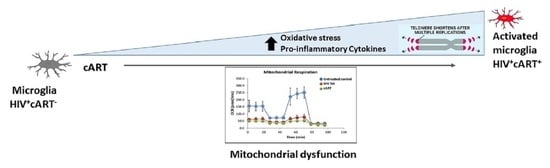
Telomere Length Shortening in Microglia: Implication for Accelerated Senescence and Neurocognitive Deficits in HIV
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Viability Assay
2.3. RNA Extraction
2.4. Gene Expression Analysis Using Real Time PCR (QPCR)
2.5. Analysis for Telomere Length
2.6. Quantification of Mitochondrial DNA (mtDNA)
2.7. Mitochondrial Respiration
2.8. Immunofluorescent Staining
2.9. Reactive Oxygen Species (ROS) Measurement
2.10. Raman Spectroscopy
2.11. Statistical Analysis
3. Results
3.1. Effect of cART on TL and Telomerase/TRF-1 Gene Expression in HIV Patients
3.2. Effect of cART on mtDNA Expression in HIV Patients
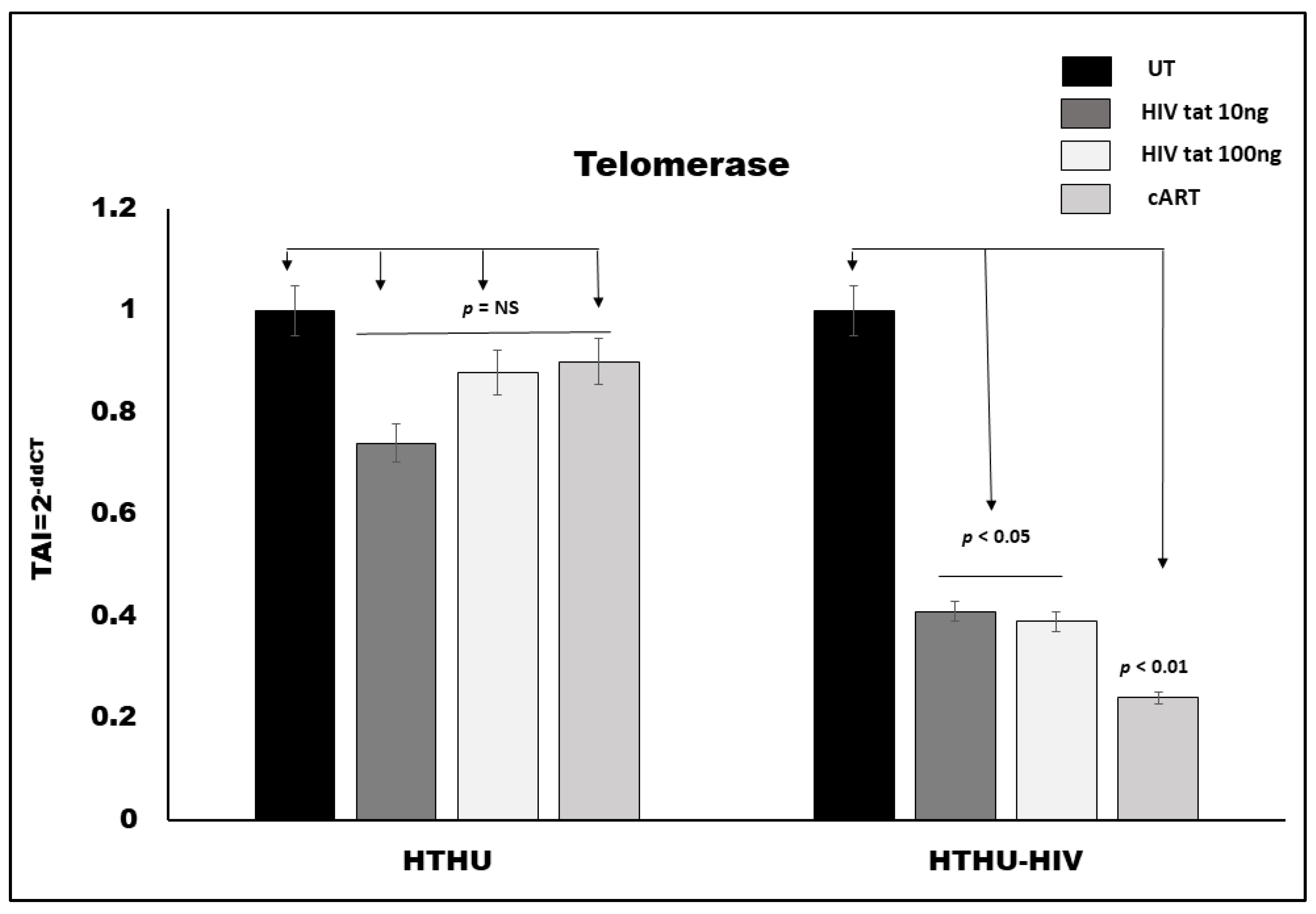
3.3. Effect of HIV Tat and cART on TL in Microglia
3.4. Effect of HIV Tat and cART on Telomerase Gene Expression in Microglia
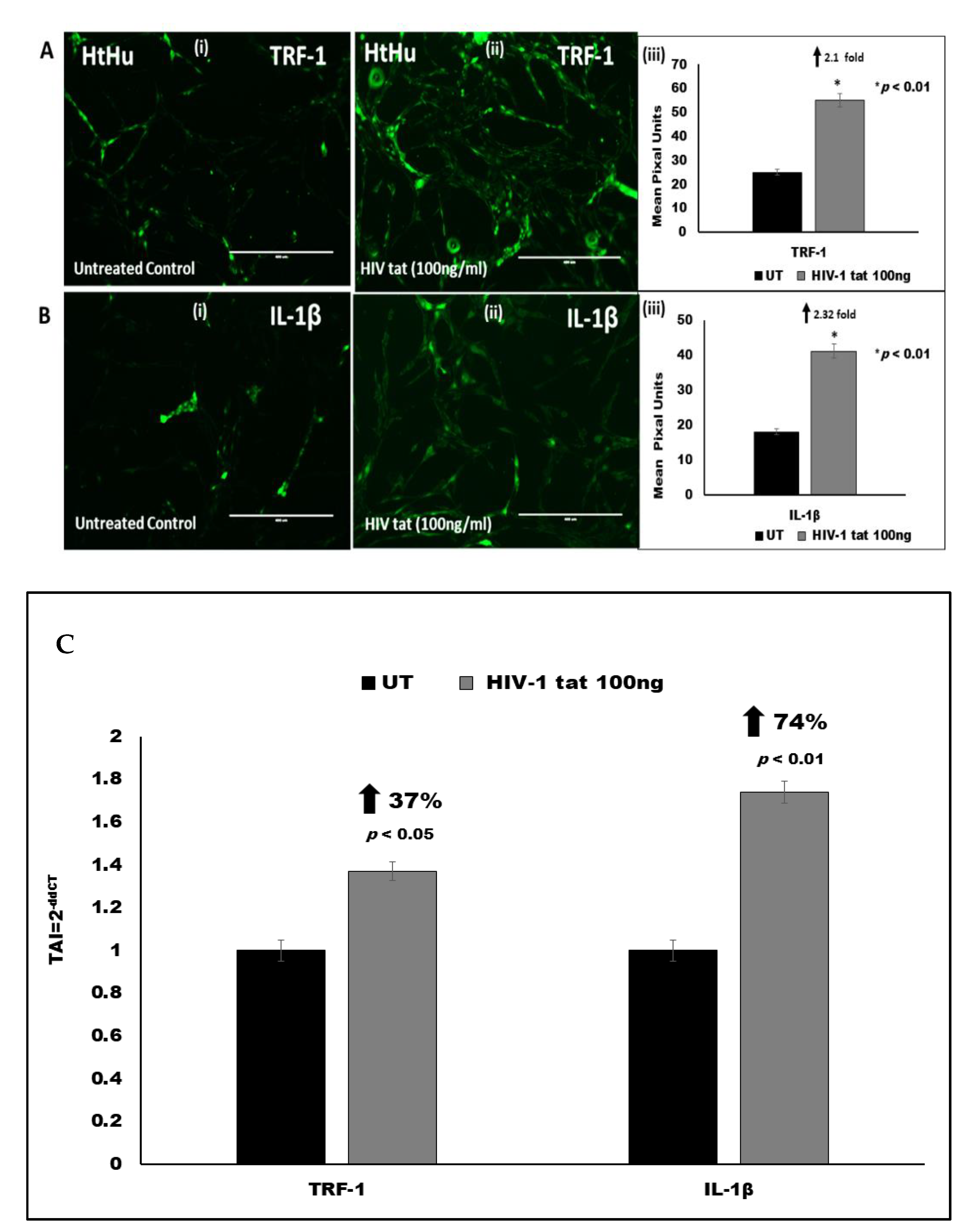
3.5. Effect of HIV Tat on TRF-1 Expression in Microglia
3.6. Effect of HIV Tat on Pro-Inflammatory Cytokine IL-1β Expression in Microglia
3.7. Effect of HIV Tat and cART on Mitochondrial Energetics
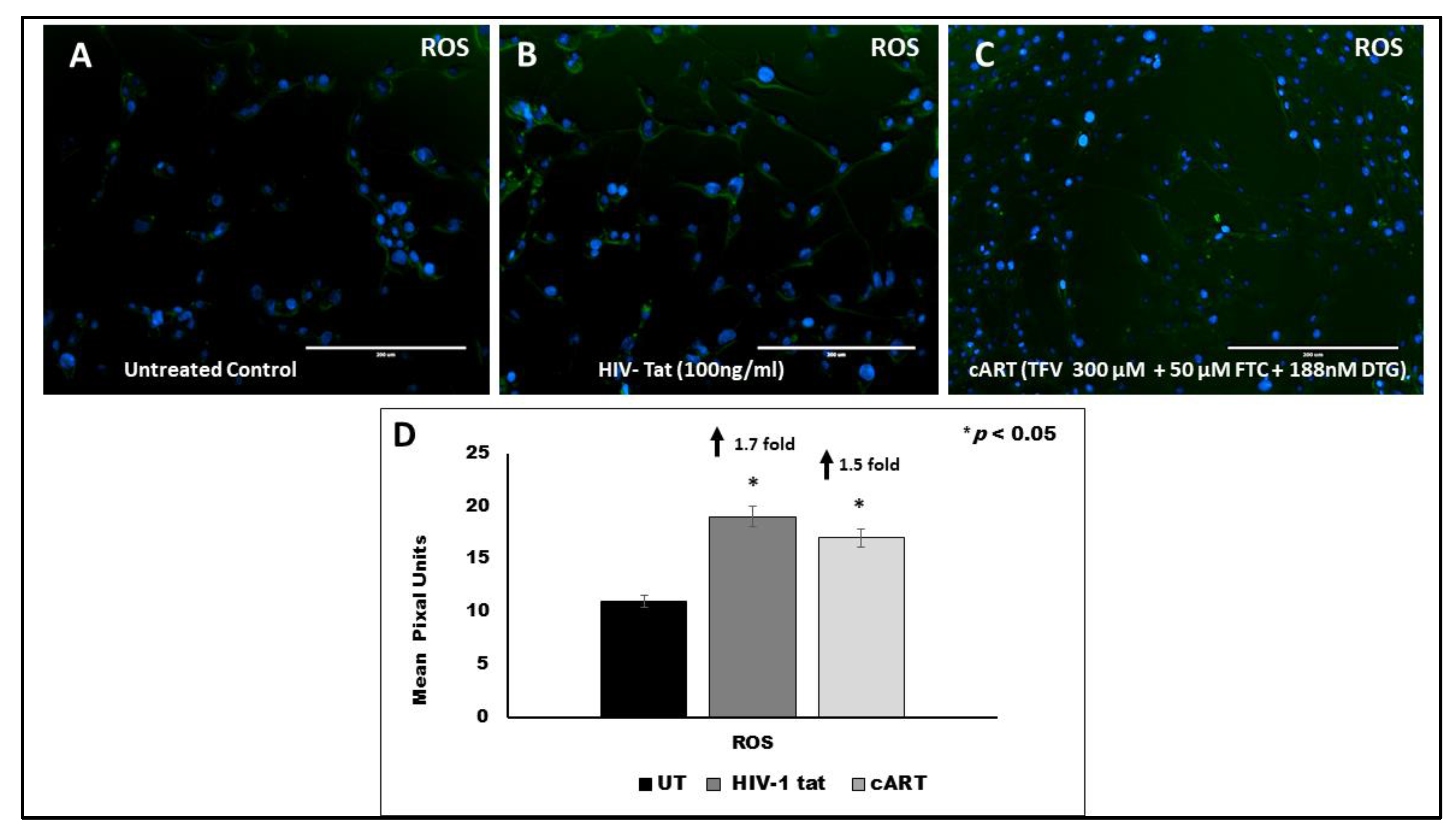
3.8. Effect of HIV Tat on ROS Production
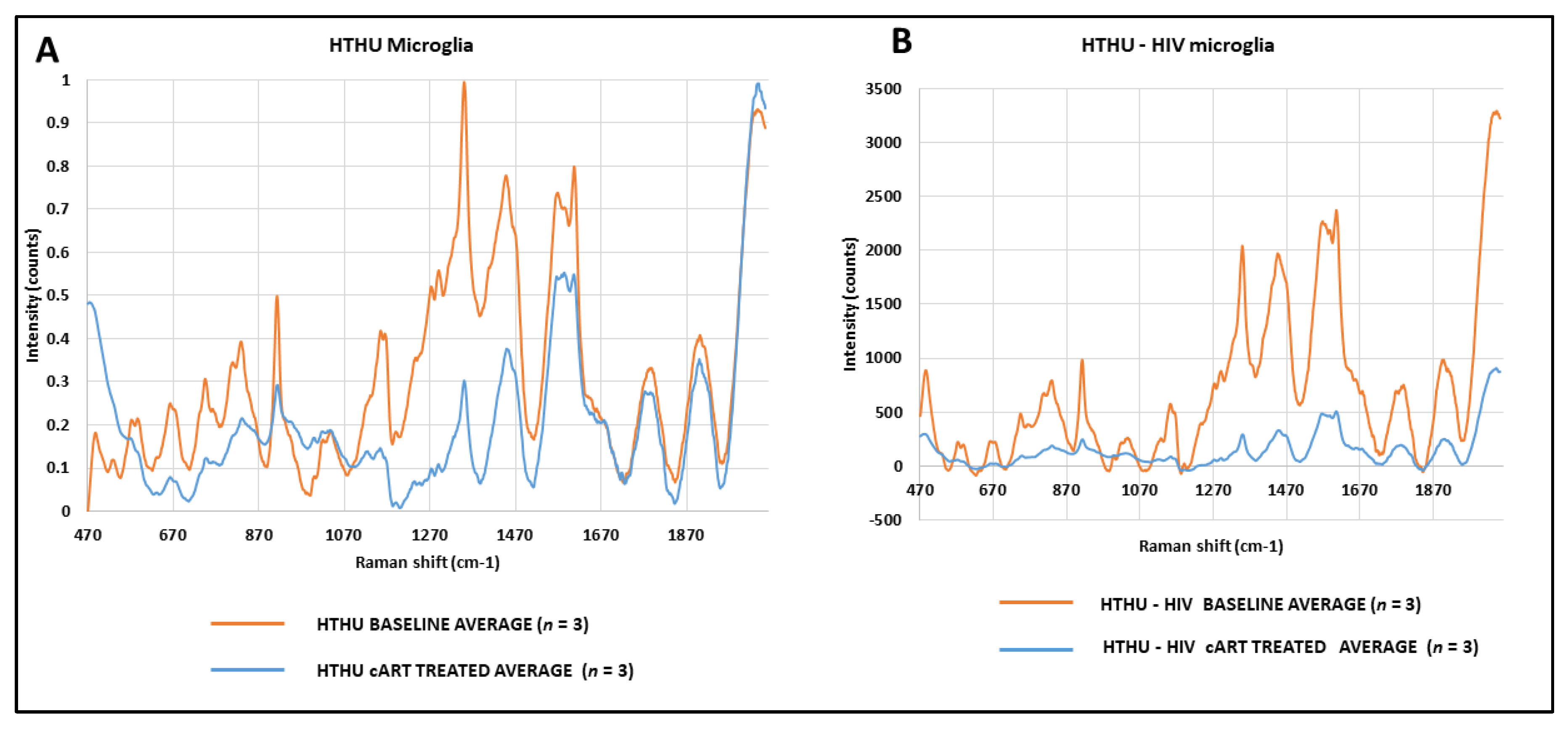
3.9. Effect of cART on Metabolic Changes in HTHU and HTHU/HIV Microglia Quantified by Raman Spectroscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gatignol, A. Transcription of HIV: Tat and Cellular Chromatin. Charact. Porous Solids III 2007, 55, 137–159. [Google Scholar] [CrossRef]
- Easley, R.; Van Duyne, R.; Coley, W.; Guendel, I.; Dadgar, S.; Kehn-Hall, K.; Kashanchi, F. Chromatin dynamics associated with HIV-1 Tat-activated transcription. Biochim. Biophys. Acta Bioenerg. 2010, 1799, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Price, T.O.; Ercal, N.; Nakaoke, R.; Banks, W.A. HIV-1 viral proteins gp120 and Tat induce oxidative stress in brain endothelial cells. Brain Res. 2005, 1045, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Kaul, M.; Lipton, S.A. Mechanisms of neuronal injury and death in HIV-1 associated dementia. Current HIV research. Curr. HIV Res. 2006, 4, 307–318. [Google Scholar] [CrossRef]
- Jin, J.; Lam, L.; Sadic, E.; Fernandez, F.; Tan, J.; Giunta, B. HIV-1 Tat-induced microglial activation and neuronal damage is inhibited via CD45 modulation: A potential new treatment target for HAND. Am. J. Transl. Res. 2012, 4, 302–315. [Google Scholar]
- Epstein, L.G.; Gendelman, H.E. Human immunodeficiency virus type 1 infection of the nervous system: Pathogenetic mecha-nisms. Ann. Neurol. 1993, 33, 429–436. [Google Scholar] [CrossRef]
- Brabers, N.A.C.H.; Nottet, H.S.L.M. Role of the pro-inflammatory cytokines TNF-alpha and IL-1beta in HIV-associated dementia. Eur. J. Clin. Investig. 2006, 36, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Breen, E.C.; Rezai, A.R.; Nakajima, K.; Beall, G.N.; Mitsuyasu, R.T.; Hirano, T.; Kishimoto, T.; Martinez-Maza, O. Infection with HIV is associated with elevated IL-6 levels and production. J. Immunol. 1990, 144, 480–484. [Google Scholar]
- Kaul, M.; Lipton, S.A. Chemokines and activated macrophages in HIV gp120–induced neuronal apotosis. Proc. Natl. Acad. Sci. USA 1999, 96, 8212–8216. [Google Scholar] [CrossRef] [PubMed]
- Kaul, M.; Garden, G.; Lipton, S.A. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nat. Cell Biol. 2001, 410, 988–994. [Google Scholar] [CrossRef]
- Caron, M.; Auclairt, M.; Vissian, A.; Vigouroux, C.; Capeau, J. Contribution of mitochondrial dysfunction and oxidative stress to cellular premature senescence induced by antiretroviral thymidine analogues. Antivir. Ther. 2008, 13, 27–38. [Google Scholar]
- Hukezalie, K.R.; Thumati, N.R.; Côté, H.C.F.; Wong, J.M.Y. In Vitro and Ex Vivo Inhibition of Human Telomerase by Anti-HIV Nucleoside Reverse Transcriptase Inhibitors (NRTIs) but Not by Non-NRTIs. PLoS ONE 2012, 7, e47505. [Google Scholar] [CrossRef] [PubMed]
- Leeansyah, E.; Cameron, P.U.; Solomon, A.; Tennakoon, S.; Velayudham, P.; Gouillou, M.; Spelman, T.; Hearps, A.C.; Fairley, C.K.; Smit, D.V.; et al. Inhibition of Telomerase Activity by Human Immunodeficiency Virus (HIV) Nucleos(t)ide Reverse Transcriptase Inhibitors: A Potential Factor Contributing to HIV-Associated Accelerated Aging. J. Infect. Dis. 2013, 207, 1157–1165. [Google Scholar] [CrossRef]
- Margot, N.; Ram, R.; Abram, M.; Haubrich, R.; Callebaut, C. Antiviral Activity of Tenofovir Alafenamide against HIV-1 with Thymidine Analog-Associated Mutations and M184V. Antimicrob. Agents Chemother. 2020, 64, e02557-19. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mesa, Y.; Jay, T.R.; Checkley, M.A.; Luttge, B.; Dobrowolski, C.; Valadkhan, S.; Landreth, G.E.; Karn, J.; Alvarez-Carbonell, D. Immortalization of primary microglia: A new platform to study HIV regulation in the central nervous system. J. NeuroVirol. 2017, 23, 47–66. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Khoo, T.C.; Tubbesing, K.; Rudkouskaya, A.; Rajoria, S.; Sharikova, A.; Barroso, M.; Khmaladze, A. Quantitative label-free imaging of iron-bound transferrin in breast cancer cells and tumors. Redox Biol. 2020, 36, 101617. [Google Scholar] [CrossRef]
- Khmaladze, A.; Jasensky, J.; Price, E.; Zhang, C.; Boughton, A.; Han, X.; Seeley, E.; Liu, X.; Banaszak Holl, M.M.; Chen, Z. Hy-perspectral imaging and characterization of live cells by broadband coherent anti-stokes Raman scattering (CARS) micros-copy with singular value decomposition (SVD) analysis. Appl. Spectrosc. 2014, 68, 1116–1122. [Google Scholar] [CrossRef]
- Khmaladze, A.; Ganguly, A.; Kuo, S.; Raghavan, M.; Kainkaryam, M.; Cole, J.H.; Marcelo, C.L.; Feinberg, S.E.; Izumi, K.; Morris, M.D. Tissue-Engineered Constructs of Human Oral Mucosa Examined by Raman Spectroscopy. Tissue Eng. Part C Methods 2013, 19, 299–306. [Google Scholar] [CrossRef]
- Sfakis, L.; Kamaldinov, T.; Khmaladze, A.; Hosseini, Z.F.; Nelson, D.A.; Larsen, M.; Castracane, J. Mesenchymal Cells Affect Salivary Epithelial Cell Morphology on PGS/PLGA Core/Shell Nanofibers. Int. J. Mol. Sci. 2018, 19, 1031. [Google Scholar] [CrossRef]
- Yu, C.; Gestl, E.; Eckert, K.; Allara, D.; Irudayaraj, J. Characterization of human breast epithelial cells by confocal Raman microspectroscopy. Cancer Detect. Prev. 2006, 30, 515–522. [Google Scholar] [CrossRef]
- Tubbesing, K.; Khoo, T.C.; Bahreini, S.J.; Sharikova, A.; Barroso, M.; Khmaladze, A. Iron-binding cellular profile of trans-ferrin using label-free Raman hyperspectral imaging and singular value decomposition (SVD). Free Radic. Biol. Med. 2021, 169, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Surmacki, J.; Musial, J.; Kordek, R.; Abramczyk, H. Raman imaging at biological interfaces: Applications in breast cancer diagnosis. Mol. Cancer 2013, 12, 1–48. [Google Scholar] [CrossRef] [PubMed]
- Jasensky, J.; Boughton, A.P.; Khmaladze, A.; Ding, J.; Zhang, C.; Swain, J.E.; Smith, G.W.; Chen, Z.; Smith, G.D. Live-cell quantification and comparison of mammalian oocyte cytosolic lipid content between species, during development, and in relation to body composition using nonlinear vibrational microscopy. Analyst 2016, 141, 4694–4706. [Google Scholar] [CrossRef] [PubMed]
- D’Brant, L.Y.; Desta, H.; Khoo, T.C.; Sharikova, A.; Mahajan, S.D.; Khmaladze, A. Methamphetamine-induced apoptosis in glial cells examined under marker-free imaging modalities. J. Biomed. Opt. 2019, 24, 046503. [Google Scholar] [CrossRef]
- Sharikova, A.; Peerzada, L.; Pisila, K.; Khoo, T.C.; Cherkinsky, A.; Khmaladze, A. Characterization of nanofibers for tissue engineering: Chemical mapping by Confocal Raman microscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 227, 117670. [Google Scholar] [CrossRef]
- Fauci, A.S.; Marston, H.D. Ending the HIV-AIDS pandemic—follow the science. N. Engl. J. Med. 2015, 373, 2197–2199. [Google Scholar] [CrossRef]
- Saylor, D.; Dickens, A.; Sacktor, N.; Haughey, N.; Slusher, B.; Pletnikov, M.; Mankowski, J.L.; Brown, A.; Volsky, D.J.; McArthur, D.S. HIV-associated neurocognitive disorder—pathogenesis and prospects for treatment. Nat. Rev. Neurol. 2016, 12, 234–248. [Google Scholar] [CrossRef]
- Heaton, R.K.; Clifford, D.B.; Franklin, D.R.; Woods, S.P.; Ake, C.; Vaida, F.; Ellis, R.J.; Letendre, S.L.; Marcotte, T.D.; Atkinson, J.H.; et al. HIV-associated neurocogni-tive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010, 75, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Maschke, M.; Kastrup, O.; Esser, S.; Ross, B.; Hengge, U.; Hufnagel, A. Incidence and prevalence of neurological disorders associat-ed with HIV since the introduction of highly active antiretroviral therapy (HAART). J. Neurol. Neurosurg. Psychiatry 2000, 69, 376–380. [Google Scholar] [CrossRef]
- Blanco, J.R.; Jarrin, I.; Martinez, A.; Siles, E.; Larrayoz, I.M.; Cañuelo, A.; Gutierrez, F.; Gonzalez-Garcia, J.; Vidal, F.; Moreno, S. Shorter telomere lengthpredicts poorer immunological recovery in virologically suppressed HIV-1-infected pa-tients treated with combined antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 2015, 68, 21–29. [Google Scholar] [CrossRef]
- Gomez, D.E.; Armando, R.G.; Farina, H.G.; Menna, P.L.; Cerrudo, C.S.; Ghiringhelli, P.D.; Alonso, D.F. Telomere structure and telomerase in health and disease (review). Int. J. Oncol. 2012, 41, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Dragović, G.; Andjić, M.; Toljić, B.; Jevtović, D.; Lukić, R.; de Luka, S.; Trbovich, A.; Milašin, J. Correlation between metabolic syn-drome and relative telomere length shortening in HIV/AIDS patients on combined antiretroviral therapy. Exp. Gerontol. 2021, 147, 111269. [Google Scholar] [CrossRef]
- Stella-Ascariz, N.; Montejano, R.; Pintado-Berninches, L.; Monge, S.; Bernardino, J.I.; Valero, I.P.; Montes, M.L.; Mingorance, J.; Perona, R.; Arribas, J.R. Brief Report: Differential Effects of Tenofovir, Abacavir, Emtricitabine, and Darunavir on Telomerase Activity In Vitro. J. Acquir. Immune Defic. Syndr. 2017, 74, 91–94. [Google Scholar] [CrossRef][Green Version]
- Blasco, M.A. Telomere length, stem cells and aging. Nat. Chem. Biol. 2007, 3, 640–649. [Google Scholar] [CrossRef]
- Montejano, R.; Stella-Ascariz, N.; Monge, S.; Bernardino, J.I.; Valero, I.P.; Montes, M.L.; Valencia, E.; Martín-Carbonero, L.; Moreno, V.; González-García, J.; et al. Impact of Antiretroviral Treatment Containing Tenofovir Difumarate on the Telomere Length of Aviremic HIV-Infected Patients. J. Acquir. Immune Defic. Syndr. 2017, 76, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, P.; Blanco, R.; de Carcer, G.; Schoeftner, S.; Benetti, R.; Flores, J.M.; Malumbres, M.; Blasco, M.A. TRF1 Controls Telomere Length and Mitotic Fidelity in Epithelial Homeostasis. Mol. Cell. Biol. 2009, 29, 1608–1625. [Google Scholar] [CrossRef] [PubMed]
- Streit, W.J.; Xue, Q.S.; Tischer, J.; Bechmann, I. Microglial pathology. Acta Neuropathol. Commun. 2014, 2, 142. [Google Scholar] [CrossRef] [PubMed]
- Perry, V.H.; Holmes, C. Microglial priming in neurodegenerative disease. Nat. Rev. Neurol. 2014, 10, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, G.; Uhlemann, R.; Schöner, J.; Wegner, S.; Boujon, V.; Deigendesch, N.; Endres, M.; Gertz, K. Repression of telomere-associated genes by microglia activation in neuropsychiatric disease. Eur. Arch. Psychiatry Clin. Neurosci. 2017, 267, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Gleichmann, M.; Cheng, A. Mitochondria in neuroplasticity and neurological disorders. Neuron 2008, 60, 748–766. [Google Scholar] [CrossRef]
- Thomas, J.B.; Brier, M.R.; Snyder, A.Z.; Vaida, F.F.; Ances, B.M. Pathways to neurodegeneration: Effects of HIV and aging on resting-state functional connectivity. Neurology 2013, 80, 1186–1193. [Google Scholar] [CrossRef]
- Lauro, C.; Limatola, C. Metabolic Reprograming of Microglia in the Regulation of the Innate Inflammatory Response. Front. Immunol. 2020, 11, 493. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Jiang, Z.; Chen, X.; Liu, M.; Li, J.; Liu, N. Electron transport chain inhibitors induce microglia activation through enhancing mitochondrial reactive oxygen species production. Exp. Cell Res. 2016, 340, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Voloboueva, L.A.; Emery, J.F.; Sun, X.; Giffard, R.G. Inflammatory response of microglial BV-2 cells includes a glyco-lytic shift and is modulated by mitochondrial glucose-regulated protein 75/mortalin. FEBS Lett. 2013, 587, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Qin, C.; Hu, Z.-W.; Zhou, L.-Q.; Yu, H.-H.; Chen, M.; Bosco, D.B.; Wang, W.; Wu, L.-J.; Tian, D.-S. Microglia reprogram metabolic profiles for phenotype and function changes in central nervous system. Neurobiol. Dis. 2021, 152, 105290. [Google Scholar] [CrossRef]
- Thangaraj, A.; Periyasamy, P.; Liao, K.; Bendi, V.S.; Callen, S.; Pendyala, G.; Buch, S. HIV-1 TAT-mediated microglial acti-vation: Role of mitochondrial dysfunction and defective mitophagy. Autophagy 2018, 14, 1596–1619. [Google Scholar] [CrossRef]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural. Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef]
- Islam, M. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, C.-B.; Bedi, H.; Gomez, R.; Khan, A.; Meciszewski, T.; Aalinkeel, R.; Khoo, T.C.; Sharikova, A.V.; Khmaladze, A.; Mahajan, S.D. Telomere Length Shortening in Microglia: Implication for Accelerated Senescence and Neurocognitive Deficits in HIV. Vaccines 2021, 9, 721. https://doi.org/10.3390/vaccines9070721
Hsiao C-B, Bedi H, Gomez R, Khan A, Meciszewski T, Aalinkeel R, Khoo TC, Sharikova AV, Khmaladze A, Mahajan SD. Telomere Length Shortening in Microglia: Implication for Accelerated Senescence and Neurocognitive Deficits in HIV. Vaccines. 2021; 9(7):721. https://doi.org/10.3390/vaccines9070721
Chicago/Turabian StyleHsiao, Chiu-Bin, Harneet Bedi, Raquel Gomez, Ayesha Khan, Taylor Meciszewski, Ravikumar Aalinkeel, Ting Chean Khoo, Anna V. Sharikova, Alexander Khmaladze, and Supriya D. Mahajan. 2021. "Telomere Length Shortening in Microglia: Implication for Accelerated Senescence and Neurocognitive Deficits in HIV" Vaccines 9, no. 7: 721. https://doi.org/10.3390/vaccines9070721
APA StyleHsiao, C.-B., Bedi, H., Gomez, R., Khan, A., Meciszewski, T., Aalinkeel, R., Khoo, T. C., Sharikova, A. V., Khmaladze, A., & Mahajan, S. D. (2021). Telomere Length Shortening in Microglia: Implication for Accelerated Senescence and Neurocognitive Deficits in HIV. Vaccines, 9(7), 721. https://doi.org/10.3390/vaccines9070721