airway nitric oxide production in pertussis. Cell. Microbiol. 1999, 1, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Flak, T.A.; Heiss, L.N.; Engle, J.T.; Goldman, W.E. Synergistic Epithelial Responses to Endotoxin and a Naturally Occurring Muramyl Peptide. Infect. Immun. 2000, 68, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Trollfors, B.; Lagergård, T.; Taranger, J.; Bergfors, E.; Schneerson, R.; Robbins, J.B. Serum immunoglobulin G antibody responses to Bordetella pertussis lipooligosaccharide and B. parapertussis lipopolysaccharide in children with pertussis and parapertussis. Clin. Diagn. Lab. Immunol. 2001, 8, 1015–1017. [Google Scholar] [CrossRef] [PubMed]
- Mountzouros, K.T.; Kimura, A.; Cowell, J.L. A bactericidal monoclonal antibody specific for the lipooligosaccharide of Bordetella pertussis reduces colonization of the respiratory tract of mice after aerosol infection with B. pertussis. Infect. Immun. 1992, 60, 5316–5318. [Google Scholar] [CrossRef]
- Weiss, A.A.; Patton, A.K.; Millen, S.H.; Chang, S.-J.; Ward, J.I.; Bernstein, D.I. Acellular pertussis vaccines and complement kill-ing of Bordetella pertussis. Infect. Immun. 2004, 72, 7346–7351. [Google Scholar] [CrossRef] [PubMed]
- Jennings, H.J.; Lugowski, C.; Ashton, F.E. Conjugation of meningococcal lipopolysaccharide R-type oligosaccharides to tetanus toxoid as route to a potential vaccine against group B Neisseria meningitidis. Infect. Immun. 1984, 43, 407–412. [Google Scholar] [CrossRef]
- Jennings, H.J.; Lugowski, C. Immunochemistry of groups A, B, and C meningococcal polysaccharide-tetanus toxoid conjugates. J. Immunol. 1981, 127, 1011–1018. [Google Scholar]
- Albitar-Nehme, S.; Basheer, S.M.; Njamkepo, E.; Brisson, J.-R.; Guiso, N.; Caroff, M. Comparison of lipopolysaccharide structures of Bordetella pertussis clinical isolates from pre- and post-vaccine era. Carbohydr. Res. 2013, 378, 56–62. [Google Scholar] [CrossRef]
- Niedziela, T.; Letowska, I.; Lukasiewicz, J.; Kaszowska, M.; Czarnecka, A.; Kenne, L.; Lugowski, C. Epitope of the Vaccine-Type Bordetella pertussis Strain 186 Lipooligosaccharide and Antiendotoxin Activity of Antibodies Directed against the Terminal Pentasaccharide-Tetanus Toxoid Conjugate. Infect. Immun. 2005, 73, 7381–7389. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kubler-Kielb, J.; Vinogradov, E.; Lagergard, T.; Ginzberg, A.; King, J.D.; Preston, A.; Maskell, D.J.; Pozsgay, V.; Keith, J.M.; Robbins, J.B.; et al. Oligosaccharide conjugates of Bordetella pertussis and bronchiseptica induce bactericidal antibodies, an addition to pertussis vaccine. Proc. Natl. Acad. Sci. USA 2011, 108, 4087–4092. [Google Scholar] [CrossRef] [PubMed]
- Koj, S.; Niedziela, T.; Lugowski, C. Bordetella pertussis LOS-Derived Oligosaccharide with Pertussis Toxin GlycoconJugate and Its Application in the Prophylaxis and Treatment of Infections Caused by Bordetella Pertussis. U.S. Patent 9,878,051, 30 January 2018. [Google Scholar]
- Caroff, M.; Brisson, J.-R.; Martin, A.; Karibian, D. Structure of the Bordetella pertussis 1414 endotoxin. FEBS Lett. 2000, 477, 8–14. [Google Scholar] [CrossRef]
- Cobb, B.A.; Wang, Q.; Tzianabos, A.O.; Kasper, D.L. Polysaccharide processing and presentation by the MHCII pathway. Cell 2004, 117, 677–687. [Google Scholar] [CrossRef]
- Duan, J.; Kasper, D.L. Oxidative depolymerization of polysaccharides by reactive oxygen/nitrogen species. Glycobiology 2010, 21, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Avci, F.Y.; Kasper, D.L. Microbial carbohydrate depolymerization by antigen-presenting cells: Deamination prior to presentation by the MHCII pathway. Proc. Natl. Acad. Sci. USA 2008, 105, 5183–5188. [Google Scholar] [CrossRef]
- Avci, F.Y.; Li, X.; Tsuji, M.; Kasper, D.L. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat. Med. 2011, 17, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Stefanetti, G.; Berti, F.; Kasper, D.L. Polysaccharide structure dictates mechanism of adaptive immune response to glycoconjugate vaccines. Proc. Natl. Acad. Sci. USA 2019, 116, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Stainer, D.W.; Scholte, M.J. A Simple Chemically Defined Medium for the Production of Phase I Bordetella pertussis. Microbiology 1970, 63, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Westphal, O.; Jann, K. Bacterial Lipopolysaccharides Extraction with Phenol-Water and Further Applications of the Procedure. Methods Carbohydr. Chem. 1965, 5, 83–91. [Google Scholar]
- Petersson, C.; Niedziela, T.; Jachymek, W.; Kenne, L.; Zarzecki, P.; Lugowski, C. Structural studies of the O-specific polysac-charide of Hafnia alvei strain PCM 1206 lipopolysaccharide containing D-allothreonine. Eur. J. Biochem. 1997, 244, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Le Blay, K.; Caroff, M.; Blanchard, F.; Perry, M.B.; Chaby, R. Epitopes of Bordetella pertussis lipopolysaccharides as potential markers for typing of isolates with monoclonal antibodies. Microbiology 1996, 142, 971–978. [Google Scholar] [CrossRef][Green Version]
- Niedziela, T.; Lukasiewicz, J.; Jachymek, W.; Dzieciatkowska, M.; Lugowski, C.; Kenne, L. Core oligosaccharides of Plesiomonas shigelloides O54:H2 (strain CNCTC 113/92): Structural and serological analysis of the lipopolysaccharide core region, the O-antigen biological repeating unit, and the linkage between them. J. Biol. Chem. 2002, 277, 11653–11663. [Google Scholar] [CrossRef] [PubMed]
- Ozcengiz, E.; Kilinç, K.; Büyüktanir, O.; Günalp, A. Rapid purification of pertussis toxin (PT) and filamentous hemagglutinin (FHA) by cation-exchange chromatography. Vaccine 2004, 22, 1570–1575. [Google Scholar] [CrossRef] [PubMed]
- Baldridge, J.R.; Crane, R.T. Monophosphoryl Lipid A (MPL) Formulations for the Next Generation of Vaccines. Methods 1999, 19, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Voller, A.; Draper, C.; Bidwell, D.; Bartlett, A. MICROPLATE ENZYME-LINKED IMMUNOSORBENT ASSAY FOR CHAGAS’ DISEASE. Lancet 1975, 305, 426–428. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-M.; Frasch, C.E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 1982, 119, 115–119. [Google Scholar] [CrossRef]
- Goddard, T.D.; Kneller, D.G. SPARKY, 3rd ed.; University of California: San Francisco, CA, USA, 2001. [Google Scholar]
- Maaheimo, H.; Kosma, P.; Brade, L.; Brade, H.; Peters, T. Mapping the Binding of Synthetic Disaccharides Representing Epitopes of Chlamydial Lipopolysaccharide to Antibodies with NMR. Biochemistry 2000, 39, 12778–12788. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koj, S.; Ucieklak, K.; Lugowski, C.; Niedziela, T. Structure and Immunogenicity of the Bordetella pertussis LOS-Derived Oligosaccharides in the Endosomal-Like Pre-Processing Mice Model. Vaccines 2021, 9, 645. https://doi.org/10.3390/vaccines9060645
Koj S, Ucieklak K, Lugowski C, Niedziela T. Structure and Immunogenicity of the Bordetella pertussis LOS-Derived Oligosaccharides in the Endosomal-Like Pre-Processing Mice Model. Vaccines. 2021; 9(6):645. https://doi.org/10.3390/vaccines9060645
Chicago/Turabian StyleKoj, Sabina, Karolina Ucieklak, Czeslaw Lugowski, and Tomasz Niedziela. 2021. "Structure and Immunogenicity of the Bordetella pertussis LOS-Derived Oligosaccharides in the Endosomal-Like Pre-Processing Mice Model" Vaccines 9, no. 6: 645. https://doi.org/10.3390/vaccines9060645
APA StyleKoj, S., Ucieklak, K., Lugowski, C., & Niedziela, T. (2021). Structure and Immunogenicity of the Bordetella pertussis LOS-Derived Oligosaccharides in the Endosomal-Like Pre-Processing Mice Model. Vaccines, 9(6), 645. https://doi.org/10.3390/vaccines9060645