Modeling the Impact of COVID-19 Vaccination in Lebanon: A Call to Speed-Up Vaccine Roll Out
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mathematical Model Description
2.2. Model Parameterization, Fitting, and Application to Lebanon
2.3. Vaccine Characteristics and Scale-Up Scenarios
2.4. Measures of Vaccine Impact
2.5. Uncertainty Analysis
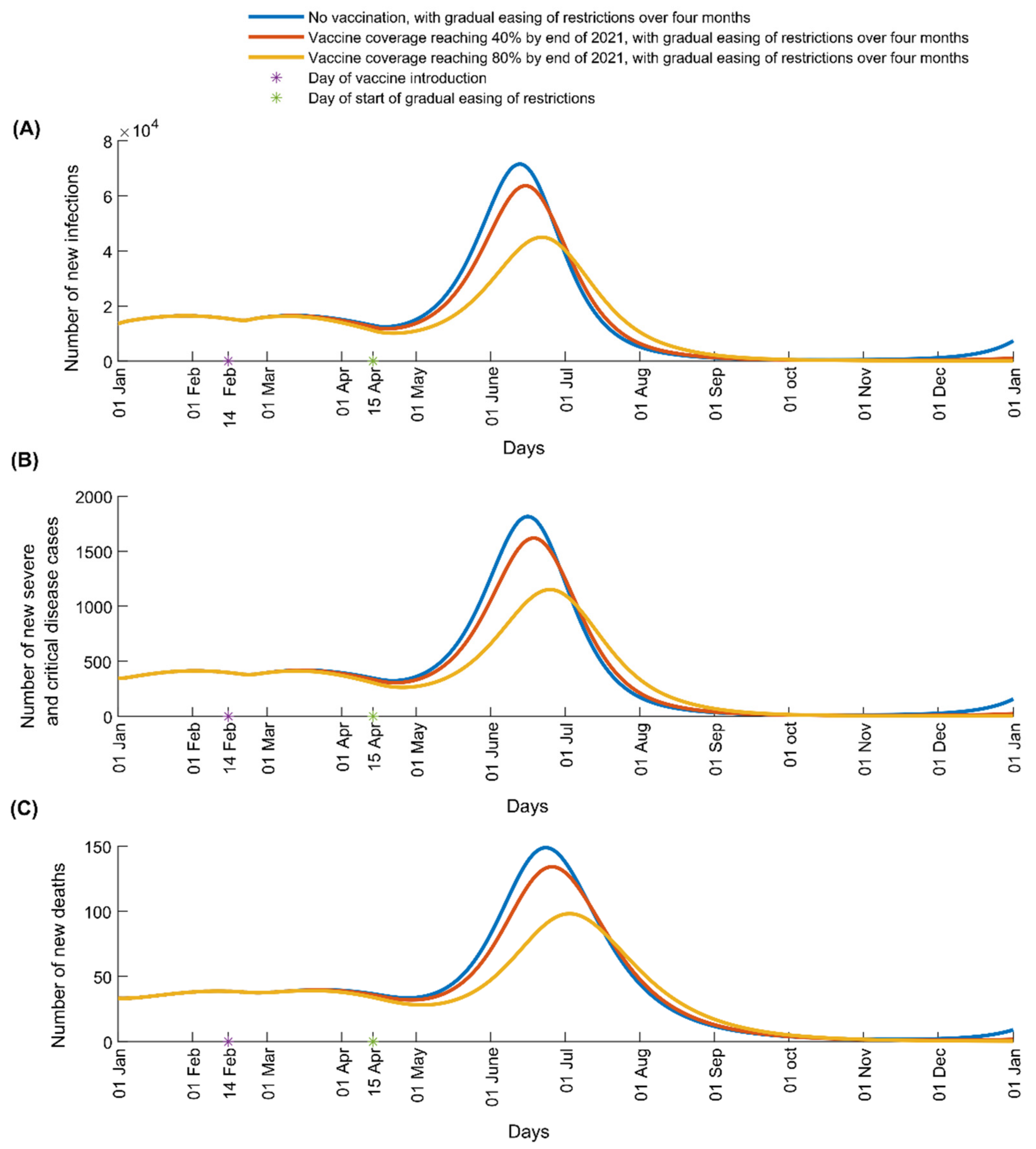
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lebanon COVID-19 Dashboard. Key Indicators Based on Publicly Available Information. Available online: https://infogram.com/lebanon-covid-19-dashboard-1h7g6k3z3jpo2oy?live (accessed on 22 April 2021).
- United Nations Department of Economic and Social Affairs Population Dynamics. The 2019 Revision of World Population Prospects. Available online: https://population.un.org/wpp/ (accessed on 29 April 2021).
- UNHCR. Syria Regional Refugee Response—4 November 2020. Available online: https://data2.unhcr.org/en/situations/syria#_ga=2.115689286.1120363714.1587065689-968033415.1586509661 (accessed on 18 November 2020).
- Fouad, F.M.; McCall, S.J.; Ayoub, H.; Abu-Raddad, L.J.; Mumtaz, G.R. Vulnerability of Syrian refugees in Lebanon to COVID-19: Quantitative insights. Confl. Health 2021, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2020, 384, 403–416. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Janssen COVID-19 Vaccine Frequently Asked Questions. Available online: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/janssen-covid-19-vaccine-frequently-asked-questions#:%7E:text=United%20States%3A%20the%20vaccine%20was,28%20days%20after%20vaccination%2C%20respectively (accessed on 22 June 2021).
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Amit, S.; Regev-Yochay, G.; Afek, A.; Kreiss, Y.; Leshem, E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet 2021, 397, 875–877. [Google Scholar] [CrossRef]
- Tande, A.J.; Pollock, B.D.; Shah, N.D.; Farrugia, G.; Virk, A.; Swift, M.; Breeher, L.; Binnicker, M.; Berbari, E.F. Impact of the COVID-19 Vaccine on Asymptomatic Infection Among Patients Undergoing Pre-Procedural COVID-19 Molecular Screening. Clin. Infect Dis. 2021. [Google Scholar] [CrossRef]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernan, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef]
- Abu-Raddad, L.J.; Chemaitelly, H.; Butt, A.A.; National Study Group for, C.-V. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef]
- Inter-Ministerial and Municipal Platform for Assessment, C.a.T.I. Available online: https://impact.cib.gov.lb/home?dashboardName=vaccine (accessed on 29 April 2021).
- Davies, N.G.; Abbott, S.; Barnard, R.C.; Jarvis, C.I.; Kucharski, A.J.; Munday, J.D.; Pearson, C.A.B.; Russell, T.W.; Tully, D.C.; Washburne, A.D.; et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science 2021. [Google Scholar] [CrossRef]
- Challen, R.; Brooks-Pollock, E.; Read, J.M.; Dyson, L.; Tsaneva-Atanasova, K.; Danon, L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: Matched cohort study. BMJ 2021, 372, n579. [Google Scholar] [CrossRef]
- Abdool Karim, S.S.; de Oliveira, T. New SARS-CoV-2 Variants—Clinical, Public Health, and Vaccine Implications. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef]
- Makhoul, M.; Ayoub, H.H.; Chemaitelly, H.; Seedat, S.; Mumtaz, G.R.; Al-Omari, S.; Abu-Raddad, L.J. Epidemiological Impact of SARS-CoV-2 Vaccination: Mathematical Modeling Analyses. Vaccines 2020, 8, 668. [Google Scholar] [CrossRef]
- Makhoul, M.; Chemaitelly, H.; Ayoub, H.H.; Seedat, S.; Abu-Raddad, L.J. Epidemiological Differences in the Impact of COVID-19 Vaccination in the United States and China. Vaccines 2021, 9, 223. [Google Scholar] [CrossRef]
- Ayoub, H.H.; Chemaitelly, H.; Makhoul, M.; Kanaani, Z.A.; Kuwari, E.A.; Butt, A.A.; Coyle, P.; Jeremijenko, A.; Kaleeckal, A.H.; Latif, A.N.; et al. Epidemiological impact of prioritising SARS-CoV-2 vaccination by antibody status: Mathematical modelling analyses. BMJ Innov. 2021, 7, 327–336. [Google Scholar] [CrossRef]
- Abu-Raddad, L.J.; Chemaitelly, H.; Malek, J.A.; Ahmed, A.A.; Mohamoud, Y.A.; Younuskunju, S.; Ayoub, H.H.; Al Kanaani, Z.; Al Khal, A.; Al Kuwari, E.; et al. Assessment of the risk of SARS-CoV-2 reinfection in an intense re-exposure setting. Clin. Infect Dis. 2020. [Google Scholar] [CrossRef]
- Wajnberg, A.; Amanat, F.; Firpo, A.; Altman, D.R.; Bailey, M.J.; Mansour, M.; McMahon, M.; Meade, P.; Mendu, D.R.; Muellers, K.; et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 2020, 370, 1227–1230. [Google Scholar] [CrossRef]
- Wajnberg, A.; Mansour, M.; Leven, E.; Bouvier, N.M.; Patel, G.; Firpo-Betancourt, A.; Mendu, R.; Jhang, J.; Arinsburg, S.; Gitman, M.; et al. Humoral response and PCR positivity in patients with COVID-19 in the New York City region, USA: An observational study. Lancet Microbe 2020, 1, e283–e289. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371. [Google Scholar] [CrossRef]
- Abu-Raddad, L.J.; Chemaitelly, H.; Coyle, P.; Malek, J.A.; Ahmed, A.A.; Mohamoud, Y.A.; Younuskunju, S.; Ayoub, H.H.; Al Kanaani, Z.; Al Kuwari, E.; et al. SARS-CoV-2 antibody-positivity protects against reinfection for at least seven months with 95% efficacy. EClinicalMedicine 2021, 35, 100861. [Google Scholar] [CrossRef]
- The Language of Technical Computing; The MathWorks, Inc.: Natick, MA, USA, 2019.
- He, W.; Yi, G.Y.; Zhu, Y. Estimation of the basic reproduction number, average incubation time, asymptomatic infection rate, and case fatality rate for COVID-19: Meta-analysis and sensitivity analysis. J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- MIDAS Online COVID-19 Portal. COVID-19 Parameter Estimates: Basic Reproduction Number. Available online: https://github.com/midas-network/COVID-19/tree/master/parameter_estimates/2019_novel_coronavirus (accessed on 19 May 2020).
- Korber, B.; Fischer, W.M.; Gnanakaran, S.; Yoon, H.; Theiler, J.; Abfalterer, W.; Hengartner, N.; Giorgi, E.E.; Bhattacharya, T.; Foley, B.; et al. Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell 2020, 182, 812–827.e819. [Google Scholar] [CrossRef]
- Grubaugh, N.D.; Hanage, W.P.; Rasmussen, A.L. Making Sense of Mutation: What D614G Means for the COVID-19 Pandemic Remains Unclear. Cell 2020, 182, 794–795. [Google Scholar] [CrossRef]
- Lauring, A.S.; Hodcroft, E.B. Genetic Variants of SARS-CoV-2-What Do They Mean? JAMA J. Am. Med. Assoc. 2021. [Google Scholar] [CrossRef]
- Hall, V.J.; Foulkes, S.; Saei, A.; Andrews, N.; Oguti, B.; Charlett, A.; Wellington, E.; Stowe, J.; Gillson, N.; Atti, A.; et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): A prospective, multicentre, cohort study. Lancet 2021. [Google Scholar] [CrossRef]
- World Health Organization. Clinical Management of COVID-19. Available online: https://www.who.int/publications-detail/clinical-management-of-covid-19 (accessed on 31 May 2020).
- World Health Organization. International Guidelines for Certification and Classification (Coding) of COVID-19 as Cause of Death. Available online: https://www.who.int/classifications/icd/Guidelines_Cause_of_Death_COVID-19-20200420-EN.pdf?ua=1.DocumentNumber:WHO/HQ/DDI/DNA/CAT (accessed on 1 June 2020).
- Bubar, K.M.; Reinholt, K.; Kissler, S.M.; Lipsitch, M.; Cobey, S.; Grad, Y.H.; Larremore, D.B. Model-informed COVID-19 vaccine prioritization strategies by age and serostatus. Science 2021. [Google Scholar] [CrossRef] [PubMed]
- Mckay, M.D.; Beckman, R.J.; Conover, W.J. A Comparison of Three Methods for Selecting Values of Input Variables in the Analysis of Output from a Computer Code. Technometrics 1979, 21, 239–245. [Google Scholar] [CrossRef]
- Sanchez, M.A.; Blower, S.M. Uncertainty and sensitivity analysis of the basic reproductive rate—Tuberculosis as an example. Am. J. Epidemiol. 1997, 145, 1127–1137. [Google Scholar] [CrossRef] [Green Version]
- India’s COVID-19 emergency. Lancet 2021, 397, 1683. [CrossRef]
- Wouters, O.J.; Shadlen, K.C.; Salcher-Konrad, M.; Pollard, A.J.; Larson, H.J.; Teerawattananon, Y.; Jit, M. Challenges in ensuring global access to COVID-19 vaccines: Production, affordability, allocation, and deployment. Lancet 2021, 397, 1023–1034. [Google Scholar] [CrossRef]
- Plotkin, S.A.; Halsey, N. Accelerate COVID-19 Vaccine Rollout by Delaying the Second Dose of mRNA Vaccines. Clin. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Overview of the Implementation of COVID-19 Vaccination Strategies and Vaccine Deployment Plans in the EU/EEA—EN; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2021.
- WHO. Why Are There Extra Doses of Vaccine in the Vaccine Vial? WHO: Geneva, Switzerland, 2021. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mumtaz, G.R.; El-Jardali, F.; Jabbour, M.; Harb, A.; Abu-Raddad, L.J.; Makhoul, M. Modeling the Impact of COVID-19 Vaccination in Lebanon: A Call to Speed-Up Vaccine Roll Out. Vaccines 2021, 9, 697. https://doi.org/10.3390/vaccines9070697
Mumtaz GR, El-Jardali F, Jabbour M, Harb A, Abu-Raddad LJ, Makhoul M. Modeling the Impact of COVID-19 Vaccination in Lebanon: A Call to Speed-Up Vaccine Roll Out. Vaccines. 2021; 9(7):697. https://doi.org/10.3390/vaccines9070697
Chicago/Turabian StyleMumtaz, Ghina R., Fadi El-Jardali, Mathilda Jabbour, Aya Harb, Laith J. Abu-Raddad, and Monia Makhoul. 2021. "Modeling the Impact of COVID-19 Vaccination in Lebanon: A Call to Speed-Up Vaccine Roll Out" Vaccines 9, no. 7: 697. https://doi.org/10.3390/vaccines9070697
APA StyleMumtaz, G. R., El-Jardali, F., Jabbour, M., Harb, A., Abu-Raddad, L. J., & Makhoul, M. (2021). Modeling the Impact of COVID-19 Vaccination in Lebanon: A Call to Speed-Up Vaccine Roll Out. Vaccines, 9(7), 697. https://doi.org/10.3390/vaccines9070697






